Are We Ready to Reclassify Crohn’s Disease Using Molecular Classification?
Abstract
:1. Introduction
1.1. Crohn’s Disease
1.2. Current Classification of CD
1.3. Problems with Current CD Classification Systems
2. Serological Markers
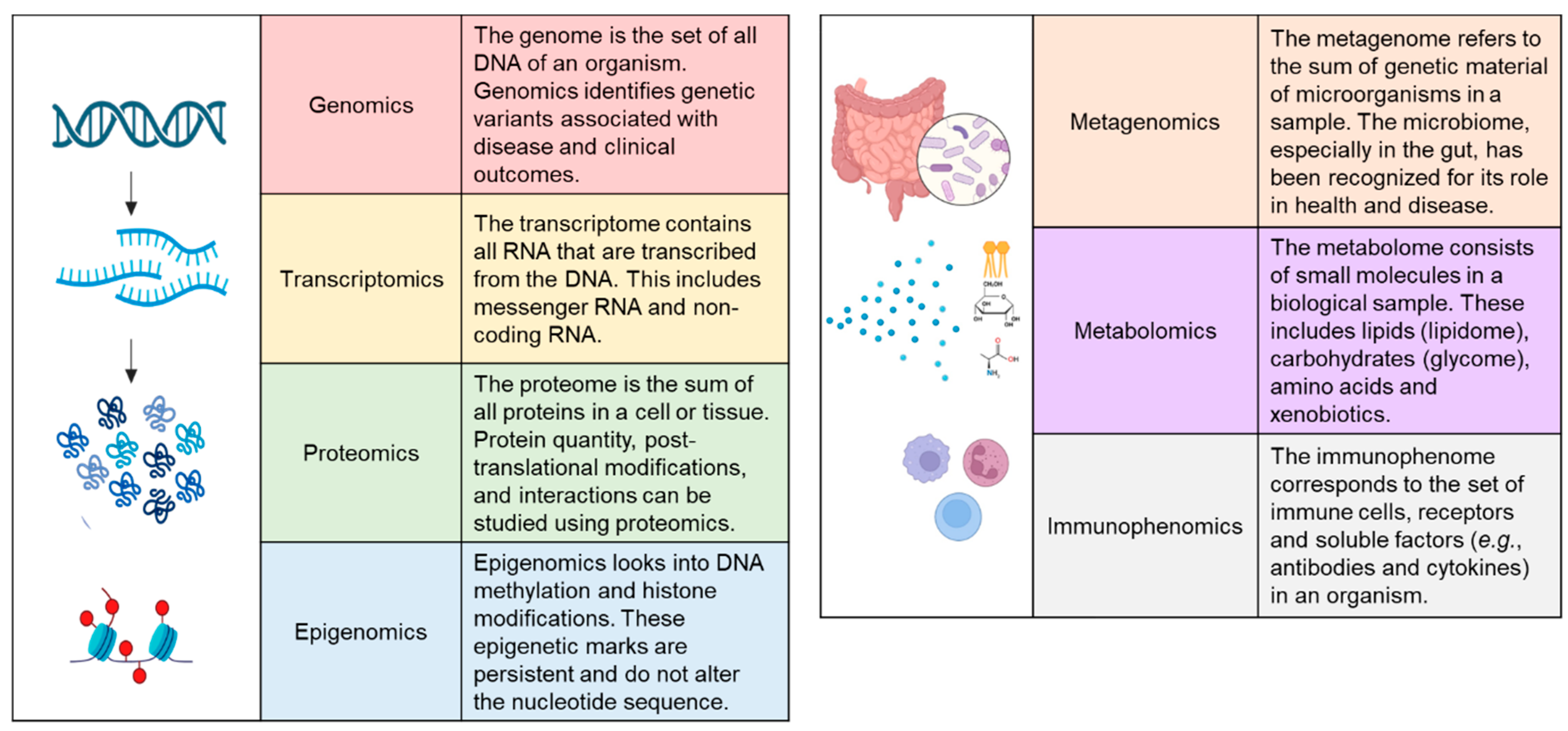
3. Omics Approaches for the Molecular Classification of CD
4. Genomics
5. Transcriptomics
6. Proteomics
7. Epigenomics
8. Metagenomics
9. Metabolomics
10. Lipidomics
11. Immunophenomics
12. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, R.; Li, Z.; Liu, S.; Zhang, D. Global, Regional and National Burden of Inflammatory Bowel Disease in 204 Countries and Territories from 1990 to 2019: A Systematic Analysis Based on the Global Burden of Disease Study 2019. BMJ Open 2023, 13, e065186. [Google Scholar] [CrossRef]
- Cho, C.W.; You, M.-W.; Oh, C.H.; Lee, C.K.; Moon, S.K. Long-Term Disease Course of Crohn’s Disease: Changes in Disease Location, Phenotype, Activities, and Predictive Factors. Gut Liver 2022, 16, 157–170. [Google Scholar] [CrossRef] [PubMed]
- Lichtenstein, G.R.; Loftus, E.V.; Isaacs, K.L.; Regueiro, M.D.; Gerson, L.B.; Sands, B.E. ACG Clinical Guideline: Management of Crohn’s Disease in Adults. Am. J. Gastroenterol. 2018, 113, 481–517. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, S.; Ekbom, A.; Rhodes, J.M. Recent Advances in Clinical Practice: A Systematic Review of Isolated Colonic Crohn’s Disease: The Third IBD? Gut 2017, 66, 362–381. [Google Scholar] [CrossRef]
- Perler, B.K.; Ungaro, R.; Baird, G.; Mallette, M.; Bright, R.; Shah, S.; Shapiro, J.; Sands, B.E. Presenting Symptoms in Inflammatory Bowel Disease: Descriptive Analysis of a Community-Based Inception Cohort. BMC Gastroenterol. 2019, 19, 47. [Google Scholar] [CrossRef]
- Singh, S.; Blanchard, A.; Walker, J.R.; Graff, L.A.; Miller, N.; Bernstein, C.N. Common Symptoms and Stressors Among Individuals With Inflammatory Bowel Diseases. Clin. Gastroenterol. Hepatol. 2011, 9, 769–775. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.-C.; Wu, T.-C.; Lo, Y.-C.; Wang, L.-S. Gastrointestinal Complications and Extraintestinal Manifestations of Inflammatory Bowel Disease in Taiwan: A Population-Based Study. J. Chin. Med. Assoc. 2017, 80, 56. [Google Scholar] [CrossRef]
- Li, Y.; Chen, B.; Gao, X.; Hu, N.; Huang, M.; Ran, Z.; Liu, Z.; Zhong, J.; Zou, D.; Wu, X.; et al. Current Diagnosis and Management of Crohn’s Disease in China: Results from a Multicenter Prospective Disease Registry. BMC Gastroenterol. 2019, 19, 145. [Google Scholar] [CrossRef]
- Shivashankar, R.; Tremaine, W.J.; Harmsen, W.S.; Loftus, E.V. Incidence and Prevalence of Crohn’s Disease and Ulcerative Colitis in Olmsted County, Minnesota From 1970 Through 2010. Clin. Gastroenterol. Hepatol. 2017, 15, 857–863. [Google Scholar] [CrossRef]
- Gasche, C.; Scholmerich, J.; Brynskov, J.; D’Haens, G.; Hanauer, S.B.; Irvine, E.J.; Jewell, D.P.; Rachmilewitz, D.; Sachar, D.B.; Sandborn, W.J.; et al. A Simple Classification of Crohn’s Disease: Report of the Working Party for the World Congresses of Gastroenterology, Vienna 1998. Inflamm. Bowel. Dis. 2000, 6, 8–15. [Google Scholar] [CrossRef]
- Silverberg, M.S.; Satsangi, J.; Ahmad, T.; Arnott, I.D.R.; Bernstein, C.N.; Brant, S.R.; Caprilli, R.; Colombel, J.-F.; Gasche, C.; Geboes, K.; et al. Toward an Integrated Clinical, Molecular and Serological Classification of Inflammatory Bowel Disease: Report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. Can. J. Gastroenterol. 2005, 19 (Suppl. A), 5A–36A. [Google Scholar] [CrossRef] [PubMed]
- Satsangi, J.; Silverberg, M.S.; Vermeire, S.; Colombel, J. The Montreal Classification of Inflammatory Bowel Disease: Controversies, Consensus, and Implications. Gut 2006, 55, 749–753. [Google Scholar] [CrossRef] [PubMed]
- Chamouard, P.; Richert, Z.; Meyer, N.; Rahmi, G.; Baumann, R. Diagnostic Value of C-Reactive Protein for Predicting Activity Level of Crohn’s Disease. Clin. Gastroenterol. Hepatol. 2006, 4, 882–887. [Google Scholar] [CrossRef]
- Mosli, M.H.; Zou, G.; Garg, S.K.; Feagan, S.G.; MacDonald, J.K.; Chande, N.; Sandborn, W.J.; Feagan, B.G. C-Reactive Protein, Fecal Calprotectin, and Stool Lactoferrin for Detection of Endoscopic Activity in Symptomatic Inflammatory Bowel Disease Patients: A Systematic Review and Meta-Analysis. Am. J. Gastroenterol. 2015, 110, 802–819, quiz 820. [Google Scholar] [CrossRef]
- Chow, D.K.L.; Leong, R.W.L.; Lai, L.H.; Wong, G.L.H.; Leung, W.-K.; Chan, F.K.L.; Sung, J.J.Y. Changes in Crohn’s Disease Phenotype over Time in the Chinese Population: Validation of the Montreal Classification System. Inflamm. Bowel. Dis. 2008, 14, 536–541. [Google Scholar] [CrossRef] [PubMed]
- Torres, U.d.S.; Rodrigues, J.O.; Junqueira, M.S.G.; Uezato, S.; Netinho, J.G. The Montreal Classification for Crohn’s Disease: Clinical Application to a Brazilian Single-Center Cohort of 90 Consecutive Patients. Arq. Gastroenterol. 2010, 47, 279–284. [Google Scholar] [CrossRef]
- Cosnes, J.; Cattan, S.; Blain, A.; Beaugerie, L.; Carbonnel, F.; Parc, R.; Gendre, J.-P. Long-Term Evolution of Disease Behavior of Crohn’s Disease. Inflamm. Bowel. Dis. 2002, 8, 244–250. [Google Scholar] [CrossRef]
- Lovasz, B.D.; Lakatos, L.; Horvath, A.; Szita, I.; Pandur, T.; Mandel, M.; Vegh, Z.; Golovics, P.A.; Mester, G.; Balogh, M.; et al. Evolution of Disease Phenotype in Adult and Pediatric Onset Crohn’s Disease in a Population-Based Cohort. World J. Gastroenterol. 2013, 19, 2217–2226. [Google Scholar] [CrossRef]
- Louis, E.; Collard, A.; Oger, A.; Degroote, E.; El Yafi, F.A.N.; Belaiche, J. Behaviour of Crohn’s Disease According to the Vienna Classification: Changing Pattern over the Course of the Disease. Gut 2001, 49, 777–782. [Google Scholar] [CrossRef] [PubMed]
- Irwin, J.; Ferguson, E.; Simms, L.A.; Hanigan, K.; Carbonnel, F.; Radford-Smith, G. A Rolling Phenotype in Crohn’s Disease. PLoS ONE 2017, 12, e0174954. [Google Scholar] [CrossRef] [PubMed]
- Sudhakar, P.; Verstockt, B.; Cremer, J.; Verstockt, S.; Sabino, J.; Ferrante, M.; Vermeire, S. Understanding the Molecular Drivers of Disease Heterogeneity in Crohn’s Disease Using Multi-Omic Data Integration and Network Analysis. Inflamm. Bowel. Dis. 2020, 27, 870–886. [Google Scholar] [CrossRef]
- Atreya, R.; Siegmund, B. Location Is Important: Differentiation between Ileal and Colonic Crohn’s Disease. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 544–558. [Google Scholar] [CrossRef] [PubMed]
- Weiser, M.; Simon, J.M.; Kochar, B.; Tovar, A.; Israel, J.W.; Robinson, A.; Gipson, G.R.; Schaner, M.S.; Herfarth, H.H.; Sartor, R.B.; et al. Molecular Classification of Crohn’s Disease Reveals Two Clinically Relevant Subtypes. Gut 2018, 67, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.-N.; Zheng, D.-P.; Qiu, Y.; Zhang, S.-H.; He, Y.; Chen, B.-L.; Zeng, Z.-R.; Mao, R.; Chen, M.-H. Classifying Crohn’s Disease into Colon-Involving versus Non-Colon-Involving Groups Is a Better Predictor of Clinical Outcomes than the Montreal Classification. Therap. Adv. Gastroenterol. 2020, 13, 1756284820968732. [Google Scholar] [CrossRef] [PubMed]
- Durko, Ł.; Stasikowska-Kanicka, O.A.; Wagrowska-Danilewicz, M.; Danilewicz, M.; Małecka-Panas, E.I. An Analysis of the Correlation of Clinical, Endoscopic and Histological Classifications in Crohn’s Disease. Prz. Gastroenterol. 2013, 8, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Weng, J.; Lin, X.; Chen, X.; Liang, Y.; Xu, Y.; Cai, J.; Lu, P.; Rong, Y.; Zou, Y.; Zhu, L. Crohn’s Disease Patients with L4-Esophagogastroduodenal Phenotype Is Associated with a Better Prognosis: A Retrospective Cohort Study. Front. Pharmacol. 2022, 13, 963892. [Google Scholar] [CrossRef]
- Mazor, Y.; Maza, I.; Kaufman, E.; Ben-Horin, S.; Karban, A.; Chowers, Y.; Eliakim, R. Prediction of Disease Complication Occurrence in Crohn’s Disease Using Phenotype and Genotype Parameters at Diagnosis. J. Crohn’s Colitis 2011, 5, 592–597. [Google Scholar] [CrossRef]
- Spekhorst, L.M.; Visschedijk, M.C.; Alberts, R.; Festen, E.A.; van der Wouden, E.-J.; Dijkstra, G.; Weersma, R.K. Performance of the Montreal Classification for Inflammatory Bowel Diseases. World J. Gastroenterol. 2014, 20, 15374–15381. [Google Scholar] [CrossRef]
- Lo, B.; Vind, I.; Vester-Andersen, M.K.; Burisch, J. Validation of Ulcerative Colitis and Crohn’s Disease and Their Phenotypes in the Danish National Patient Registry Using a Population-Based Cohort. Scand J. Gastroenterol. 2020, 55, 1171–1175. [Google Scholar] [CrossRef]
- Gradishar, W.J.; Moran, M.S.; Abraham, J.; Aft, R.; Agnese, D.; Allison, K.H.; Anderson, B.; Burstein, H.J.; Chew, H.; Dang, C.; et al. Breast Cancer, Version 3.2022, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Canc. Netw. 2022, 20, 691–722. [Google Scholar] [CrossRef]
- Vermeire, S.; Van Assche, G.; Rutgeerts, P. Classification of Inflammatory Bowel Disease: The Old and the New. Curr. Opin. Gastroenterol. 2012, 28, 321. [Google Scholar] [CrossRef] [PubMed]
- Ferrante, M.; Henckaerts, L.; Joossens, M.; Pierik, M.; Joossens, S.; Dotan, N.; Norman, G.L.; Altstock, R.T.; Van Steen, K.; Rutgeerts, P.; et al. New Serological Markers in Inflammatory Bowel Disease Are Associated with Complicated Disease Behaviour. Gut 2007, 56, 1394–1403. [Google Scholar] [CrossRef] [PubMed]
- Dotan, I.; Fishman, S.; Dgani, Y.; Schwartz, M.; Karban, A.; Lerner, A.; Weishauss, O.; Spector, L.; Shtevi, A.; Altstock, R.T.; et al. Antibodies against Laminaribioside and Chitobioside Are Novel Serologic Markers in Crohn’s Disease. Gastroenterology 2006, 131, 366–378. [Google Scholar] [CrossRef] [PubMed]
- Vasiliauskas, E.; Kam, L.; Karp, L.; Gaiennie, J.; Yang, H.; Targan, S. Marker Antibody Expression Stratifies Crohn’s Disease into Immunologically Homogeneous Subgroups with Distinct Clinical Characteristics. Gut 2000, 47, 487–496. [Google Scholar] [CrossRef] [PubMed]
- Arnott, I.D.R.; Landers, C.J.; Nimmo, E.J.; Drummond, H.E.; Smith, B.K.R.; Targan, S.R.; Satsangi, J. Sero-Reactivity to Microbial Components in Crohn’s Disease Is Associated with Disease Severity and Progression, but Not NOD2/CARD15 Genotype. Am. J. Gastroenterol. 2004, 99, 2376–2384. [Google Scholar] [CrossRef]
- Prideaux, L.; De Cruz, P.; Ng, S.C.; Kamm, M.A. Serological Antibodies in Inflammatory Bowel Disease: A Systematic Review. Inflamm. Bowel Dis. 2012, 18, 1340–1355. [Google Scholar] [CrossRef]
- Mow, W.S.; Vasiliauskas, E.A.; Lin, Y.-C.; Fleshner, P.R.; Papadakis, K.A.; Taylor, K.D.; Landers, C.J.; Abreu-Martin, M.T.; Rotter, J.I.; Yang, H.; et al. Association of Antibody Responses to Microbial Antigens and Complications of Small Bowel Crohn’s Disease. Gastroenterology 2004, 126, 414–424. [Google Scholar] [CrossRef]
- Targan, S.R.; Landers, C.J.; Yang, H.; Lodes, M.J.; Cong, Y.; Papadakis, K.A.; Vasiliauskas, E.; Elson, C.O.; Hershberg, R.M. Antibodies to CBir1 Flagellin Define a Unique Response That Is Associated Independently with Complicated Crohn’s Disease. Gastroenterology 2005, 128, 2020–2028. [Google Scholar] [CrossRef]
- Zholudev, A.; Zurakowski, D.; Young, W.; Leichtner, A.; Bousvaros, A. Serologic Testing with ANCA, ASCA, and Anti-OmpC in Children and Young Adults with Crohn’s Disease and Ulcerative Colitis: Diagnostic Value and Correlation with Disease Phenotype. Am. J. Gastroenterol. 2004, 99, 2235–2241. [Google Scholar] [CrossRef]
- Cleynen, I.; Boucher, G.; Jostins, L.; Schumm, L.P.; Zeissig, S.; Ahmad, T.; Andersen, V.; Andrews, J.M.; Annese, V.; Brand, S.; et al. Inherited Determinants of Crohn’s Disease and Ulcerative Colitis Phenotypes: A Genetic Association Study. Lancet 2016, 387, 156–167. [Google Scholar] [CrossRef]
- Hugot, J.P.; Chamaillard, M.; Zouali, H.; Lesage, S.; Cézard, J.P.; Belaiche, J.; Almer, S.; Tysk, C.; O’Morain, C.A.; Gassull, M.; et al. Association of NOD2 Leucine-Rich Repeat Variants with Susceptibility to Crohn’s Disease. Nature 2001, 411, 599–603. [Google Scholar] [CrossRef] [PubMed]
- Ogura, Y.; Bonen, D.K.; Inohara, N.; Nicolae, D.L.; Chen, F.F.; Ramos, R.; Britton, H.; Moran, T.; Karaliuskas, R.; Duerr, R.H.; et al. A Frameshift Mutation in NOD2 Associated with Susceptibility to Crohn’s Disease. Nature 2001, 411, 603–606. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, T.; Armuzzi, A.; Bunce, M.; Mulcahy-Hawes, K.; Marshall, S.E.; Orchard, T.R.; Crawshaw, J.; Large, O.; de Silva, A.; Cook, J.T.; et al. The Molecular Classification of the Clinical Manifestations of Crohn’s Disease. Gastroenterology 2002, 122, 854–866. [Google Scholar] [CrossRef] [PubMed]
- Jostins, L.; Ripke, S.; Weersma, R.K.; Duerr, R.H.; McGovern, D.P.; Hui, K.Y.; Lee, J.C.; Schumm, L.P.; Sharma, Y.; Anderson, C.A.; et al. Host-Microbe Interactions Have Shaped the Genetic Architecture of Inflammatory Bowel Disease. Nature 2012, 491, 119–124. [Google Scholar] [CrossRef] [PubMed]
- de Lange, K.M.; Moutsianas, L.; Lee, J.C.; Lamb, C.A.; Luo, Y.; Kennedy, N.A.; Jostins, L.; Rice, D.L.; Gutierrez-Achury, J.; Ji, S.-G.; et al. Genome-Wide Association Study Implicates Immune Activation of Multiple Integrin Genes in Inflammatory Bowel Disease. Nat. Genet. 2017, 49, 256–261. [Google Scholar] [CrossRef]
- Sazonovs, A.; Stevens, C.R.; Venkataraman, G.R.; Yuan, K.; Avila, B.; Abreu, M.T.; Ahmad, T.; Allez, M.; Ananthakrishnan, A.N.; Atzmon, G.; et al. Large-Scale Sequencing Identifies Multiple Genes and Rare Variants Associated with Crohn’s Disease Susceptibility. Nat. Genet. 2022, 54, 1275–1283. [Google Scholar] [CrossRef]
- Cortes, A.; Brown, M.A. Promise and Pitfalls of the Immunochip. Arthritis Res Ther 2011, 13, 101. [Google Scholar] [CrossRef]
- Wray, N.R.; Lin, T.; Austin, J.; McGrath, J.J.; Hickie, I.B.; Murray, G.K.; Visscher, P.M. From Basic Science to Clinical Application of Polygenic Risk Scores: A Primer. JAMA Psychiatry 2021, 78, 101–109. [Google Scholar] [CrossRef]
- Abakkouy, Y.; Cleynen, I. The Promise of Polygenic Risk Scores as a Research Tool to Analyse the Genetics Underlying IBD Phenotypes. J. Crohns Colitis 2021, 15, 877–878. [Google Scholar] [CrossRef]
- Voskuil, M.D.; Spekhorst, L.M.; van der Sloot, K.W.J.; Jansen, B.H.; Dijkstra, G.; van der Woude, C.J.; Hoentjen, F.; Pierik, M.J.; van der Meulen, A.E.; de Boer, N.K.H.; et al. Genetic Risk Scores Identify Genetic Aetiology of Inflammatory Bowel Disease Phenotypes. J. Crohns Colitis 2021, 15, 930–937. [Google Scholar] [CrossRef]
- Lee, J.C.; Biasci, D.; Roberts, R.; Gearry, R.B.; Mansfield, J.C.; Ahmad, T.; Prescott, N.J.; Satsangi, J.; Wilson, D.C.; Jostins, L.; et al. Genome-Wide Association Study Identifies Distinct Genetic Contributions to Prognosis and Susceptibility in Crohn’s Disease. Nat. Genet. 2017, 49, 262–268. [Google Scholar] [CrossRef]
- Lawrance, I.C.; Fiocchi, C.; Chakravarti, S. Ulcerative Colitis and Crohn’s Disease: Distinctive Gene Expression Profiles and Novel Susceptibility Candidate Genes. Hum. Mol. Genet. 2001, 10, 445–456. [Google Scholar] [CrossRef] [PubMed]
- Burczynski, M.E.; Peterson, R.L.; Twine, N.C.; Zuberek, K.A.; Brodeur, B.J.; Casciotti, L.; Maganti, V.; Reddy, P.S.; Strahs, A.; Immermann, F.; et al. Molecular Classification of Crohn’s Disease and Ulcerative Colitis Patients Using Transcriptional Profiles in Peripheral Blood Mononuclear Cells. J. Mol. Diagn. 2006, 8, 51–61. [Google Scholar] [CrossRef]
- Hong, S.N.; Joung, J.-G.; Bae, J.S.; Lee, C.S.; Koo, J.S.; Park, S.J.; Im, J.P.; Kim, Y.S.; Kim, J.W.; Park, W.Y.; et al. RNA-Seq Reveals Transcriptomic Differences in Inflamed and Noninflamed Intestinal Mucosa of Crohn’s Disease Patients Compared with Normal Mucosa of Healthy Controls. Inflamm. Bowel. Dis. 2017, 23, 1098–1108. [Google Scholar] [CrossRef] [PubMed]
- Kaser, A.; Ludwiczek, O.; Holzmann, S.; Moschen, A.R.; Weiss, G.; Enrich, B.; Graziadei, I.; Dunzendorfer, S.; Wiedermann, C.J.; Mürzl, E.; et al. Increased Expression of CCL20 in Human Inflammatory Bowel Disease. J. Clin. Immunol. 2004, 24, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Dobre, M.; Milanesi, E.; Mănuc, T.E.; Arsene, D.E.; Ţieranu, C.G.; Maj, C.; Becheanu, G.; Mănuc, M. Differential Intestinal Mucosa Transcriptomic Biomarkers for Crohn’s Disease and Ulcerative Colitis. J. Immunol. Res. 2018, 2018, 9208274. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.C.; Chang, C.; Boschetti, G.; Ungaro, R.; Giri, M.; Grout, J.A.; Gettler, K.; Chuang, L.-S.; Nayar, S.; Greenstein, A.J.; et al. Single-Cell Analysis of Crohn’s Disease Lesions Identifies a Pathogenic Cellular Module Associated with Resistance to Anti-TNF Therapy. Cell 2019, 178, 1493–1508.e20. [Google Scholar] [CrossRef]
- Kong, L.; Pokatayev, V.; Lefkovith, A.; Carter, G.T.; Creasey, E.A.; Krishna, C.; Subramanian, S.; Kochar, B.; Ashenberg, O.; Lau, H.; et al. The Landscape of Immune Dysregulation in Crohn’s Disease Revealed through Single-Cell Transcriptomic Profiling in the Ileum and Colon. Immunity 2023, 56, 444–458.e5. [Google Scholar] [CrossRef]
- Burke, J.P.; Ferrante, M.; Dejaegher, K.; Watson, R.W.G.; Docherty, N.G.; De Hertogh, G.; Vermeire, S.; Rutgeerts, P.; D’Hoore, A.; Penninckx, F.; et al. Transcriptomic Analysis of Intestinal Fibrosis-Associated Gene Expression in Response to Medical Therapy in Crohn’s Disease. Inflamm. Bowel. Dis. 2008, 14, 1197–1204. [Google Scholar] [CrossRef]
- Arnauts, K.; Verstockt, B.; Ramalho, A.S.; Vermeire, S.; Verfaillie, C.; Ferrante, M. Ex Vivo Mimicking of Inflammation in Organoids Derived From Patients With Ulcerative Colitis. Gastroenterology 2020, 159, 1564–1567. [Google Scholar] [CrossRef]
- d’Aldebert, E.; Quaranta, M.; Sébert, M.; Bonnet, D.; Kirzin, S.; Portier, G.; Duffas, J.-P.; Chabot, S.; Lluel, P.; Allart, S.; et al. Characterization of Human Colon Organoids From Inflammatory Bowel Disease Patients. Front. Cell Dev. Biol. 2020, 8, 363. [Google Scholar] [CrossRef] [PubMed]
- Niklinska-Schirtz, B.J.; Venkateswaran, S.; Anbazhagan, M.; Kolachala, V.L.; Prince, J.; Dodd, A.; Chinnadurai, R.; Gibson, G.; Denson, L.A.; Cutler, D.J.; et al. Ileal Derived Organoids From Crohn’s Disease Patients Show Unique Transcriptomic and Secretomic Signatures. Cell. Mol. Gastroenterol. Hepatol. 2021, 12, 1267–1280. [Google Scholar] [CrossRef] [PubMed]
- Angus, H.C.K.; Butt, A.G.; Schultz, M.; Kemp, R.A. Intestinal Organoids as a Tool for Inflammatory Bowel Disease Research. Front. Med. 2019, 6, 334. [Google Scholar] [CrossRef] [PubMed]
- Timp, W.; Timp, G. Beyond Mass Spectrometry, the next Step in Proteomics. Sci. Adv. 2020, 6, eaax8978. [Google Scholar] [CrossRef]
- Nanni, P.; Parisi, D.; Roda, G.; Casale, M.; Belluzzi, A.; Roda, E.; Mayer, L.; Roda, A. Serum Protein Profiling in Patients with Inflammatory Bowel Diseases Using Selective Solid-Phase Bulk Extraction, Matrix-Assisted Laser Desorption/Ionization Time-of-Flight Mass Spectrometry and Chemometric Data Analysis. Rapid Commun. Mass Spectrom. 2007, 21, 4142–4148. [Google Scholar] [CrossRef]
- Basso, D.; Padoan, A.; D’Incà, R.; Arrigoni, G.; Scapellato, M.L.; Contran, N.; Franchin, C.; Lorenzon, G.; Mescoli, C.; Moz, S.; et al. Peptidomic and Proteomic Analysis of Stool for Diagnosing IBD and Deciphering Disease Pathogenesis. Clin. Chem. Lab. Med. (CCLM) 2020, 58, 968–979. [Google Scholar] [CrossRef]
- Klein, O.; Fogt, F.; Hollerbach, S.; Nebrich, G.; Boskamp, T.; Wellmann, A. Classification of Inflammatory Bowel Disease from Formalin-Fixed, Paraffin-Embedded Tissue Biopsies via Imaging Mass Spectrometry. Proteomics Clin. Appl. 2020, 14, e1900131. [Google Scholar] [CrossRef]
- Starr, A.E.; Deeke, S.A.; Ning, Z.; Chiang, C.-K.; Zhang, X.; Mottawea, W.; Singleton, R.; Benchimol, E.I.; Wen, M.; Mack, D.R.; et al. Proteomic Analysis of Ascending Colon Biopsies from a Paediatric Inflammatory Bowel Disease Inception Cohort Identifies Protein Biomarkers That Differentiate Crohn’s Disease from UC. Gut 2017, 66, 1573–1583. [Google Scholar] [CrossRef]
- Andersson, E.; Bergemalm, D.; Kruse, R.; Neumann, G.; D’Amato, M.; Repsilber, D.; Halfvarson, J. Subphenotypes of Inflammatory Bowel Disease Are Characterized by Specific Serum Protein Profiles. PLoS ONE 2017, 12, e0186142. [Google Scholar] [CrossRef]
- Rukmangadachar, L.A.; Makharia, G.K.; Mishra, A.; Das, P.; Hariprasad, G.; Srinivasan, A.; Gupta, S.D.; Ahuja, V.; Acharya, S.K. Proteome Analysis of the Macroscopically Affected Colonic Mucosa of Crohn’s Disease and Intestinal Tuberculosis. Sci. Rep. 2016, 6, 23162. [Google Scholar] [CrossRef]
- Leibovitzh, H.; Lee, S.-H.; Raygoza Garay, J.A.; Espin-Garcia, O.; Xue, M.; Neustaeter, A.; Goethel, A.; Huynh, H.Q.; Griffiths, A.M.; Turner, D.; et al. Immune Response and Barrier Dysfunction-Related Proteomic Signatures in Preclinical Phase of Crohn’s Disease Highlight Earliest Events of Pathogenesis. Gut 2023, 72, 1462–1471. [Google Scholar] [CrossRef] [PubMed]
- Meuwis, M.-A.; Fillet, M.; Lutteri, L.; Marée, R.; Geurts, P.; de Seny, D.; Malaise, M.; Chapelle, J.-P.; Wehenkel, L.; Belaiche, J.; et al. Proteomics for Prediction and Characterization of Response to Infliximab in Crohn’s Disease: A Pilot Study. Clin. Biochem. 2008, 41, 960–967. [Google Scholar] [CrossRef] [PubMed]
- Hatsugai, M.; Kurokawa, M.S.; Kouro, T.; Nagai, K.; Arito, M.; Masuko, K.; Suematsu, N.; Okamoto, K.; Itoh, F.; Kato, T. Protein Profiles of Peripheral Blood Mononuclear Cells Are Useful for Differential Diagnosis of Ulcerative Colitis and Crohn’s Disease. J. Gastroenterol. 2010, 45, 488–500. [Google Scholar] [CrossRef] [PubMed]
- Townsend, P.; Zhang, Q.; Shapiro, J.; Webb-Robertson, B.-J.; Bramer, L.; Schepmoes, A.A.; Weitz, K.K.; Mallette, M.; Moniz, H.; Bright, R.; et al. Serum Proteome Profiles in Stricturing Crohn’s Disease: A Pilot Study. Inflamm. Bowel. Dis. 2015, 21, 1935–1941. [Google Scholar] [CrossRef]
- Vitali, R.; Palone, F.; Armuzzi, A.; Fulci, V.; Negroni, A.; Carissimi, C.; Cucchiara, S.; Stronati, L. Proteomic Analysis Identifies Three Reliable Biomarkers of Intestinal Inflammation in the Stools of Patients With Inflammatory Bowel Disease. J. Crohns Colitis 2023, 17, 92–102. [Google Scholar] [CrossRef] [PubMed]
- D’Haens, G.; Kelly, O.; Battat, R.; Silverberg, M.S.; Laharie, D.; Louis, E.; Savarino, E.; Bodini, G.; Yarur, A.; Boland, B.S.; et al. Development and Validation of a Test to Monitor Endoscopic Activity in Patients With Crohn’s Disease Based on Serum Levels of Proteins. Gastroenterology 2020, 158, 515–526.e10. [Google Scholar] [CrossRef]
- Kalla, R.; Adams, A.T.; Bergemalm, D.; Vatn, S.; Kennedy, N.A.; Ricanek, P.; Lindstrom, J.; Ocklind, A.; Hjelm, F.; Ventham, N.T.; et al. Serum Proteomic Profiling at Diagnosis Predicts Clinical Course, and Need for Intensification of Treatment in Inflammatory Bowel Disease. J. Crohn’s Colitis 2021, 15, 699–708. [Google Scholar] [CrossRef]
- Lee, J.W.J.; Plichta, D.; Hogstrom, L.; Borren, N.Z.; Lau, H.; Gregory, S.M.; Tan, W.; Khalili, H.; Clish, C.; Vlamakis, H.; et al. Multi-Omics Reveal Microbial Determinants Impacting Responses to Biologic Therapies in Inflammatory Bowel Disease. Cell Host. Microbe 2021, 29, 1294–1304.e4. [Google Scholar] [CrossRef]
- Bourgonje, A.R.; Hu, S.; Spekhorst, L.M.; Zhernakova, D.V.; Vich Vila, A.; Li, Y.; Voskuil, M.D.; van Berkel, L.A.; Bley Folly, B.; Charrout, M.; et al. The Effect of Phenotype and Genotype on the Plasma Proteome in Patients with Inflammatory Bowel Disease. J. Crohns Colitis 2021, 16, 414–429. [Google Scholar] [CrossRef]
- van Zalm, P.W.; Ahmed, S.; Fatou, B.; Schreiber, R.; Barnaby, O.; Boxer, A.; Zetterberg, H.; Steen, J.A.; Steen, H. Meta-Analysis of Published Cerebrospinal Fluid Proteomics Data Identifies and Validates Metabolic Enzyme Panel as Alzheimer’s Disease Biomarkers. Cell Rep. Med. 2023, 4, 101005. [Google Scholar] [CrossRef]
- Tabb, D.L.; Vega-Montoto, L.; Rudnick, P.A.; Variyath, A.M.; Ham, A.-J.L.; Bunk, D.M.; Kilpatrick, L.E.; Billheimer, D.D.; Blackman, R.K.; Cardasis, H.L.; et al. Repeatability and Reproducibility in Proteomic Identifications by Liquid Chromatography—Tandem Mass Spectrometry. J. Proteome Res. 2010, 9, 761. [Google Scholar] [CrossRef] [PubMed]
- Noble, A.J.; Nowak, J.K.; Adams, A.T.; Uhlig, H.H.; Satsangi, J. Defining Interactions Between the Genome, Epigenome, and the Environment in Inflammatory Bowel Disease: Progress and Prospects. Gastroenterology 2023, 165, 44–60.e2. [Google Scholar] [CrossRef] [PubMed]
- Ventham, N.T.; Kennedy, N.A.; Nimmo, E.R.; Satsangi, J. Beyond Gene Discovery in Inflammatory Bowel Disease: The Emerging Role of Epigenetics. Gastroenterology 2013, 145, 293–308. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Xia, P.; Su, P. High-Dimensional DNA Methylation Mediates the Effect of Smoking on Crohn’s Disease. Front. Genet. 2022, 13, 831885. [Google Scholar] [CrossRef]
- Wiklund, P.; Karhunen, V.; Richmond, R.C.; Parmar, P.; Rodriguez, A.; De Silva, M.; Wielscher, M.; Rezwan, F.I.; Richardson, T.G.; Veijola, J.; et al. DNA Methylation Links Prenatal Smoking Exposure to Later Life Health Outcomes in Offspring. Clin. Epigenetics 2019, 11, 97. [Google Scholar] [CrossRef]
- Vieujean, S.; Caron, B.; Haghnejad, V.; Jouzeau, J.-Y.; Netter, P.; Heba, A.-C.; Ndiaye, N.C.; Moulin, D.; Barreto, G.; Danese, S.; et al. Impact of the Exposome on the Epigenome in Inflammatory Bowel Disease Patients and Animal Models. Int. J. Mol. Sci. 2022, 23, 7611. [Google Scholar] [CrossRef]
- Nimmo, E.R.; Prendergast, J.G.; Aldhous, M.C.; Kennedy, N.A.; Henderson, P.; Drummond, H.E.; Ramsahoye, B.H.; Wilson, D.C.; Semple, C.A.; Satsangi, J. Genome-Wide Methylation Profiling in Crohn’s Disease Identifies Altered Epigenetic Regulation of Key Host Defense Mechanisms Including the Th17 Pathway. Inflamm. Bowel Dis. 2012, 18, 889–899. [Google Scholar] [CrossRef]
- Hornschuh, M.; Wirthgen, E.; Wolfien, M.; Singh, K.P.; Wolkenhauer, O.; Däbritz, J. The Role of Epigenetic Modifications for the Pathogenesis of Crohn’s Disease. Clin. Epigenetics 2021, 13, 108. [Google Scholar] [CrossRef]
- Joustra, V.; Hageman, I.L.; Satsangi, J.; Adams, A.; Ventham, N.T.; de Jonge, W.J.; Henneman, P.; D’Haens, G.R.; Li Yim, A.Y.F. Systematic Review and Meta-Analysis of Peripheral Blood DNA Methylation Studies in Inflammatory Bowel Disease. J. Crohns Colitis 2023, 17, 185–198. [Google Scholar] [CrossRef]
- Ventham, N.T.; Kennedy, N.A.; Adams, A.T.; Kalla, R.; Heath, S.; O’Leary, K.R.; Drummond, H.; Wilson, D.C.; Gut, I.G.; Nimmo, E.R.; et al. Integrative Epigenome-Wide Analysis Demonstrates That DNA Methylation May Mediate Genetic Risk in Inflammatory Bowel Disease. Nat. Commun. 2016, 7, 13507. [Google Scholar] [CrossRef]
- Kalla, R.; Adams, A.T.; Nowak, J.K.; Bergemalm, D.; Vatn, S.; Ventham, N.T.; Kennedy, N.A.; Ricanek, P.; Lindstrom, J.; Söderholm, J.; et al. Analysis of Systemic Epigenetic Alterations in Inflammatory Bowel Disease: Defining Geographical, Genetic and Immune-Inflammatory Influences on the Circulating Methylome. J. Crohns Colitis 2022, 17, 170–184. [Google Scholar] [CrossRef] [PubMed]
- Adams, A.T.; Kennedy, N.A.; Hansen, R.; Ventham, N.T.; O’Leary, K.R.; Drummond, H.E.; Noble, C.L.; El-Omar, E.; Russell, R.K.; Wilson, D.C.; et al. Two-Stage Genome-Wide Methylation Profiling in Childhood-Onset Crohn’s Disease Implicates Epigenetic Alterations at the VMP1/MIR21 and HLA Loci. Inflamm. Bowel. Dis. 2014, 20, 1784–1793. [Google Scholar] [CrossRef] [PubMed]
- Sadler, T.; Bhasin, J.M.; Xu, Y.; Barnholz-Sloan, J.; Chen, Y.; Ting, A.H.; Stylianou, E. Genome-Wide Analysis of DNA Methylation and Gene Expression Defines Molecular Characteristics of Crohn’s Disease-Associated Fibrosis. Clin. Epigenetics 2016, 8, 30. [Google Scholar] [CrossRef] [PubMed]
- Li Yim, A.Y.F.; de Bruyn, J.R.; Duijvis, N.W.; Sharp, C.; Ferrero, E.; de Jonge, W.J.; Wildenberg, M.E.; Mannens, M.M.A.M.; Buskens, C.J.; D’Haens, G.R.; et al. A Distinct Epigenetic Profile Distinguishes Stenotic from Non-Inflamed Fibroblasts in the Ileal Mucosa of Crohn’s Disease Patients. PLoS ONE 2018, 13, e0209656. [Google Scholar] [CrossRef]
- Ventham, N.T.; Kennedy, N.A.; Kalla, R.; Adams, A.T.; Noble, A.; Ennis, H.; Mowat, C.; Dunlop, M.G.; Satsangi, J. Genome-Wide Methylation Profiling in 229 Patients With Crohn’s Disease Requiring Intestinal Resection: Epigenetic Analysis of the Trial of Prevention of Post-Operative Crohn’s Disease (TOPPIC). Cell. Mol. Gastroenterol. Hepatol. 2023, 16, 431–450. [Google Scholar] [CrossRef]
- Howell, K.J.; Kraiczy, J.; Nayak, K.M.; Gasparetto, M.; Ross, A.; Lee, C.; Mak, T.N.; Koo, B.-K.; Kumar, N.; Lawley, T.; et al. DNA Methylation and Transcription Patterns in Intestinal Epithelial Cells From Pediatric Patients With Inflammatory Bowel Diseases Differentiate Disease Subtypes and Associate With Outcome. Gastroenterology 2018, 154, 585–598. [Google Scholar] [CrossRef]
- Joustra, V.; Li Yim, A.; Hageman, I.; Levin, E.; Noble, A.; Chapman, T.; McGregor, C.; Adams, A.; Satsangi, J.; de Jonge, W.; et al. OP03 Highly Stable Epigenome-Wide Peripheral Blood DNA Methylation Signatures Accurately Predict Endoscopic Response to Adalimumab, Vedolizumab and Ustekinumab in Crohn’s Disease Patients: The EPIC-CD Study. J. Crohn’s Colitis 2023, 17, i6–i8. [Google Scholar] [CrossRef]
- Joustra, V.; Hageman, I.; Li Yim, A.; Levin, E.; Satsangi, J.; Adams, A.; De Jonge, W.; Henneman, P.; D’Haens, G.; on behalf of the EPIC consortium. OP29 Peripheral Blood DNA Methylation Biomarkers Accurately Predict Clinical- and Endoscopic Response to Vedolizumab in a Real-Life Cohort of Crohn’s Disease Patients. J. Crohn’s Colitis 2022, 16, i032–i033. [Google Scholar] [CrossRef]
- Joustra, V.; Li Yim, A.Y.F.; Hageman, I.; Levin, E.; Adams, A.; Satsangi, J.; de Jonge, W.J.; Henneman, P.; D’Haens, G. Long-Term Temporal Stability of Peripheral Blood DNA Methylation Profiles in Patients With Inflammatory Bowel Disease. Cell Mol. Gastroenterol. Hepatol. 2022, 15, 869–885. [Google Scholar] [CrossRef]
- Somineni, H.K.; Venkateswaran, S.; Kilaru, V.; Marigorta, U.M.; Mo, A.; Okou, D.T.; Kellermayer, R.; Mondal, K.; Cobb, D.; Walters, T.D.; et al. Blood-Derived DNA Methylation Signatures of Crohn’s Disease and Severity of Intestinal Inflammation. Gastroenterology 2019, 156, 2254–2265.e3. [Google Scholar] [CrossRef]
- Lloyd-Price, J.; Abu-Ali, G.; Huttenhower, C. The Healthy Human Microbiome. Genome Med. 2016, 8, 51. [Google Scholar] [CrossRef] [PubMed]
- Santana, P.T.; Rosas, S.L.B.; Ribeiro, B.E.; Marinho, Y.; de Souza, H.S.P. Dysbiosis in Inflammatory Bowel Disease: Pathogenic Role and Potential Therapeutic Targets. Int. J. Mol. Sci. 2022, 23, 3464. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.S.; Spakowicz, D.J.; Hong, B.-Y.; Petersen, L.M.; Demkowicz, P.; Chen, L.; Leopold, S.R.; Hanson, B.M.; Agresta, H.O.; Gerstein, M.; et al. Evaluation of 16S RRNA Gene Sequencing for Species and Strain-Level Microbiome Analysis. Nat. Commun. 2019, 10, 5029. [Google Scholar] [CrossRef] [PubMed]
- Pascal, V.; Pozuelo, M.; Borruel, N.; Casellas, F.; Campos, D.; Santiago, A.; Martinez, X.; Varela, E.; Sarrabayrouse, G.; Machiels, K.; et al. A Microbial Signature for Crohn’s Disease. Gut 2017, 66, 813–822. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Rahman, L.I.H.; Morgan, X.C. Searching for a Consensus Among Inflammatory Bowel Disease Studies: A Systematic Meta-Analysis. Inflamm. Bowel. Dis. 2023, 29, 125–139. [Google Scholar] [CrossRef]
- Amos, G.C.A.; Sergaki, C.; Logan, A.; Iriarte, R.; Bannaga, A.; Chandrapalan, S.; Wellington, E.M.H.; Rijpkema, S.; Arasaradnam, R.P. Exploring How Microbiome Signatures Change across Inflammatory Bowel Disease Conditions and Disease Locations. Sci. Rep. 2021, 11, 18699. [Google Scholar] [CrossRef]
- Gonzalez, C.G.; Mills, R.H.; Zhu, Q.; Sauceda, C.; Knight, R.; Dulai, P.S.; Gonzalez, D.J. Location-Specific Signatures of Crohn’s Disease at a Multi-Omics Scale. Microbiome 2022, 10, 133. [Google Scholar] [CrossRef]
- Martinez-Medina, M.; Aldeguer, X.; Lopez-Siles, M.; González-Huix, F.; López-Oliu, C.; Dahbi, G.; Blanco, J.E.; Blanco, J.; Garcia-Gil, L.J.; Darfeuille-Michaud, A. Molecular Diversity of Escherichia Coli in the Human Gut: New Ecological Evidence Supporting the Role of Adherent-Invasive E. Coli (AIEC) in Crohn’s Disease. Inflamm. Bowel. Dis. 2009, 15, 872–882. [Google Scholar] [CrossRef]
- Kugathasan, S.; Denson, L.A.; Walters, T.D.; Kim, M.-O.; Marigorta, U.M.; Schirmer, M.; Mondal, K.; Liu, C.; Griffiths, A.; Noe, J.D.; et al. Prediction of Complicated Disease Course for Children Newly Diagnosed with Crohn’s Disease: A Multicentre Inception Cohort Study. Lancet 2017, 389, 1710–1718. [Google Scholar] [CrossRef]
- Lopez, J.; Grinspan, A. Fecal Microbiota Transplantation for Inflammatory Bowel Disease. Gastroenterol. Hepatol. 2016, 12, 374–379. [Google Scholar]
- Knox, N.C.; Forbes, J.D.; Van Domselaar, G.; Bernstein, C.N. The Gut Microbiome as a Target for IBD Treatment: Are We There Yet? Curr. Treat. Options Gastroenterol. 2019, 17, 115–126. [Google Scholar] [CrossRef]
- Patti, G.J.; Yanes, O.; Siuzdak, G. Metabolomics: The Apogee of the Omic Triology. Nat. Rev. Mol. Cell Biol. 2012, 13, 263–269. [Google Scholar] [CrossRef]
- Griffiths, W.J.; Koal, T.; Wang, Y.; Kohl, M.; Enot, D.P.; Deigner, H.-P. Targeted Metabolomics for Biomarker Discovery. Angew. Chem. Int. Ed. 2010, 49, 5426–5445. [Google Scholar] [CrossRef] [PubMed]
- Chetwynd, A.J.; Dunn, W.B.; Rodriguez-Blanco, G. Collection and Preparation of Clinical Samples for Metabolomics. In Metabolomics: From Fundamentals to Clinical Applications; Sussulini, A., Ed.; Advances in Experimental Medicine and Biology; Springer International Publishing: Cham, Switzerland, 2017; pp. 19–44. ISBN 978-3-319-47656-8. [Google Scholar]
- Aldars-García, L.; Gisbert, J.P.; Chaparro, M. Metabolomics Insights into Inflammatory Bowel Disease: A Comprehensive Review. Pharmaceuticals 2021, 14, 1190. [Google Scholar] [CrossRef] [PubMed]
- Marchesi, J.R.; Holmes, E.; Khan, F.; Kochhar, S.; Scanlan, P.; Shanahan, F.; Wilson, I.D.; Wang, Y. Rapid and Noninvasive Metabonomic Characterization of Inflammatory Bowel Disease. J. Proteome Res. 2007, 6, 546–551. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, K.; Catesson, A.; Griffin, J.L.; Holmes, E.; Williams, H.R.T. Metabolomic Analysis in Inflammatory Bowel Disease: A Systematic Review. J. Crohns Colitis 2021, 15, 813–826. [Google Scholar] [CrossRef]
- Vila, A.V.; Hu, S.; Andreu-Sánchez, S.; Collij, V.; Jansen, B.H.; Augustijn, H.E.; Bolte, L.A.; Ruigrok, R.A.A.A.; Abu-Ali, G.; Giallourakis, C.; et al. Faecal Metabolome and Its Determinants in Inflammatory Bowel Disease. Gut 2023. [Google Scholar] [CrossRef]
- Mossotto, E.; Boberska, J.; Ashton, J.J.; Stafford, I.S.; Cheng, G.; Baker, J.; Borca, F.; Phan, H.T.T.; Coelho, T.F.; Beattie, R.M.; et al. Evidence of a Genetically Driven Metabolomic Signature in Actively Inflamed Crohn’s Disease. Sci. Rep. 2022, 12, 14101. [Google Scholar] [CrossRef]
- Xu, X.; Ocansey, D.K.W.; Hang, S.; Wang, B.; Amoah, S.; Yi, C.; Zhang, X.; Liu, L.; Mao, F. The Gut Metagenomics and Metabolomics Signature in Patients with Inflammatory Bowel Disease. Gut Pathogens 2022, 14, 26. [Google Scholar] [CrossRef]
- Shimizu, T. Lipid Mediators in Health and Disease: Enzymes and Receptors as Therapeutic Targets for the Regulation of Immunity and Inflammation. Annu. Rev. Pharmacol. Toxicol. 2009, 49, 123–150. [Google Scholar] [CrossRef]
- Shores, D.R.; Binion, D.G.; Freeman, B.A.; Baker, P.R.S. New Insights into the Role of Fatty Acids in the Pathogenesis and Resolution of Inflammatory Bowel Disease. Inflamm. Bowel. Dis. 2011, 17, 2192–2204. [Google Scholar] [CrossRef]
- Fahy, E.; Cotter, D.; Sud, M.; Subramaniam, S. Lipid Classification, Structures and Tools. Biochim. Biophys. Acta 2011, 1811, 637–647. [Google Scholar] [CrossRef] [PubMed]
- Vale, G.; Martin, S.A.; Mitsche, M.A.; Thompson, B.M.; Eckert, K.M.; McDonald, J.G. Three-Phase Liquid Extraction: A Simple and Fast Method for Lipidomic Workflows. J. Lipid Res. 2019, 60, 694–706. [Google Scholar] [CrossRef]
- Cajka, T.; Fiehn, O. Comprehensive Analysis of Lipids in Biological Systems by Liquid Chromatography-Mass Spectrometry. Trends Analyt. Chem. 2014, 61, 192–206. [Google Scholar] [CrossRef] [PubMed]
- Manfredi, M.; Conte, E.; Barberis, E.; Buzzi, A.; Robotti, E.; Caneparo, V.; Cecconi, D.; Brandi, J.; Vanni, E.; Finocchiaro, M.; et al. Integrated Serum Proteins and Fatty Acids Analysis for Putative Biomarker Discovery in Inflammatory Bowel Disease. J. Proteom. 2019, 195, 138–149. [Google Scholar] [CrossRef]
- Fan, F.; Mundra, P.A.; Fang, L.; Galvin, A.; Moore, X.L.; Weir, J.M.; Wong, G.; White, D.A.; Chin-Dusting, J.; Sparrow, M.P.; et al. Lipidomic Profiling in Inflammatory Bowel Disease: Comparison Between Ulcerative Colitis and Crohn’s Disease. Inflamm. Bowel. Dis. 2015, 21, 1511–1518. [Google Scholar] [CrossRef]
- Iwatani, S.; Iijima, H.; Otake, Y.; Amano, T.; Tani, M.; Yoshihara, T.; Tashiro, T.; Tsujii, Y.; Inoue, T.; Hayashi, Y.; et al. Novel Mass Spectrometry-Based Comprehensive Lipidomic Analysis of Plasma from Patients with Inflammatory Bowel Disease. J. Gastroenterol. Hepatol. 2020, 35, 1355–1364. [Google Scholar] [CrossRef] [PubMed]
- Brown, E.M.; Ke, X.; Hitchcock, D.; Jeanfavre, S.; Avila-Pacheco, J.; Nakata, T.; Arthur, T.D.; Fornelos, N.; Heim, C.; Franzosa, E.A.; et al. Bacteroides-Derived Sphingolipids Are Critical for Maintaining Intestinal Homeostasis and Symbiosis. Cell Host. Microbe 2019, 25, 668–680.e7. [Google Scholar] [CrossRef]
- Guan, S.; Jia, B.; Chao, K.; Zhu, X.; Tang, J.; Li, M.; Wu, L.; Xing, L.; Liu, K.; Zhang, L.; et al. UPLC-QTOF-MS-Based Plasma Lipidomic Profiling Reveals Biomarkers for Inflammatory Bowel Disease Diagnosis. J. Proteome Res. 2020, 19, 600–609. [Google Scholar] [CrossRef]
- Scoville, E.A.; Allaman, M.M.; Brown, C.T.; Motley, A.K.; Horst, S.N.; Williams, C.S.; Koyama, T.; Zhao, Z.; Adams, D.W.; Beaulieu, D.B.; et al. Alterations in Lipid, Amino Acid, and Energy Metabolism Distinguish Crohn’s Disease from Ulcerative Colitis and Control Subjects by Serum Metabolomic Profiling. Metabolomics 2018, 14, 17. [Google Scholar] [CrossRef]
- Jansson, J.; Willing, B.; Lucio, M.; Fekete, A.; Dicksved, J.; Halfvarson, J.; Tysk, C.; Schmitt-Kopplin, P. Metabolomics Reveals Metabolic Biomarkers of Crohn’s Disease. PLoS ONE 2009, 4, e6386. [Google Scholar] [CrossRef]
- Tefas, C.; Ciobanu, L.; Tanțău, M.; Moraru, C.; Socaciu, C. The Potential of Metabolic and Lipid Profiling in Inflammatory Bowel Diseases: A Pilot Study. Bosn. J. Basic Med. Sci. 2020, 20, 262–270. [Google Scholar] [CrossRef]
- Horta, D.; Moreno-Torres, M.; Ramírez-Lázaro, M.J.; Lario, S.; Kuligowski, J.; Sanjuan-Herráez, J.D.; Quintas, G.; Villoria, A.; Calvet, X. Analysis of the Association between Fatigue and the Plasma Lipidomic Profile of Inflammatory Bowel Disease Patients. J. Proteome Res. 2021, 20, 381–392. [Google Scholar] [CrossRef]
- Lee, Y.; Choo, J.; Kim, S.J.; Heo, G.; Pothoulakis, C.; Kim, Y.-H.; Im, E. Analysis of Endogenous Lipids during Intestinal Wound Healing. PLoS ONE 2017, 12, e0183028. [Google Scholar] [CrossRef]
- Wang, R.; Gu, X.; Dai, W.; Ye, J.; Lu, F.; Chai, Y.; Fan, G.; Gonzalez, F.J.; Duan, G.; Qi, Y. A Lipidomics Investigation into the Intervention of Celastrol in Experimental Colitis. Mol. Biosyst. 2016, 12, 1436–1444. [Google Scholar] [CrossRef]
- Cecerska-Heryć, E.; Ronkowski, B.; Heryć, R.; Serwin, N.; Grygorcewicz, B.; Roszak, M.; Galant, K.; Dołęgowska, B. Proteomic and Lipidomic Biomarkers in the Diagnosis and Progression of Inflammatory Bowel Disease—A Review. Proteom. Clin. Appl. 2023, 17, e2200003. [Google Scholar] [CrossRef]
- Lee, E.G.; Yoon, Y.C.; Yoon, J.; Lee, S.J.; Oh, Y.-K.; Kwon, S.W. Systematic Review of Recent Lipidomics Approaches Toward Inflammatory Bowel Disease. Biomol. Ther. 2021, 29, 582–595. [Google Scholar] [CrossRef]
- Ahluwalia, B.; Moraes, L.; Magnusson, M.K.; Öhman, L. Immunopathogenesis of Inflammatory Bowel Disease and Mechanisms of Biological Therapies. Scand. J. Gastroenterol. 2018, 53, 379–389. [Google Scholar] [CrossRef]
- Verdier, J.; Begue, B.; Cerf-Bensussan, N.; Ruemmele, F.M. Compartmentalized Expression of Th1 and Th17 Cytokines in Pediatric Inflammatory Bowel Diseases. Inflamm. Bowel. Dis. 2012, 18, 1260–1266. [Google Scholar] [CrossRef]
- Lee, H.B.; Kim, J.H.; Yim, C.Y.; Kim, D.G.; Ahn, D.S. Differences in Immunophenotyping of Mucosal Lymphocytes between Ulcerative Colitis and Crohn’s Disease. Korean J. Intern. Med. 1997, 12, 7–15. [Google Scholar] [CrossRef]
- Kosoy, R.; Kim-Schulze, S.; Rahman, A.; Friedman, J.R.; Huang, R.; Peters, L.A.; Amir, E.-A.; Perrigoue, J.; Stojmirovic, A.; Song, W.-M.; et al. Deep Analysis of the Peripheral Immune System in IBD Reveals New Insight in Disease Subtyping and Response to Monotherapy or Combination Therapy. Cell Mol. Gastroenterol. Hepatol. 2021, 12, 599–632. [Google Scholar] [CrossRef] [PubMed]
- Kredel, L.I.; Jödicke, L.J.; Scheffold, A.; Gröne, J.; Glauben, R.; Erben, U.; Kühl, A.A.; Siegmund, B. T-Cell Composition in Ileal and Colonic Creeping Fat—Separating Ileal from Colonic Crohn’s Disease. J. Crohns Colitis 2019, 13, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Levitte, S.; Jhun, I.; Neighbors, M.; Peale, F.; Grimbaldeston, M.; Haileselassie, Y.; Habtezion, A.; Park, K.; Rogalla, S. Quantitative immunohistochemical analysis of immune cells reveals immunophenotypes associated with intestinal fibrosis and postoperative stricture recurrence in crohn’s disease. Gastroenterology 2022, 162, S639–S640. [Google Scholar] [CrossRef]
- Smids, C.; Horjus Talabur Horje, C.S.; Drylewicz, J.; Roosenboom, B.; Groenen, M.J.M.; van Koolwijk, E.; van Lochem, E.G.; Wahab, P.J. Intestinal T Cell Profiling in Inflammatory Bowel Disease: Linking T Cell Subsets to Disease Activity and Disease Course. J. Crohns Colitis 2018, 12, 465–475. [Google Scholar] [CrossRef]
- McKinney, E.F.; Lyons, P.A.; Carr, E.J.; Hollis, J.L.; Jayne, D.R.W.; Willcocks, L.C.; Koukoulaki, M.; Brazma, A.; Jovanovic, V.; Kemeny, D.M.; et al. A CD8+ T Cell Transcription Signature Predicts Prognosis in Autoimmune Disease. Nat. Med. 2010, 16, 586–591, 1p following 591. [Google Scholar] [CrossRef]
- Lee, J.C.; Lyons, P.A.; McKinney, E.F.; Sowerby, J.M.; Carr, E.J.; Bredin, F.; Rickman, H.M.; Ratlamwala, H.; Hatton, A.; Rayner, T.F.; et al. Gene Expression Profiling of CD8+ T Cells Predicts Prognosis in Patients with Crohn Disease and Ulcerative Colitis. J. Clin. Invest. 2011, 121, 4170–4179. [Google Scholar] [CrossRef]
- Noor, N.; Brezina, B.; Negro, J.D.L.R.; Dowling, F.; Bond, S.; Whitehead, L.; Lee, J.; Lyons, P.; McKinney, E.; Smith, K.; et al. Predicting Outcomes for Crohn’s Disease Using a Molecular Biomarker: Profile Trial. Clin. Med. 2022, 22, 22–23. [Google Scholar] [CrossRef]
- Parkes, M.; Noor, N.M.; Dowling, F.; Leung, H.; Bond, S.; Whitehead, L.; Upponi, S.; Kinnon, P.; Sandham, A.P.; Lyons, P.A.; et al. PRedicting Outcomes For Crohn’s DIsease Using a MoLecular BiomarkEr (PROFILE): Protocol for a Multicentre, Randomised, Biomarker-Stratified Trial. BMJ Open 2018, 8, e026767. [Google Scholar] [CrossRef]
- Gómez-Cebrián, N.; Domingo-Ortí, I.; Poveda, J.L.; Vicent, M.J.; Puchades-Carrasco, L.; Pineda-Lucena, A. Multi-Omic Approaches to Breast Cancer Metabolic Phenotyping: Applications in Diagnosis, Prognosis, and the Development of Novel Treatments. Cancers 2021, 13, 4544. [Google Scholar] [CrossRef]
- Babu, M.; Snyder, M. Multi-Omics Profiling for Health. Mol. Cell Proteom. 2023, 22, 100561. [Google Scholar] [CrossRef]
| Vienna Classification | Montreal Classification | |
|---|---|---|
| Age at diagnosis | A1 below 40 y A2 above 40 y | A1 below 16 y A2 between 17 and 40 y A3 above 40 y |
| Location | L1 ileal L2 colonic L3 ileocolonic L4 upper | L1 ileal L2 colonic L3 ileocolonic L4 isolated upper disease * |
| Behaviour | B1 non-stricturing, non-penetrating B2 stricturing B3 penetrating | B1 non-stricturing, non-penetrating B2 stricturing B3 penetrating p perianal disease modifier † |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamal, S.; Parkash, N.; Beattie, W.; Christensen, B.; Segal, J.P. Are We Ready to Reclassify Crohn’s Disease Using Molecular Classification? J. Clin. Med. 2023, 12, 5786. https://doi.org/10.3390/jcm12185786
Kamal S, Parkash N, Beattie W, Christensen B, Segal JP. Are We Ready to Reclassify Crohn’s Disease Using Molecular Classification? Journal of Clinical Medicine. 2023; 12(18):5786. https://doi.org/10.3390/jcm12185786
Chicago/Turabian StyleKamal, Shahed, Nikita Parkash, William Beattie, Britt Christensen, and Jonathan P. Segal. 2023. "Are We Ready to Reclassify Crohn’s Disease Using Molecular Classification?" Journal of Clinical Medicine 12, no. 18: 5786. https://doi.org/10.3390/jcm12185786
APA StyleKamal, S., Parkash, N., Beattie, W., Christensen, B., & Segal, J. P. (2023). Are We Ready to Reclassify Crohn’s Disease Using Molecular Classification? Journal of Clinical Medicine, 12(18), 5786. https://doi.org/10.3390/jcm12185786







