The Natural History and Management of Hepatic Hemangioma
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Methods
2.3. Statistical Analysis
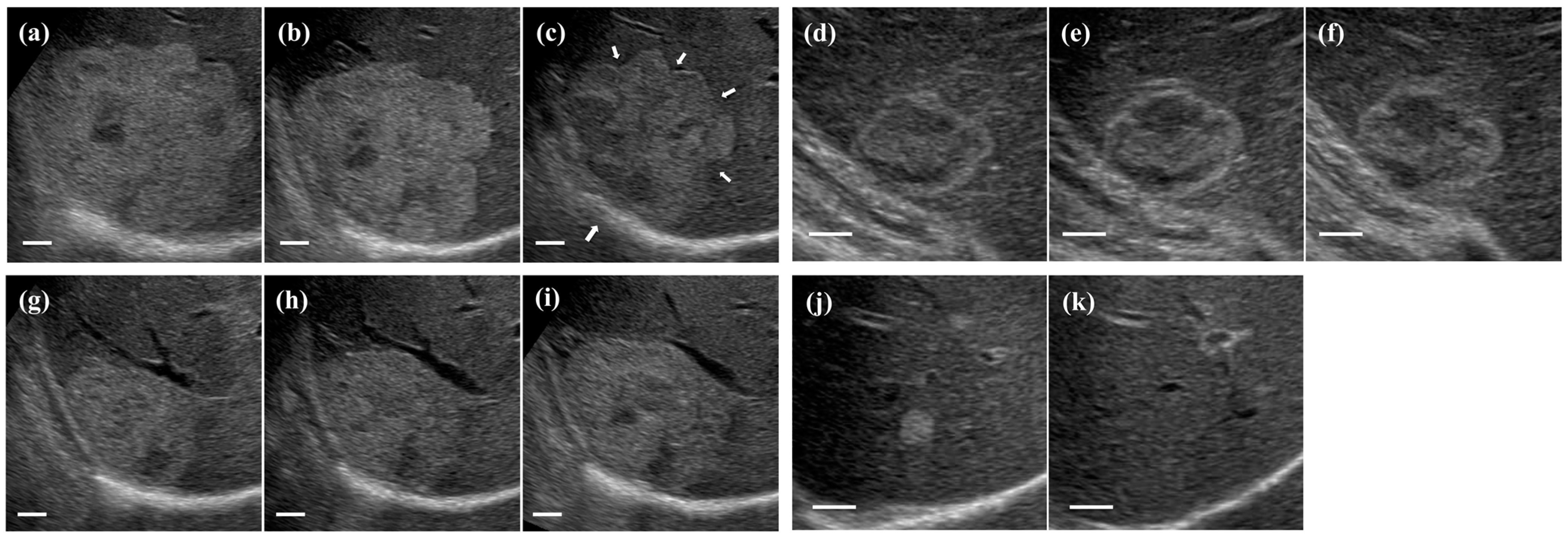
3. Results
3.1. Characteristics of Patients and Hemangiomas
3.2. Relationship between Changes in Hemangioma Size and Clinical Parameters
3.3. Ultrasonographic Follow-Up
3.4. Comparison of Clinical Parameters at the First Examination with Those at the Last Examination in the Follow-Up Period by Change in Hemangioma Size
3.5. Growth Pattern of Hepatic Hemangiomas
3.6. Effects of Age, Liver Disease, and Hemangioma Size on the Changes in Values of M2BPGi
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PT | Prothrombin time |
| TAT | Thrombin–antithrombin III complex |
| FDP | Fibrin and fibrinogen degradation products |
| US | Ultrasonography |
| CT | Computed tomography |
| AFP | α-fetoprotein |
| PIVKA-II | Protein induced by vitamin K absence or antagonist-II |
| M2BPGi | Mac-2 binding protein glycosylation isomer |
| KMS | Kasabach–Merritt syndrome |
References
- Li, J.; Huang, L.; Liu, C.; Yan, J.; Xu, F.; Wu, M.; Yan, Y. New recognition of the natural history and growth pattern of hepatic hemangioma in adults. Hepatol. Res. 2016, 46, 727–733. [Google Scholar]
- Maruyama, S.; Koda, M.; Matono, T.; Isomoto, H. Association of tumor size and internal echo pattern with coagulopathy associated with hepatic hemangiomas. Mol. Clin. Oncol. 2021, 14, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, S.; Matono, T.; Koda, M. Prevalence and characteristics of hepatic hemangioma associated with coagulopathy and its predictive risk factors. J. Clin. Med. 2022, 11, 4347. [Google Scholar] [CrossRef] [PubMed]
- Gandolfi, L.; Leo, P.; Solmi, L.; Verros, G.; Colecchia, A. Natural history of hepatic hemangiomas: Clinical and ultrasound study. Gut 1991, 32, 677–680. [Google Scholar] [CrossRef]
- Isidori, D.C.; Renol, K.; Saif, A.M.; Annalisa, A.; Gaetano, B.; Adriana, T. Giant carvenous liver hemangiomas: Is it the time to change the size categories? Hepatobiliary Pancreat. Dis. Int. 2016, 15, 21–29. [Google Scholar]
- Okano, H.; Shiraki, K.; Inoue, H.; Ito, T.; Yamanaka, T.; Deguchi, T.; Sugimoto, K.; Sakai, T.; Ohmori, S.; Murata, K.; et al. Natural course of cavernous hepatic hemangioma. Oncol. Rep. 2001, 8, 411–414. [Google Scholar] [CrossRef]
- Yeh, W.C.; Yang, P.M.; Huang, G.T.; Sheu, J.C.; Chen, D.S. Long-term follow-up of hepatic hemangiomas by ultrasonography: With emphasis on the growth rate of the tumor. Hepato-gastroenterology 2007, 54, 475–479. [Google Scholar]
- Ito, H.; Tsujimoto, F.; Nakajima, Y.; Igarashi, G.; Okamura, T.; Sakurai, M.; Nobuoka, S.; Otsubo, T. Sonographic characterization of 271 hepatic hemangiomas with typical appearance on CT imaging. J. Med. Ultrason. 2012, 39, 61–68. [Google Scholar] [CrossRef]
- Deha, E.; Olivier, C.B.; Otto, M.D.; Roelof, J.B.; Fiebo, J.W.K.; Dirk, J.G.; Thomas, M.G. Management of liver hemangiomas according to size and symptoms. J. Gastroenterol. Hepatol. 2007, 22, 1953–1958. [Google Scholar]
- Paulo, H.; Marcelo, L.V.C.; Marcel, A.C.M.; Vincenzo, P.; Luis, A.C.M.; Marcel, C.C.M.; Joaquim, J.G.R.; William, A.S. Management of hepatic hemangiomas: A 14-year experience. J. Gastrointest. Surg. 2005, 9, 853–859. [Google Scholar]
- Arye, B.; Michael, P.F.; Jacob, S. Liver lesions with hepatic capsular retraction. Semin. Ultrasound CT MRI 2009, 30, 426–435. [Google Scholar]
- Aibe, H.; Honda, H.; Kuroiwa, T.; Yoshimitsu, H.; Irie, H.; Tajima, T.; Shinozaki, K.; Asayama, Y.; Taguchi, K.; Masuda, K. Sclerosed hemangioma of the liver. Abdom. Imaging 2001, 26, 496–499. [Google Scholar] [CrossRef] [PubMed]
- Goodman, Z.D. Benign tumors of the liver. In Neoplasms of the Liver; Okuda, K., Ishak, K.G., Eds.; Springer: Tokyo, Japan, 1987; pp. 105–125. [Google Scholar]
- Tsumaki, N.; Waguri, N.; Yoneyama, O.; Hama, I.; Kawahisa, J.; Yokoo, T.; Aiba, T.; Furukawa, K.; Sugimura, K.; Igarashi, K.; et al. A case of sclerosed hemangioma with a significant morphological change over a period of 17 years. Kanzo 2008, 49, 268–274. [Google Scholar] [CrossRef]
- Ogawa, K.; Takeuchi, K.; Okuda, C.; Tamura, T.; Koizumi, Y.; Koyama, R.; Imamura, T.; Inoue, Y.; Kuwayama, M.; Arase, Y. Change in size of hepatic hemangiomas during long-term observation: 80 lesions with observation for over 10 years. Jpn. J. Med. Ultrason. 2014, 41, 749–756. [Google Scholar] [CrossRef]
- Miyaki, D.; Aikata, H.; Waki, K.; Murakami, H.; Hashimoto, Y.; Nagaoki, Y.; Katamura, Y.; Kataoka, T.; Takagi, S.; Hiramatsu, K.; et al. Significant regression of a cavernous hepatic hemangioma to a sclerosed hemangioma over 12 years: A case study. Nihon Shokakibyo Gakkai Zasshi 2011, 108, 954–961. [Google Scholar] [PubMed]
- Yang, D.M.; Kim, H.S.; Cho, S.W.; Kim, H.S. Various causes of hepatic capsular retraction: CT and MR findings. Br. J. Radiol. 2002, 75, 994–1002. [Google Scholar] [CrossRef]
- Bos, A.J.G.; Yuan, P.; Maruta, A.; Matsuyama, S.; Arai, M.; Okazaki, I. Effects of aging on urinary secretion of 3-hydroxyproline and its importance for cancer screening in the elderly with ROC analysis. Jpn. J. Hyg. 1994, 49, 797–806. [Google Scholar] [CrossRef]
- Nicoletta, G.; Beatrice, A.; Fabio, A.; Serge, M.; Jacopo, T.; Nicola, D.; Carlo, V.; Giorgio, A. Reduced collagenolytic activity of matrix metalloproteinases and development of liver fibrosis in the aging rat. Mech. Aging Dev. 2002, 123, 413–425. [Google Scholar]
- Okazaki, I.; Inagaki, Y.; Higashiyama, R. Aging and hepatic fibrogenesis. KAN TAN SUI 2006, 53, 81–86. [Google Scholar]
- Toshima, T.; Shirabe, K.; Ikegami, T.; Yoshizumi, T.; Kuno, A.; Togayachi, A.; Gotoh, M.; Narimatsu, H.; Korenaga, M.; Mizokami, M.; et al. A novel serum marker, glycosylated Wisteria floribunda agglutinin-positive Mac-2 binding protein (WFA+-M2BP), for assessing liver fibrosis. J. Gastroenterol. 2015, 50, 76–84. [Google Scholar] [CrossRef]
- Yamasaki, K.; Tateyama, M.; Abiru, S.; Komori, A.; Nagaoka, S.; Saeki, S.; Hashimoto, S.; Sasaki, R.; Bekki, S.; Kugiyama, Y.; et al. Elevated serum levels of Wisteria Floribunda agglutinin-positive human Mac-2 binding protein predict the development of hepatocellular carcinoma in hepatitis C patients. Hepatology 2014, 60, 1563–1570. [Google Scholar] [CrossRef] [PubMed]
- Nagakawa, M.; Nawa, N.; Takeichi, E.; Shimizu, T.; Tsuchiya, J.; Sato, A.; Miyoshi, M.; Kawai, F.; Murawaki, M.; Nitta, S.; et al. Mac-2 binding protein glycosylation isomer as a novel predictive biomarker for patient survival after hepatitis C virus eradication by DAAs. J. Gastroenterol. 2020, 55, 990–999. [Google Scholar] [CrossRef] [PubMed]
- Shirabe, K.; Bekki, Y.; Gantumur, D.; Araki, K.; Ishii, N.; Kuno, A.; Narimatsu, H.; Mizokami, M. Mac-2 binding protein glycan isomer (M2BPGi) is a new serum biomarker for assessing liver fibrosis: More than a biomarker of liver fibrosis. J. Gastroenterol. 2018, 53, 819–826. [Google Scholar] [CrossRef]
- Ishii, N.; Harimoto, N.; Araki, K.; Muranushi, R.; Hoshino, K.; Hagiwara, K.; Gantumur, D.; Yamasaki, T.; Tsukagoshi, M.; Igarashi, T.; et al. Preoperative Mac-2 binding protein glycosylation isomer level predicts postoperative ascites in patients with hepatic resection for hepatocellular carcinoma. Hepatol. Res. 2019, 49, 1398–1405. [Google Scholar] [CrossRef] [PubMed]
- Terkivatan, T.; Vrijiland, W.W.; Hoed, P.T.; Man, R.A.; Hussain, S.M.; Tilanus, H.W.; IJzermans, J.N.M. Size of lesion is not a criterion for resection during management of giant liver haemangioma. Br. J. Surg. 2002, 89, 1240–1244. [Google Scholar] [CrossRef]
- Wei, Z.; Zhi-Yong, H.; Chang-Shu, K.; Chao, W.; Zhi-Wei, Z.; Bi-Xiang, Z.; Yi-Fa, C.; Wan-Guang, Z.; Peng, Z.; Xiao-Ping, C. Surgical treatment of giant liver hemangioma larger than 10 cm: A single center’s experience with 86 patients. Medicine 2015, 94, e1420. [Google Scholar]
- Vilmarie, R.; Adrianna, L.; Patricia, M.V.; Peter, A.A. Kasabach-Merritt phenomenon: Case series and retrospective review of the Mayo Clinic experience. J. Pediatr. Hematol. Oncol. 2009, 31, 522–526. [Google Scholar]
- Aslan, A.; Vilsendorf, A.M.Z.; Kleine, M.; Bredt, M.; Bektas, H. Adult Kasabach-Merritt syndrome due to hepatic giant hemangioma. Case Rep. Gastroenterol. 2009, 3, 306–313. [Google Scholar] [CrossRef]
- Pietrabissa, A.; Giulianotti, P.; Campatelli, A.; Dicandio, G.; Farina, F.; Signori, S.; Mosca, F. Management and follow-up of 78 giant haemangiomas of the liver. Br. J. Surg. 1996, 83, 915–918. [Google Scholar] [CrossRef]
- Marcello, D.; Gregor, A.S.; Angelo, D.; Karl, J.O. The risk of spontaneous rupture of liver hemangiomas: A critical review of the literature. J. Hepatobiliary Pancreat. Sci. 2011, 18, 797–805. [Google Scholar]
- Ebina, Y.; Hazama, R.; Nishimoto, M.; Tanimura, K.; Miyahara, Y.; Morizane, M.; Nakabayashi, K.; Fukumoto, T.; Ku, Y.; Yamada, H. Resection of giant liver hemangioma in a pregnant woman with coagulopathy: Case report and literature review. J. Prenatal. Med. 2011, 5, 93–96. [Google Scholar]

| Parameters | Value | |
|---|---|---|
| Age (years) | 57 ± 14 | |
| Male/female (n) | 77/134 | |
| Follow-up period (months) | 56.5 ± 14.5 | |
| Size of hemangioma (mm) | 22.1 ± 17.8 | |
| Small | <20 | 123 (58.3%) |
| Medium | 20–40 | 63 (29.9%) |
| Large | >40 | 25 (11.8%) |
| Location of hemangioma (n) | ||
| Right lobe | 184 (87.2%) | |
| Left lobe | 25 (11.8%) | |
| Bilateral lobe | 2 (1.0%) | |
| Number of hemangiomas (n) | ||
| Single | 180 (85.3%) | |
| Multiple | 31 (14.7%) | |
| Biochemistry | ||
| Total bilirubin (mg/dL) | 0.6 ± 0.2 | |
| Albumin (g/dL) | 4.2 ± 0.2 | |
| ALT (U/L) | 20 ± 13 | |
| GGT (U/L) | 42 ± 51 | |
| ALP (U/L) | 232 ± 72 | |
| BUN (mg/dL) | 14.6 ± 3.7 | |
| Cr (mg/dL) | 0.72 ± 0.18 | |
| Hematology | ||
| Hemoglobin (g/dL) | 13.6 ± 1.3 | |
| Platelets (104/μL) | 22.3 ± 5.5 | |
| Coagulation | ||
| PT (%) | 94.0 ± 12.8 | |
| Fibrinogen (mg/dL) | 276 ± 67 | |
| TAT (ng/mL) | 1.42 ± 1.06 | |
| D-dimer (μg/mL) | 0.72 ± 0.69 | |
| FDP (μg/mL) | 1.63 ± 1.00 | |
| Serology | ||
| M2BPGi (COI) | 0.61 ± 0.33 | |
| AFP (ng/mL) | 3.5 ± 1.5 | |
| PIVKA-II (mAU/mL) | 20.4 ± 5.8 | |
| Associated liver diseases (n) | ||
| Hepatitis B | 12 (5.7%) | |
| Hepatitis C | 5 (2.4%) | |
| Autoimmune hepatitis | 7 (3.3%) | |
| Primary biliary cholangitis | 2 (0.9%) | |
| Alcoholic liver disease | 16 (7.6%) | |
| Nonalcoholic steatohepatitis | 5 (2.4%) | |
| Parameters | Small (n = 123) | Medium (n = 63) | Large (n = 25) | p Value |
|---|---|---|---|---|
| Age (years) | 55 ± 15 | 57 ± 14 | 64 ±12 | 0.0309 |
| Male/female (n) | 41/82 | 27/36 | 11/14 | 0.4988 |
| Biochemistry | ||||
| Total bilirubin (mg/dL) | 0.6 ± 0.2 | 0.6 ± 0.3 | 0.6 ± 0.2 | 0.7739 |
| Albumin (g/dL) | 4.2 ± 0.2 | 4.3 ± 0.2 | 4.0 ± 0.3 | 0.0036 |
| ALT (U/L) | 21 ± 16 | 20 ± 10 | 16 ± 6 | 0.1880 |
| GGT (U/L) | 43 ± 59 | 43 ± 40 | 30 ± 29 | 0.1582 |
| ALP (U/L) | 230 ± 73 | 234 ± 68 | 236 ± 82 | 0.9203 |
| BUN (mg/dL) | 14.2 ± 3.5 | 14.6 ± 3.8 | 16.4 ± 3.8 | 0.0314 |
| Cr (mg/dL) | 0.71 ± 0.15 | 0.73 ± 0.22 | 0.77 ± 0.18 | 0.3940 |
| Hematology | ||||
| Hemoglobin (g/dL) | 13.7 ± 1.2 | 13.7 ±1.2 | 12.8 ± 1.7 | 0.0246 |
| Platelets (104/μL) | 22.7 ± 5.2 | 21.6 ± 5.5 | 20.0 ± 6.5 | 0.0159 |
| Coagulation | ||||
| PT (%) | 93.0 ± 12.9 | 95.9 ± 12.8 | 95.2 ± 10.4 | 0.4029 |
| Fibrinogen (mg/dL) | 284 ± 66 | 276 ± 66 | 238 ± 63 | 0.0104 |
| TAT (ng/mL) | 1.11 ± 0.52 | 1.26 ± 0.62 | 3.36 ± 1.71 | <0.0001 |
| D-dimer (μg/mL) | 0.50 ± 0.30 | 0.61 ± 0.34 | 2.06 ± 1.13 | <0.0001 |
| FDP (μg/mL) | 1.33 ± 0.22 | 1.55 ± 0.63 | 3.33 ± 1.92 | <0.0001 |
| Serology | ||||
| M2BPGi (COI) | 0.53 ± 0.27 | 0.67 ± 0.35 | 0.88 ± 0.32 | <0.0001 |
| AFP (ng/mL) | 3.3 ± 1.5 | 4.3 ± 1.5 | 3.4 ± 1.8 | 0.0021 |
| PIVKA-II (mAU/mL) | 19.5 ± 5.5 | 20.8 ± 5.9 | 23.6 ± 6.4 | 0.0077 |
| Ultrasound findings | ||||
| Echo pattern | <0.0001 | |||
| Homogeneous type (n) | 122 (99.2%) | 27 (42.9%) | 0 (0%) | |
| Mixed type (n) | 1 (0.8%) | 36 (57.1%) | 25 (100%) | |
| Portal vein diameter (mm) | 10.3 ± 2.0 | 11.2 ± 1.7 | 12.8 ± 1.9 | <0.0001 |
| Spleen index (mm2) | 1220 ± 490 | 1259 ± 468 | 1702 ± 639 | 0.0013 |
| Parameters | Decrease (n = 82) | No Change (n = 66) | Increase (n = 63) | p Value |
|---|---|---|---|---|
| Age (years) | 64 ± 11 | 55 ± 13 | 48 ± 14 | <0.0001 |
| Male/female (n) | 27/55 | 23/43 | 27/36 | 0.5881 |
| Follow-up period (months) | 58.0 ± 10.9 | 52.8 ± 11.9 | 50.9 ± 10.5 | 0.0041 |
| Biochemistry | ||||
| Total bilirubin (mg/dL) | 0.6 ± 0.2 | 0.6 ± 0.3 | 0.6 ± 0.2 | 0.6692 |
| Albumin (g/dL) | 4.1 ± 0.3 | 4.3 ± 0.2 | 4.3 ± 0.2 | 0.0037 |
| ALT (U/L) | 22 ± 18 | 19 ± 9 | 19 ± 10 | 0.6414 |
| GGT (U/L) | 45 ± 69 | 33 ± 27 | 46 ± 41 | 0.3001 |
| ALP (U/L) | 244 ± 66 | 233 ± 87 | 215 ± 57 | 0.0463 |
| BUN (mg/dL) | 15.4 ± 3.8 | 14.6 ± 3.3 | 13.6 ± 3.7 | 0.0501 |
| Cr (mg/dL) | 0.74 ± 0.19 | 0.69 ± 0.14 | 0.72 ± 0.20 | 0.1558 |
| Hematology | ||||
| Hemoglobin (g/dL) | 13.4 ± 1.3 | 13.7 ± 1.2 | 13.6 ± 1.3 | 0.3195 |
| Platelets (104/μL) | 21.0 ± 5.4 | 22.7 ± 5.5 | 23.2 ± 5.3 | 0.0033 |
| Coagulation | ||||
| PT (%) | 95.3 ± 13.1 | 92.9 ± 13.0 | 93.5 ± 11.8 | 0.5096 |
| Fibrinogen (mg/dL) | 268 ± 65 | 284 ± 69 | 279 ± 67 | 0.3484 |
| TAT (ng/mL) | 1.81 ± 1.37 | 1.08 ± 0.50 | 1.27 ± 0.86 | 0.0006 |
| D-dimer (μg/mL) | 0.99 ± 0.96 | 0.49 ± 0.33 | 0.58 ± 0.35 | <0.0001 |
| FDP (μg/mL) | 1.96 ± 1.42 | 1.37 ± 0.38 | 1.47 ± 0.51 | 0.0002 |
| Serology | ||||
| M2BPGi (COI) | 0.72 ± 0.37 | 0.48 ± 0.22 | 0.60 ± 0.30 | 0.0003 |
| AFP (ng/mL) | 3.5 ± 1.5 | 3.6 ± 1.6 | 3.5 ± 1.5 | 0.9777 |
| PIVKA-II (mAU/mL) | 20.4 ± 6.2 | 20.8 ± 5.6 | 19.8 ± 5.5 | 0.5597 |
| Ultrasound findings | ||||
| Tumor size (mm) | 30.8 ± 22.8 | 14.1 ± 7.9 | 18.6 ± 10.4 | <0.0001 |
| Echo pattern | <0.0001 | |||
| Homogeneous type (n) | 47 (57.3%) | 59 (89.4%) | 44 (69.8%) | |
| Mixed type (n) | 35 (42.7%) | 7 (10.6%) | 19 (30.2%) | |
| Portal vein diameter (mm) | 11.0 ± 2.2 | 10.5 ± 2.1 | 11.2 ± 1.8 | 0.0219 |
| Spleen index (mm2) | 1273 ± 525 | 1180 ± 469 | 1432 ± 554 | 0.0143 |
| Decrease (n = 82) | No Change (n = 66) | Increase (n = 63) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Parameters | First | Last | p Value | First | Last | p Value | First | Last | p Value |
| Tumor size (mm) | 30.6 ± 22.8 | 23.3 ± 20.0 | 0.0054 | 14.1 ± 7.9 | 13.8 ± 8.3 | 0.4412 | 18.6 ± 10.4 | 24.5 ± 13.2 | 0.0034 |
| Biochemistry | |||||||||
| Total bilirubin (mg/dL) | 0.6 ± 0.2 | 0.6 ± 0.2 | 0.2129 | 0.6 ± 0.3 | 0.6 ± 0.2 | 0.2749 | 0.6 ± 0.2 | 0.6 ± 0.2 | 0.0539 |
| Albumin (g/dL) | 4.1 ± 0.3 | 4.1 ± 0.3 | 0.4147 | 4.3 ± 0.2 | 4.3 ± 0.2 | 0.1409 | 4.3 ± 0.2 | 4.3 ± 0.2 | 0.0647 |
| ALT (U/L) | 22 ± 18 | 21 ± 10 | 0.8174 | 19 ± 9 | 21 ± 10 | 0.0196 | 19 ± 10 | 21 ± 10 | 0.0083 |
| GGT (U/L) | 45 ± 69 | 47 ± 58 | 0.6030 | 33 ± 27 | 38 ± 32 | 0.0137 | 46 ± 41 | 49 ± 45 | 0.4218 |
| ALP (U/L) | 244 ± 66 | 265 ± 65 | 0.0031 | 233 ± 87 | 241 ± 85 | 0.0857 | 215 ± 57 | 227 ± 57 | 0.1314 |
| Hematology | |||||||||
| Hemoglobin (g/dL) | 13.4 ± 1.3 | 13.4 ± 1.3 | 0.3156 | 13.7 ± 1.2 | 13.9 ± 1.1 | 0.4698 | 13.6 ± 1.3 | 13.7 ± 1.5 | 0.1506 |
| Platelets (104/μL) | 21.0 ± 5.4 | 21.0 ± 5.6 | 0.6953 | 22.7 ± 5.5 | 21.1 ± 4.6 | 0.0020 | 23.2 ± 5.3 | 21.8 ± 4.7 | 0.0064 |
| Coagulation | |||||||||
| PT (%) | 95.3 ± 13.1 | 99.0 ± 12.6 | 0.2421 | 92.9 ± 13.0 | 91.6 ± 10.0 | 0.3815 | 93.5 ± 11.8 | 94.7 ± 11.1 | 0.2745 |
| Fibrinogen (mg/dL) | 268 ± 65 | 281 ± 54 | 0.4427 | 284 ± 69 | 272 ± 69 | 0.3506 | 279 ± 67 | 264 ± 55 | 0.0154 |
| TAT (ng/mL) | 1.81 ± 1.37 | 1.39 ± 1.00 | 0.0455 | 1.08 ± 0.50 | 1.11 ± 0.67 | 0.5944 | 1.27 ± 0.86 | 1.72 ± 1.13 | 0.0102 |
| D-dimer (μg/mL) | 0.99 ± 0.96 | 0.67 ± 0.58 | <0.0001 | 0.49 ± 0.33 | 0.46 ± 0.31 | 0.5170 | 0.58 ± 0.35 | 0.73 ± 0.38 | 0.0003 |
| FDP (μg/mL) | 1.96 ± 1.42 | 1.66 ± 0.94 | 0.0003 | 1.37 ± 0.38 | 1.44 ± 0.49 | 0.5159 | 1.47 ± 0.51 | 1.64 ± 0.74 | 0.0129 |
| Serology | |||||||||
| M2BPGi (COI) | 0.72 ± 0.37 | 1.05 ± 0.49 | <0.0001 | 0.48 ± 0.22 | 0.56 ± 0.28 | 0.0610 | 0.60 ± 0.30 | 0.53 ± 0.24 | 0.0438 |
| AFP (ng/mL) | 3.5 ± 1.5 | 3.9 ± 1.8 | 0.0319 | 3.6 ± 1.6 | 3.9 ± 1.6 | 0.0221 | 3.5 ± 1.5 | 3.6 ± 1.7 | 0.8687 |
| PIVKA-II (mAU/mL) | 20.4 ± 6.2 | 21.7 ± 7.5 | 0.3236 | 20.8 ± 5.6 | 21.1 ± 5.8 | 0.1656 | 19.8 ± 5.5 | 20.5 ± 6.5 | 0.2711 |
| Ultrasound findings | |||||||||
| Portal vein diameter (mm) | 11.0 ± 2.2 | 11.1 ± 2.3 | 0.3156 | 10.5 ± 2.1 | 10.5 ± 1.8 | 0.9435 | 11.2 ± 1.8 | 11.1 ± 2.1 | 0.5667 |
| Spleen index (mm2) | 1273 ± 525 | 1306 ± 515 | 0.9915 | 1180 ± 469 | 1162 ± 473 | 0.7020 | 1432 ± 554 | 1409 ± 513 | 0.8461 |
| >60 y | 40–60 y | <40 y | p Value | |
|---|---|---|---|---|
| All patients (n = 321) | 0.64 ± 0.33 (n = 105) | 0.53 ± 0.29 (n = 156) | 0.41 ± 0.18 (n = 60) | 0.00002 |
| Liver disease (−) (n = 274) | 0.61 ± 0.33 (n = 87) | 0.50 ± 0.28 (n = 128) | 0.41 ± 0.19 (n = 59) | 0.00013 |
| Liver disease (+) (n = 47) | 0.77 ± 0.32 (n = 18) | 0.65 ± 0.28 (n = 29) | 0.157 | |
| Small (n = 211) | 0.59 ± 0.28 (n = 64) | 0.46 ± 0.24 (n = 100) | 0.36 ± 0.13 (n = 47) | 0.00002 |
| Medium (n = 83) | 0.63 ± 0.41 (n = 30) | 0.60 ± 0.31 (n = 42) | 0.55 ± 0.22 (n = 11) | 0.713 |
| Large (n = 27) | 0.89 ± 0.23 (n = 12) | 0.82 ± 0.31 (n = 13) | 0.80 ± 0.14 (n = 2) | 0.235 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maruyama, S.; Matono, T.; Koda, M. The Natural History and Management of Hepatic Hemangioma. J. Clin. Med. 2023, 12, 5703. https://doi.org/10.3390/jcm12175703
Maruyama S, Matono T, Koda M. The Natural History and Management of Hepatic Hemangioma. Journal of Clinical Medicine. 2023; 12(17):5703. https://doi.org/10.3390/jcm12175703
Chicago/Turabian StyleMaruyama, Shigeo, Tomomitsu Matono, and Masahiko Koda. 2023. "The Natural History and Management of Hepatic Hemangioma" Journal of Clinical Medicine 12, no. 17: 5703. https://doi.org/10.3390/jcm12175703




