Where Are We and Where to Next?—The Future of Perianal Crohn’s Disease Management
Abstract
:1. Background
2. Current Management of Perianal Fistulizing CD (pCD)
2.1. Classification
2.2. Assessment
2.3. Medical Treatment
Surgery
3. The Future of Treating Perianal CD
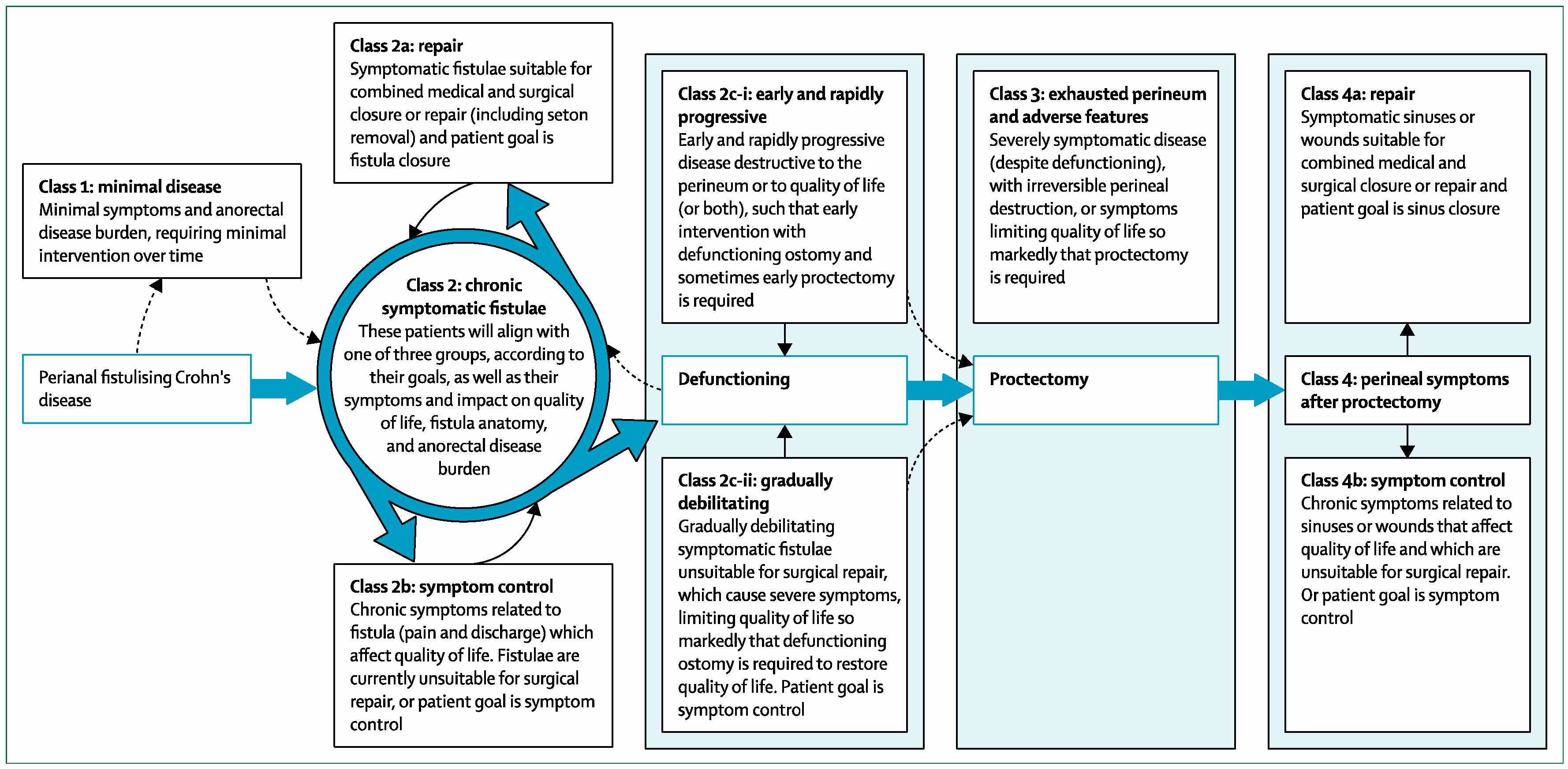
3.1. Better Classification of Perianal CD
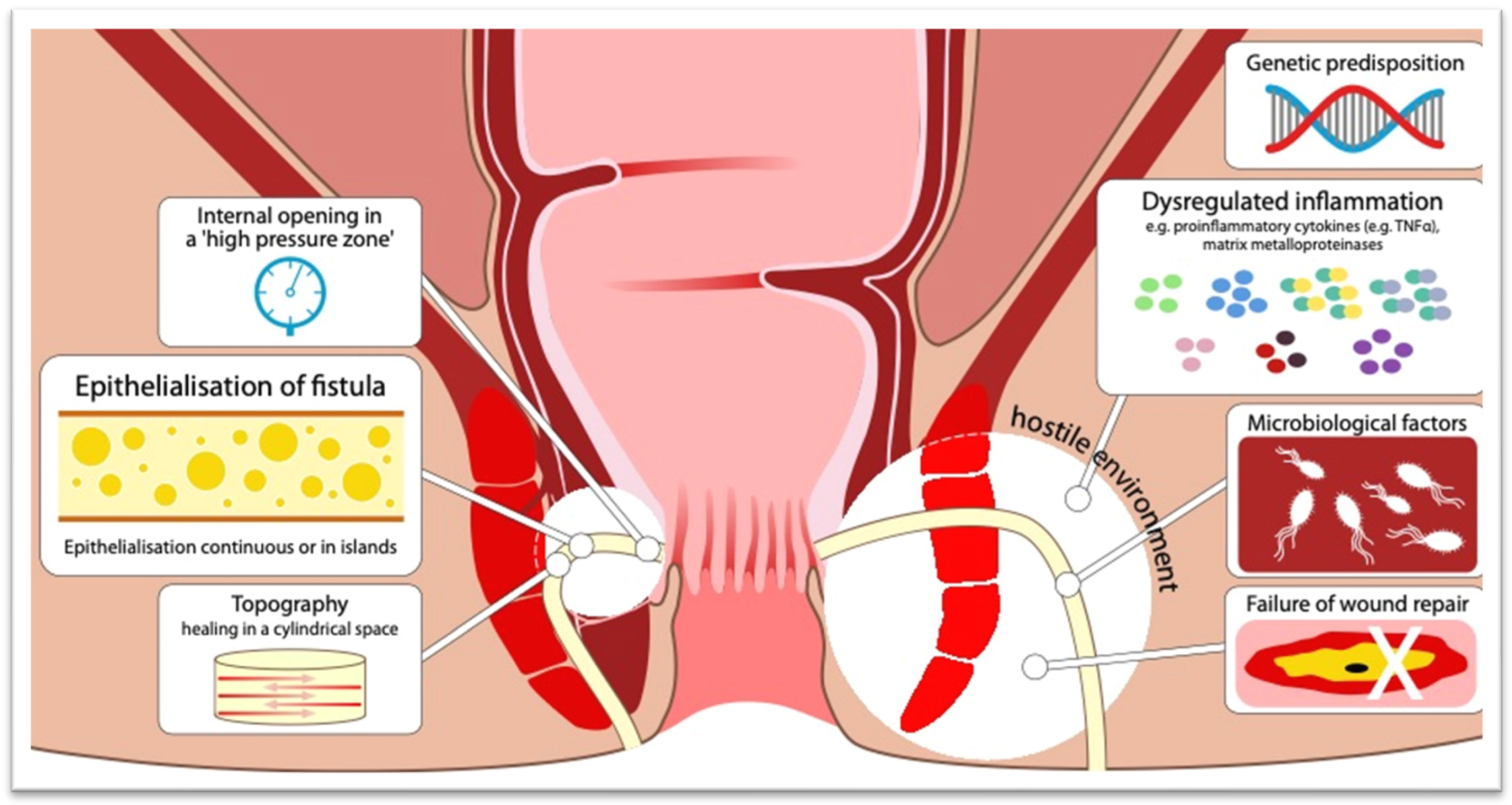
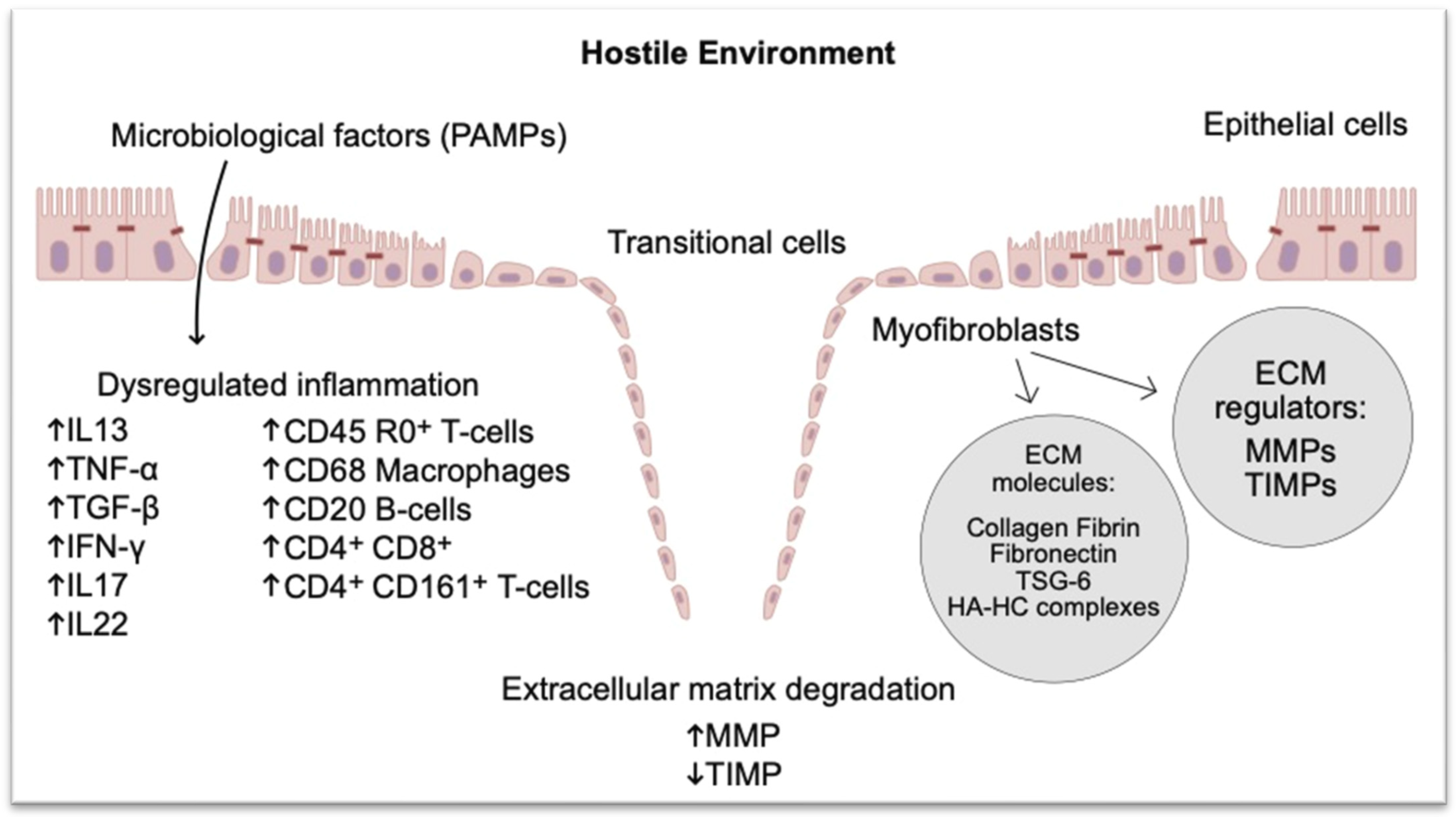
3.2. Better Understanding of the Pathophysiology of Perianal CD
3.3. Improved Management May Need All Aspects of Pathogenesis to Be Addressed—Combining Therapies
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Torres, J.; Mehandru, S.; Colombel, J.F.; Peyrin-Biroulet, L. Crohn’s disease. Lancet 2017, 389, 1741–1755. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, D.A.; Loftus, E.V., Jr.; Tremaine, W.J.; Panaccione, R.; Harmsen, W.S.; Zinsmeister, A.R.; Sandborn, W.J. The natural history of fistulizing Crohn’s disease in Olmsted County, Minnesota. Gastroenterology 2002, 122, 875–880. [Google Scholar] [CrossRef] [PubMed]
- Ingle, S.B.; Loftus, E.V., Jr. The natural history of perianal Crohn’s disease. Dig. Liver Dis. 2007, 39, 963–969. [Google Scholar] [CrossRef]
- Eglinton, T.W.; Barclay, M.L.; Gearry, R.B.; Frizelle, F.A. The spectrum of perianal Crohn’s disease in a population-based cohort. Dis. Colon Rectum 2012, 55, 773–777. [Google Scholar] [CrossRef] [PubMed]
- Hellers, G.; Bergstrand, O.; Ewerth, S.; Holmström, B. Occurrence and outcome after primary treatment of anal fistulae in Crohn’s disease. Gut 1980, 21, 525–527. [Google Scholar] [CrossRef]
- Lightner, A.L.; Faubion, W.A.; Fletcher, J.G. Interdisciplinary Management of Perianal Crohn’s Disease. Gastroenterol. Clin. N. Am. 2017, 46, 547–562. [Google Scholar] [CrossRef]
- Siegmund, B.; Feakins, R.M.; Barmias, G.; Ludvig, J.C.; Teixeira, F.V.; Rogler, G.; Scharl, M. Results of the Fifth Scientific Workshop of the ECCO (II): Pathophysiology of Perianal Fistulizing Disease. J. Crohn’s Colitis 2016, 10, 377–386. [Google Scholar] [CrossRef]
- American Gastroenterological Association Clinical Practice Committee. American Gastroenterological Association medical position statement: Perianal Crohn’s disease. Gastroenterology 2003, 125, 1503–1507. [Google Scholar]
- Schwartz, D.A.; Herdman, C.R. Review article: The medical treatment of Crohn’s perianal fistulas. Aliment. Pharmacol. Ther. 2004, 19, 953–967. [Google Scholar] [CrossRef]
- Adegbola, S.O.; Dibley, L.; Sahnan, K.; Wade, T.; Verjee, A.; Sawyer, R.; Mannick, S.; McCluskey, D.; Bassett, P.; Yassin, N.; et al. Development and initial psychometric validation of a patient-reported outcome measure for Crohn’s perianal fistula: The Crohn’s Anal Fistula Quality of Life (CAF-QoL) scale. Gut 2021, 70, 1649–1656. [Google Scholar] [CrossRef]
- Panés, J.; Rimola, J. Perianal fistulizing Crohn’s disease: Pathogenesis, diagnosis and therapy. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 652–664. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, D.A.; Wiersema, M.J.; Dudiak, K.M.; Fletcher, J.G.; Clain, J.E.; Tremaine, W.J.; Zinsmeister, A.R.; Norton, I.D.; Boardman, L.A.; Devine, R.M.; et al. A comparison of endoscopic ultrasound, magnetic resonance imaging, and exam under anesthesia for evaluation of Crohn’s perianal fistulas. Gastroenterology 2001, 121, 1064–1072. [Google Scholar] [CrossRef] [PubMed]
- Sahni, V.A.; Ahmad, R.; Burling, D. Which method is best for imaging of perianal fistula? Abdom. Imaging 2008, 33, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, M.R.; Ashrafian, H.; Tozer, P.; Daulatzai, N.; Burling, D.; Hart, A.; Athanasiou, T.; Phillips, R.K. A diagnostic accuracy meta-analysis of endoanal ultrasound and MRI for perianal fistula assessment. Dis. Colon Rectum 2012, 55, 576–585. [Google Scholar] [CrossRef] [PubMed]
- Adamina, M.; Bonovas, S.; Raine, T.; Spinelli, A.; Warusavitarne, J.; Armuzzi, A.; Bachmann, O.; Bager, P.; Biancone, L.; Bokemeyer, B.; et al. ECCO Guidelines on Therapeutics in Crohn’s Disease: Surgical Treatment. J. Crohn’s Colitis 2020, 14, 155–168. [Google Scholar] [CrossRef] [PubMed]
- Aguilera-Castro, L.; Ferre-Aracil, C.; Garcia-Garcia-de-Paredes, A.; Rodriguez-de-Santiago, E.; Lopez-Sanroman, A. Management of complex perianal Crohn’s disease. Ann. Gastroenterol. 2017, 30, 33–44. [Google Scholar] [CrossRef]
- Marzo, M.; Felice, C.; Pugliese, D.; Andrisani, G.; Mocci, G.; Armuzzi, A.; Guidi, L. Management of perianal fistulas in Crohn’s disease: An up-to-date review. World J. Gastroenterol. 2015, 21, 1394–1403. [Google Scholar] [CrossRef]
- Kaur, M.; Panikkath, D.; Yan, X.; Liu, Z.; Berel, D.; Li, D.; Vasiliauskas, E.A.; Ippoliti, A.; Dubinsky, M.; Shih, D.Q.; et al. Perianal Crohn’s Disease is Associated with Distal Colonic Disease, Stricturing Disease Behavior, IBD-Associated Serologies and Genetic Variation in the JAK-STAT Pathway. Inflamm. Bowel Dis. 2016, 22, 862–869. [Google Scholar] [CrossRef]
- Present, D.H.; Rutgeerts, P.; Targan, S.; Hanauer, S.B.; Mayer, L.; van Hogezand, R.A.; Podolsky, D.K.; Sands, B.E.; Braakman, T.; DeWoody, K.L.; et al. Infliximab for the treatment of fistulas in patients with Crohn’s disease. N. Engl. J. Med. 1999, 340, 1398–1405. [Google Scholar] [CrossRef]
- Sands, B.E.; Anderson, F.H.; Bernstein, C.N.; Chey, W.Y.; Feagan, B.G.; Fedorak, R.N.; Kamm, M.A.; Korzenik, J.R.; Lashner, B.A.; Onken, J.E.; et al. Infliximab maintenance therapy for fistulizing Crohn’s disease. N. Engl. J. Med. 2004, 350, 876–885. [Google Scholar] [CrossRef]
- Yarur, A.J.; Kanagala, V.; Stein, D.J.; Czul, F.; Quintero, M.A.; Agrawal, D.; Patel, A.; Best, K.; Fox, C.; Idstein, K.; et al. Higher infliximab trough levels are associated with perianal fistula healing in patients with Crohn’s disease. Aliment. Pharmacol. Ther. 2017, 45, 933–940. [Google Scholar] [CrossRef]
- Davidov, Y.; Ungar, B.; Bar-Yoseph, H.; Carter, D.; Haj-Natour, O.; Yavzori, M.; Chowers, Y.; Eliakim, R.; Ben-Horin, S.; Kopylov, U. Association of Induction Infliximab Levels With Clinical Response in Perianal Crohn’s Disease. J. Crohn’s Colitis 2017, 11, 549–555. [Google Scholar] [CrossRef]
- Papamichael, K.; Vande Casteele, N.; Jeyarajah, J.; Jairath, V.; Osterman, M.T.; Cheifetz, A.S. Higher Postinduction Infliximab Concentrations Are Associated With Improved Clinical Outcomes in Fistulizing Crohn’s Disease: An ACCENT-II Post Hoc Analysis. Am. J. Gastroenterol. 2021, 116, 1007–1014. [Google Scholar] [CrossRef]
- De Gregorio, M.; Lee, T.; Krishnaprasad, K.; Amos, G.; An, Y.K.; Bastian-Jordan, M.; Begun, J.; Borok, N.; Brown, D.J.M.; Cheung, W.; et al. Higher Anti-tumor Necrosis Factor-α Levels Correlate With Improved Radiologic Outcomes in Crohn’s Perianal Fistulas. Clin. Gastroenterol. Hepatol. 2022, 20, 1306–1314. [Google Scholar] [CrossRef]
- Zhu, M.; Xu, X.; Feng, Q.; Cui, Z.; Wang, T.; Yan, Y.; Ran, Z. Effectiveness of Infliximab on Deep Radiological Remission in Chinese Patients with Perianal Fistulizing Crohn’s Disease. Dig. Dis. Sci. 2021, 66, 1658–1668. [Google Scholar] [CrossRef] [PubMed]
- Plevris, N.; Jenkinson, P.W.; Arnott, I.D.; Jones, G.R.; Lees, C.W. Higher anti-tumor necrosis factor levels are associated with perianal fistula healing and fistula closure in Crohn’s disease. Eur. J. Gastroenterol. Hepatol. 2020, 32, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Strik, A.S.; Löwenberg, M.; Buskens, C.J.; Gecse, B.K.; Ponsioen, I.C.; Bemelman, W.A.; D’Haens, G.R. Higher anti-TNF serum levels are associated with perianal fistula closure in Crohn’s disease patients. Scand. J. Gastroenterol. 2019, 54, 453–458. [Google Scholar] [CrossRef] [PubMed]
- Colombel, J.F.; Sandborn, W.J.; Rutgeerts, P.; Enns, R.; Hanauer, S.B.; Panaccione, R.; Schreiber, S.; Byczkowski, D.; Li, J.; Kent, J.D.; et al. Adalimumab for maintenance of clinical response and remission in patients with Crohn’s disease: The CHARM trial. Gastroenterology 2007, 132, 52–65. [Google Scholar] [CrossRef] [PubMed]
- Lichtiger, S.; Binion, D.G.; Wolf, D.C.; Present, D.H.; Bensimon, A.G.; Wu, E.; Yu, A.P.; Cardoso, A.T.; Chao, J.; Mulani, P.M.; et al. The CHOICE trial: Adalimumab demonstrates safety, fistula healing, improved quality of life and increased work productivity in patients with Crohn’s disease who failed prior infliximab therapy. Aliment. Pharmacol. Ther. 2010, 32, 1228–1239. [Google Scholar] [CrossRef]
- Panaccione, R.; Loftus, E.V., Jr.; Binion, D.; McHugh, K.; Alam, S.; Chen, N.; Guerette, B.; Mulani, P.; Chao, J. Efficacy and safety of adalimumab in Canadian patients with moderate to severe Crohn’s disease: Results of the Adalimumab in Canadian SubjeCts with ModErate to Severe Crohn’s DiseaSe (ACCESS) trial. Can. J. Gastroenterol. 2011, 25, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Adegbola, S.O.; Sahnan, K.; Tozer, P.J.; Phillips, R.K.; Faiz, O.D.; Warusavitarne, J.; Hart, A. Review of local injection of anti-TNF for perianal fistulizing Crohn’s disease. Int. J. Color. Dis. 2017, 32, 1539–1544. [Google Scholar] [CrossRef] [PubMed]
- Sands, B.O.; Gasink, C.; Jacobstein, D.; Gao, L.L.; Johanns, J.; Colombel, J.F.; de Villiers, W.J.; Sandborn, W.J. Fistula healing in pivotal studies of Ustekinumab in Crohn’s disease. Gastroenterology 2017, 152, S185. [Google Scholar] [CrossRef]
- Laurent, P.; Panaccione, R.; Gasink, C.; Hoops, T.; Izanec, J.L.; Ma, T.; Nazar, M.; Bravata, I.; Lahaye, M.; Irving, P.M.; et al. O30 Closure of perianal fistula in patients receiving ustekinumab in the SEAVUE and STARDUST trials. Gut 2022, 71, A17. [Google Scholar]
- Biemans, V.B.C.; van der Meulen-de Jong, A.E.; van der Woude, C.J.; Löwenberg, M.; Dijkstra, G.; Oldenburg, B.; de Boer, N.K.H.; van der Marel, S.; Bodelier, A.G.L.; Jansen, J.M.; et al. Ustekinumab for Crohn’s Disease: Results of the ICC Registry, a Nationwide Prospective Observational Cohort Study. J. Crohn’s Colitis 2020, 14, 33–45. [Google Scholar] [CrossRef]
- Chapuis-Biron, C.; Kirchgesner, J.; Pariente, B.; Bouhnik, Y.; Amiot, A.; Viennot, S.; Serrero, M.; Fumery, M.; Allez, M.; Siproudhis, L.; et al. Ustekinumab for Perianal Crohn’s Disease: The BioLAP Multicenter Study From the GETAID. Am. J. Gastroenterol. 2020, 115, 1812–1820. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Zhang, H.; Su, T.; Peng, X.; Zhao, J.; Liu, T.; Wang, W.; Hu, P.; Zhi, M.; Zhang, M. Ustekinumab Promotes Radiological Fistula Healing in Perianal Fistulizing Crohn’s Disease: A Retrospective Real-World Analysis. J. Clin. Med. 2023, 12, 939. [Google Scholar] [CrossRef] [PubMed]
- Chapuis-Biron, C.; Bourrier, A.; Nachury, M.; Nancey, S.; Bouhnik, Y.; Serrero, M.; Armengol-Debeir, L.; Buisson, A.; Tran-Minh, M.L.; Zallot, C.; et al. Vedolizumab for perianal Crohn’s disease: A multicentre cohort study in 151 patients. Aliment. Pharmacol. Ther. 2020, 51, 719–727. [Google Scholar] [CrossRef]
- Schwartz, D.A.; Peyrin-Biroulet, L.; Lasch, K.; Adsul, S.; Danese, S. Efficacy and Safety of 2 Vedolizumab Intravenous Regimens for Perianal Fistulizing Crohn’s Disease: ENTERPRISE Study. Clin. Gastroenterol. Hepatol. 2021, 20, 1059–1067.e9. [Google Scholar] [CrossRef]
- Reinisch, W.; Colombel, J.F.; D’Haens, G.; Rimola, J.; DeHaas-Amatsaleh, A.; McKevitt, M.; Ren, X.; Serone, A.; Schwartz, D.A.; Gecse, K.B. OP18 efficacy and safety of filgotinib for the treatment of perianal fistulizing Crohn’s disease: Results from the phase 2 divergence 2 study. J. Crohn’s Colitis 2022, 16, i019–i021. [Google Scholar] [CrossRef]
- Colombel, J.F.; Irving, P.; Rieder, F.; Panaccione, R.; Schwartz, D.; Hayashi, R.; Zhu, X.; Lacerda, A.P.; Dubcenco, E.; Marced, E.; et al. Efficacy and safety of upadacitinib for the treatment of fistulas and fissures in patients with Crohn’s disease. J. Crohn’s Colitis 2023, 17, i620–i622. [Google Scholar] [CrossRef]
- Mak, J.W.Y.; Tang, W.; Yip, T.C.F.; Ran, Z.H.; Wei, S.C.; Ahuja, V.; Kumar, S.; Leung, W.K.; Hilmi, I.; Limsrivilai, J.; et al. Stopping anti-tumour necrosis factor therapy in patients with perianal Crohn’s disease. Aliment. Pharmacol. Ther. 2019, 50, 1195–1203. [Google Scholar] [CrossRef] [PubMed]
- Huinink, S.T.B.; Thomassen, D.; Steyerberg, E.W.; Pauwels, R.W.M.; Casanova, M.J.; Bouguen, G.; Mak, J.W.Y.; Molnár, T.; Lobo, A.J.; Seidelin, J.B.; et al. Discontinuation of Anti-Tumour Necrosis Factor Therapy in Patients with Perianal Fistulizing Crohn’s Disease: Individual Participant Data Meta-Analysis of 309 patients from 12 studies. J. Crohn’s Colitis 2023. online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.J.; Heywood, N.; Sagar, P.M.; Brown, S.R.; Fearnhead, N.S.; pCD Collaborators. Surgical management of fistulating perianal Crohn’s disease: A UK survey. Color. Dis. 2017, 19, 266–273. [Google Scholar] [CrossRef]
- Lamb, C.A.; Kennedy, N.A.; Raine, T.; Hendy, P.A.; Smith, P.J.; Limdi, J.K.; Hayee, B.; Lomer, M.C.E.; Parkes, G.C.; Selinger, C.; et al. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut 2019, 68 (Suppl. 3), s1–s106. [Google Scholar] [CrossRef]
- Regueiro, M.; Mardini, H. Treatment of perianal fistulizing Crohn’s disease with infliximab alone or as an adjunct to exam under anesthesia with seton placement. Inflamm. Bowel Dis. 2003, 9, 98–103. [Google Scholar] [CrossRef]
- Gaertner, W.B.; Decanini, A.; Mellgren, A.; Lowry, A.C.; Goldberg, S.M.; Madoff, R.D.; Spencer, M.P. Does infliximab infusion impact results of operative treatment for Crohn’s perianal fistulas? Dis. Colon Rectum 2007, 50, 1754–1760. [Google Scholar] [CrossRef]
- Stellingwerf, M.E.; van Praag, E.M.; Tozer, P.J.; Bemelman, W.A.; Buskens, C.J. Systematic review and meta-analysis of endorectal advancement flap and ligation of the intersphincteric fistula tract for cryptoglandular and Crohn’s high perianal fistulas. BJS Open 2019, 3, 231–241. [Google Scholar] [CrossRef]
- Soltani, A.; Kaiser, A.M. Endorectal advancement flap for cryptoglandular or Crohn’s fistula-in-ano. Dis. Colon Rectum 2010, 53, 486–495. [Google Scholar] [CrossRef] [PubMed]
- Wood, T.; Truong, A.; Mujukian, A.; Zaghiyan, K.; Fleshner, P. Increasing experience with the LIFT procedure in Crohn’s disease patients with complex anal fistula. Tech. Coloproctol. 2022, 26, 205–212. [Google Scholar] [CrossRef]
- Wasmann, K.A.; de Groof, E.J.; Stellingwerf, M.E.; D’Haens, G.R.; Ponsioen, C.Y.; Gecse, K.B.; Dijkgraaf, M.G.W.; Gerhards, M.F.; Jansen, J.M.; Pronk, A.; et al. Treatment of Perianal Fistulas in Crohn’s Disease, Seton Versus Anti-TNF Versus Surgical Closure Following Anti-TNF [PISA]: A Randomised Controlled Trial. J. Crohn’s Colitis 2020, 14, 1049–1056. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.; Li, Y.L.; Nan, S.J.; Xiu, W.C.; Wang, Y.Q. Video-assisted anal fistula treatment for complex anorectal fistulas in adults: A systematic review and meta-analysis. Tech. Coloproctol. 2022, 26, 783–795. [Google Scholar] [CrossRef]
- Chase, T.J.G.; Quddus, A.; Selvakumar, D.; Cunha, P.; Cuming, T. VAAFT for complex anal fistula: A useful tool, however, cure is unlikely. Tech. Coloproctol. 2021, 25, 1115–1121. [Google Scholar] [CrossRef]
- Schwandner, O. Video-assisted anal fistula treatment (VAAFT) combined with advancement flap repair in Crohn’s disease. Tech. Coloproctol. 2013, 17, 221–225. [Google Scholar] [CrossRef] [PubMed]
- Adegbola, S.O.; Sahnan, K.; Tozer, P.J.; Strouhal, R.; Hart, A.L.; Lung, P.F.C.; Phillips, R.K.S.; Faiz, O.; Warusavitarne, J. Symptom Amelioration in Crohn’s Perianal Fistulas Using Video-Assisted Anal Fistula Treatment (VAAFT). J. Crohn’s Colitis 2018, 12, 1067–1072. [Google Scholar] [CrossRef]
- Cao, D.; Li, W.; Ji, Y.; Wang, X.; Cui, Z. Efficacy and safety of FiLaC™ for perianal fistulizing Crohn’s disease: A systematic review and meta-analysis. Tech. Coloproctol. 2022, 26, 775–781. [Google Scholar] [CrossRef]
- Senéjoux, A.; Siproudhis, L.; Abramowitz, L.; Munoz-Bongrand, N.; Desseaux, K.; Bouguen, G.; Bourreille, A.; Dewit, O.; Stefanescu, C.; Vernier, G.; et al. Fistula Plug in Fistulising Ano-Perineal Crohn’s Disease: A Randomised Controlled Trial. J. Crohn’s Colitis 2016, 10, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Grimaud, J.C.; Munoz-Bongrand, N.; Siproudhis, L.; Abramowitz, L.; Sénéjoux, A.; Vitton, V.; Gambiez, L.; Flourié, B.; Hébuterne, X.; Louis, E.; et al. Fibrin glue is effective healing perianal fistulas in patients with Crohn’s disease. Gastroenterology 2010, 138, 2275–2281.e1. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.J.; Parker, C.E.; Taylor, S.R.; Guizzetti, L.; Feagan, B.G.; Lobo, A.J.; Jairath, V. Efficacy of Medical Therapies for Fistulizing Crohn’s Disease: Systematic Review and Meta-analysis. Clin. Gastroenterol. Hepatol. 2018, 16, 1879–1892. [Google Scholar] [CrossRef]
- de Groof, E.J.; Sahami, S.; Lucas, C.; Ponsioen, C.Y.; Bemelman, W.A.; Buskens, C.J. Treatment of perianal fistula in Crohn’s disease: A systematic review and meta-analysis comparing seton drainage and anti-tumour necrosis factor treatment. Color. Dis. 2016, 18, 667–675. [Google Scholar] [CrossRef]
- Yassin, N.A.; Askari, A.; Warusavitarne, J.; Faiz, O.D.; Athanasiou, T.; Phillips, R.K.; Hart, A.L. Systematic review: The combined surgical and medical treatment of fistulizing perianal Crohn’s disease. Aliment. Pharmacol. Ther. 2014, 40, 741–749. [Google Scholar] [CrossRef]
- Gecse, K.B.; Sebastian, S.; Hertogh, G.D.; Yassin, N.A.; Kotze, P.G.; Reinisch, W.; Spinelli, A.; Koutroubakis, I.E.; Katsanos, K.H.; Hart, A.; et al. Results of the Fifth Scientific Workshop of the ECCO [II]: Clinical Aspects of Perianal Fistulizing Crohn’s Disease-the Unmet Needs. J. Crohn’s Colitis 2016, 10, 758–765. [Google Scholar] [CrossRef] [PubMed]
- Meima-van Praag, E.M.; van Rijn, K.L.; Wasmann, K.A.T.G.M.; Snijder, H.J.; Stoker, J.; D’Haens, G.R.; Gecse, K.B.; Gerhards, M.F.; Jansen, J.M.; Dijkgraaf, M.G.W.; et al. Short-term anti-TNF therapy with surgical closure versus anti-TNF therapy in the treatment of perianal fistulas in Crohn’s disease (PISA-II): A patient preference randomised trial. Lancet Gastroenterol. Hepatol. 2022, 7, 617–626. [Google Scholar] [CrossRef] [PubMed]
- Tozer, P.; Ng, S.C.; Siddiqui, M.R.; Plamondon, S.; Burling, D.; Gupta, A.; Swatton, A.; Tripoli, S.; Vaizey, C.J.; Kamm, M.A.; et al. Long-term MRI-guided combined anti-TNF-α and thiopurine therapy for Crohn’s perianal fistulas. Inflamm. Bowel Dis. 2012, 18, 1825–1834. [Google Scholar] [CrossRef] [PubMed]
- Chambaz, M.; Verdalle-Cazes, M.; Desprez, C.; Thomassin, L.; Charpentier, C.; Grigioni, S.; Armengol-Debeir, L.; Bridoux, V.; Savoye, G.; Savoye-Collet, C. Deep remission on magnetic resonance imaging impacts outcomes of perianal fistulizing Crohn’s disease. Dig. Liver Dis. 2019, 51, 358–363. [Google Scholar] [CrossRef]
- Panés, J.; García-Olmo, D.; Van Assche, G.; Colombel, J.F.; Reinisch, W.; Baumgart, D.C.; Dignass, A.; Nachury, M.; Ferrante, M.; Kazemi-Shirazi, L.; et al. Expanded allogeneic adipose-derived mesenchymal stem cells (Cx601) for complex perianal fistulas in Crohn’s disease: A phase 3 randomised, double-blind controlled trial. Lancet 2016, 388, 1281–1290. [Google Scholar] [CrossRef]
- Panés, J.; Bouma, G.; Ferrante, M.; Kucharzik, T.; Nachury, M.; de la Portilla de Juan, F.; Reinisch, W.; Selvaggi, F.; Tschmelitsch, J.; Brett, N.R.; et al. INSPECT: A Retrospective Study to Evaluate Long-term Effectiveness and Safety of Darvadstrocel in Patients With Perianal Fistulizing Crohn’s Disease Treated in the ADMIRE-CD Trial. Inflamm. Bowel Dis. 2022, 28, 1737–1745. [Google Scholar] [CrossRef]
- Geldof, J.; Iqbal, N.; LeBlanc, J.F.; Anandabaskaran, S.; Sawyer, R.; Buskens, C.; Bemelman, W.; Gecse, K.; Lundby, L.; Lightner, A.L.; et al. Classifying perianal fistulising Crohn’s disease: An expert consensus to guide decision-making in daily practice and clinical trials. Lancet Gastroenterol. Hepatol. 2022, 7, 576–584. [Google Scholar] [CrossRef]
- Scharl, M.; Frei, S.; Pesch, T.; Kellermeier, S.; Arikkat, J.; Frei, P.; Fried, M.; Weber, A.; Jehle, E.; Rühl, A.; et al. Interleukin-13 and transforming growth factor β synergise in the pathogenesis of human intestinal fistulae. Gut 2013, 62, 63–72. [Google Scholar] [CrossRef]
- Bataille, F.; Rohrmeier, C.; Bates, R.; Weber, A.; Rieder, F.; Brenmoehl, J.; Strauch, U.; Farkas, S.; Fürst, A.; Hofstädter, F.; et al. Evidence for a role of epithelial mesenchymal transition during pathogenesis of fistulae in Crohn’s disease. Inflamm. Bowel Dis. 2008, 14, 1514–1527. [Google Scholar] [CrossRef]
- Scharl, M.; Weber, A.; Fürst, A.; Farkas, S.; Jehle, E.; Pesch, T.; Kellermeier, S.; Fried, M.; Rogler, G. Potential role for SNAIL family transcription factors in the etiology of Crohn’s disease-associated fistulae. Inflamm. Bowel Dis. 2011, 17, 1907–1916. [Google Scholar] [CrossRef]
- Frei, S.M.; Pesch, T.; Lang, S.; Weber, A.; Jehle, E.; Vavricka, S.R.; Fried, M.; Rogler, G.; Scharl, M. A role for tumor necrosis factor and bacterial antigens in the pathogenesis of Crohn’s disease-associated fistulae. Inflamm. Bowel Dis. 2013, 19, 2878–2887. [Google Scholar] [CrossRef] [PubMed]
- Goffin, L.; Fagagnini, S.; Vicari, A.; Mamie, C.; Melhem, H.; Weder, B.; Lutz, C.; Lang, S.; Scharl, M.; Rogler, G.; et al. Anti-MMP-9 Antibody: A Promising Therapeutic Strategy for Treatment of Inflammatory Bowel Disease Complications with Fibrosis. Inflamm. Bowel Dis. 2016, 22, 2041–2057. [Google Scholar] [CrossRef]
- Kirkegaard, T.; Hansen, A.; Bruun, E.; Brynskov, J. Expression and localisation of matrix metalloproteinases and their natural inhibitors in fistulae of patients with Crohn’s disease. Gut 2004, 53, 701–709. [Google Scholar] [CrossRef]
- Rizzo, G.; Rubbino, F.; Elangovan, S.; Sammarco, G.; Lovisa, S.; Restelli, S.; Pineda Chavez, S.E.; Massimino, L.; Lamparelli, L.; Paulis, M.; et al. Dysfunctional Extracellular Matrix Remodeling Supports Perianal Fistulizing Crohn’s Disease by a Mechanoregulated Activation of the Epithelial-to-Mesenchymal Transition. Cell. Mol. Gastroenterol. Hepatol. 2023, 15, 741–764. [Google Scholar] [CrossRef] [PubMed]
- Tozer, P.J.; Lung, P.; Lobo, A.J.; Sebastian, S.; Brown, S.R.; Hart, A.L.; Fearnhead, N.; ENiGMA Collaboration. Review article: Pathogenesis of Crohn’s perianal fistula-understanding factors impacting on success and failure of treatment strategies. Aliment. Pharmacol. Ther. 2018, 48, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Toyonaga, T.; Matsushima, M.; Tanaka, Y.; Shimojima, Y.; Matsumura, N.; Kannyama, H.; Nozawa, M.; Hatakeyama, T.; Suzuki, K.; Yanagita, K.; et al. Microbiological analysis and endoanal ultrasonography for diagnosis of anal fistula in acute anorectal sepsis. Int. J. Color. Dis. 2007, 22, 209–213. [Google Scholar] [CrossRef] [PubMed]
- Tozer, P.J.; Rayment, N.; Hart, A.L.; Daulatzai, N.; Murugananthan, A.U.; Whelan, K.; Phillips, R.K. What role do bacteria play in persisting fistula formation in idiopathic and Crohn’s anal fistula? Color. Dis. 2015, 17, 235–241. [Google Scholar] [CrossRef] [PubMed]
- van Onkelen, R.S.; Mitalas, L.E.; Gosselink, M.P.; van Belkum, A.; Laman, J.D.; Schouten, W.R. Assessment of microbiota and peptidoglycan in perianal fistulas. Diagn. Microbiol. Infect. Dis. 2013, 75, 50–54. [Google Scholar] [CrossRef]
- van Onkelen, R.S.; Gosselink, M.P.; van Meurs, M.; Melief, M.J.; Schouten, W.R.; Laman, J.D. Pro-inflammatory cytokines in cryptoglandular anal fistulas. Tech. Coloproctol. 2016, 20, 619–625. [Google Scholar] [CrossRef]
- Singh, S.; Ding, N.S.; Mathis, K.L.; Dulai, P.S.; Farrell, A.M.; Pemberton, J.H.; Hart, A.L.; Sandborn, W.J.; Loftus, E.V., Jr. Systematic review with meta-analysis: Faecal diversion for management of perianal Crohn’s disease. Aliment. Pharmacol. Ther. 2015, 42, 783–792. [Google Scholar] [CrossRef]
- Goren, I.; Ian, W.; Reshef, L.; Sharar Fischler, T.; Pauker, M.; Silverman, J.; Dotan, I.; Yanai, H. P683 Patients with newly diagnosed fistulizing perianal Crohn’s disease have a distinct microbial signature. J. Crohn’s Colitis 2021, 15, S602. [Google Scholar] [CrossRef]
- Breton, J.; Tanes, C.; Tu, V.; Albenberg, L.; Rowley, S.; Devas, N.; Hwang, R.; Kachelries, K.; Wu, G.D.; Baldassano, R.N.; et al. A Microbial Signature for Paediatric Perianal Crohn’s Disease. J. Crohn’s Colitis 2022, 16, 1281–1292. [Google Scholar] [CrossRef] [PubMed]
- Schnitzler, F.; Friedrich, M.; Wolf, C.; Stallhofer, J.; Angelberger, M.; Diegelmann, J.; Olszak, T.; Tillack, C.; Beigel, F.; Göke, B.; et al. The NOD2 Single Nucleotide Polymorphism rs72796353 (IVS4+10 A>C) Is a Predictor for Perianal Fistulas in Patients with Crohn’s Disease in the Absence of Other NOD2 Mutations. PLoS ONE 2015, 10, e0116044. [Google Scholar] [CrossRef] [PubMed]
- Vermeire, S.; Pierik, M.; Hlavaty, T.; Claessens, G.; van Schuerbeeck, N.; Joossens, S.; Ferrante, M.; Henckaerts, L.; Bueno de Mesquita, M.; Vlietinck, R.; et al. Association of organic cation transporter risk haplotype with perianal penetrating Crohn’s disease but not with susceptibility to IBD. Gastroenterology 2005, 129, 1845–1853. [Google Scholar] [CrossRef]
- Palmieri, O.; Latiano, A.; Valvano, R.; D’Incà, R.; Vecchi, M.; Sturniolo, G.C.; Saibeni, S.; Peyvandi, F.; Bossa, F.; Zagaria, C.; et al. Variants of OCTN1-2 cation transporter genes are associated with both Crohn’s disease and ulcerative colitis. Aliment. Pharmacol. Ther. 2006, 23, 497–506. [Google Scholar] [CrossRef]
- Eglinton, T.W.; Roberts, R.; Pearson, J.; Barclay, M.; Merriman, T.R.; Frizelle, F.A.; Gearry, R.B. Clinical and genetic risk factors for perianal Crohn’s disease in a population-based cohort. Am. J. Gastroenterol. 2012, 107, 589–596. [Google Scholar] [CrossRef]
- Latiano, A.; Palmieri, O.; Cucchiara, S.; Castro, M.; D’Incà, R.; Guariso, G.; Dallapiccola, B.; Valvano, M.R.; Latiano, T.; Andriulli, A.; et al. Polymorphism of the IRGM gene might predispose to fistulizing behavior in Crohn’s disease. Am. J. Gastroenterol. 2009, 104, 110–116. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, X.; Jiang, X.; Yang, X.; Wen, C.; Zhi, M.; Gao, X.; Hu, P.; Liu, H. Polymorphisms of the TNF Gene and Three Susceptibility Loci Are Associated with Crohn’s Disease and Perianal Fistula Crohn’s Disease: A Study among the Han Population from South China. Med. Sci. Monit. 2019, 25, 9637–9650. [Google Scholar] [CrossRef]
- Beser, O.F.; Conde, C.D.; Serwas, N.K.; Cokugras, F.C.; Kutlu, T.; Boztug, K.; Erkan, T. Clinical features of interleukin 10 receptor gene mutations in children with very early-onset inflammatory bowel disease. J. Pediatr. Gastroenterol. Nutr. 2015, 60, 332–338. [Google Scholar] [CrossRef]
- Yang, D.H.; Yang, S.K.; Song, K.; Hong, M.; Park, S.H.; Lee, H.S.; Kim, J.B.; Lee, H.J.; Park, S.K.; Jung, K.W.; et al. TNFSF15 is an independent predictor for the development of Crohn’s disease-related complications in Koreans. J. Crohn’s Colitis 2014, 8, 1315–1326. [Google Scholar] [CrossRef]
- de Ridder, L.; Weersma, R.K.; Dijkstra, G.; van der Steege, G.; Benninga, M.A.; Nolte, I.M.; Taminiau, J.A.; Hommes, D.W.; Stokkers, P.C. Genetic susceptibility has a more important role in pediatric-onset Crohn’s disease than in adult-onset Crohn’s disease. Inflamm. Bowel Dis. 2007, 13, 1083–1092. [Google Scholar] [CrossRef] [PubMed]
- Henckaerts, L.; Van Steen, K.; Verstreken, I.; Cleynen, I.; Franke, A.; Schreiber, S.; Rutgeerts, P.; Vermeire, S. Genetic risk profiling and prediction of disease course in Crohn’s disease patients. Clin. Gastroenterol. Hepatol. 2009, 7, 972–980.e2. [Google Scholar] [CrossRef] [PubMed]
- Quaranta, M.; Burden, A.D.; Griffiths, C.E.; Worthington, J.; Barker, J.N.; Trembath, R.C.; Capon, F. Differential contribution of CDKAL1 variants to psoriasis, Crohn’s disease and type II diabetes. Genes Immun. 2009, 10, 654–658. [Google Scholar] [CrossRef]
- Song, J.; Zhuang, Y.; Zhu, C.; Meng, H.; Lu, B.; Xie, B.; Peng, J.; Li, M.; Yi, C. Differential roles of human PUS10 in miRNA processing and tRNA pseudouridylation. Nat. Chem. Biol. 2020, 16, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Cleynen, I.; González, J.R.; Figueroa, C.; Franke, A.; McGovern, D.; Bortlík, M.; Crusius, B.J.; Vecchi, M.; Artieda, M.; Szczypiorska, M.; et al. Genetic factors conferring an increased susceptibility to develop Crohn’s disease also influence disease phenotype: Results from the IBDchip European Project. Gut 2013, 62, 1556–1565. [Google Scholar] [CrossRef] [PubMed]
- Bataille, F.; Klebl, F.; Rümmele, P.; Schroeder, J.; Farkas, S.; Wild, P.J.; Fürst, A.; Hofstädter, F.; Schölmerich, J.; Herfarth, H.; et al. Morphological characterisation of Crohn’s disease fistulae. Gut 2004, 53, 1314–1321. [Google Scholar] [CrossRef]
- Bruckner, R.S.; Spalinger, M.R.; Barnhoorn, M.C.; Feakins, R.; Fuerst, A.; Jehle, E.C.; Rickenbacher, A.; Turina, M.; Niechcial, A.; Lang, S.; et al. Contribution of CD3+CD8- and CD3+CD8+ T Cells to TNF-α Overexpression in Crohn Disease-Associated Perianal Fistulas and Induction of Epithelial-Mesenchymal Transition in HT-29 Cells. Inflamm. Bowel Dis. 2021, 27, 538–549. [Google Scholar] [CrossRef]
- Maggi, L.; Capone, M.; Giudici, F.; Santarlasci, V.; Querci, V.; Liotta, F.; Ficari, F.; Maggi, E.; Tonelli, F.; Annunziato, F.; et al. CD4+CD161+ T lymphocytes infiltrate Crohn’s disease-associated perianal fistulas and are reduced by anti-TNF-α local therapy. Int. Arch. Allergy Immunol. 2013, 161, 81–86. [Google Scholar] [CrossRef]
- van Unen, V.; Li, N.; Molendijk, I.; Temurhan, M.; Höllt, T.; van der Meulen-de Jong, A.E.; Verspaget, H.W.; Mearin, M.L.; Mulder, C.J.; van Bergen, J.; et al. Mass Cytometry of the Human Mucosal Immune System Identifies Tissue- and Disease-Associated Immune Subsets. Immunity 2016, 44, 1227–1239. [Google Scholar] [CrossRef]
- Kotze, P.G.; Shen, B.; Lightner, A.; Yamamoto, T.; Spinelli, A.; Ghosh, S.; Panaccione, R. Modern management of perianal fistulas in Crohn’s disease: Future directions. Gut 2018, 67, 1181–1194. [Google Scholar] [CrossRef]
- García-Olmo, D.; García-Arranz, M.; Herreros, D.; Pascual, I.; Peiro, C.; Rodríguez-Montes, J.A. A phase I clinical trial of the treatment of Crohn’s fistula by adipose mesenchymal stem cell transplantation. Dis. Colon Rectum 2005, 48, 1416–1423. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Olmo, D.; Herreros, D.; Pascual, I.; Pascual, J.A.; Del-Valle, E.; Zorrilla, J.; De-La-Quintana, P.; Garcia-Arranz, M.; Pascual, M. Expanded adipose-derived stem cells for the treatment of complex perianal fistula: A phase II clinical trial. Dis. Colon Rectum 2009, 52, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Su, Q.; Zhang, B.; Shen, F.; Li, S. Efficacy of stem cells therapy for Crohn’s fistula: A meta-analysis and systematic review. Stem Cell Res. Ther. 2021, 12, 32. [Google Scholar] [CrossRef]
- Kou, M.; Huang, L.; Yang, J.; Chiang, Z.; Chen, S.; Liu, J.; Guo, L.; Zhang, X.; Zhou, X.; Xu, X.; et al. Mesenchymal stem cell-derived extracellular vesicles for immunomodulation and regeneration: A next generation therapeutic tool? Cell Death Dis. 2022, 13, 580. [Google Scholar] [CrossRef] [PubMed]
- Guillamat-Prats, R. The Role of MSC in Wound Healing, Scarring and Regeneration. Cells 2021, 10, 1729. [Google Scholar] [CrossRef]
- Carvello, M.; Lightner, A.; Yamamoto, T.; Kotze, P.G.; Spinelli, A. Mesenchymal Stem Cells for Perianal Crohn’s Disease. Cells 2019, 8, 764. [Google Scholar] [CrossRef]
- Li, N.; Hua, J. Interactions between mesenchymal stem cells and the immune system. Cell. Mol. Life Sci. 2017, 74, 2345–2360. [Google Scholar] [CrossRef] [PubMed]
- Lightner, A.L.; Faubion, W.A. Mesenchymal Stem Cell Injections for the Treatment of Perianal Crohn’s Disease: What We Have Accomplished and What We Still Need to Do. J. Crohn’s Colitis 2017, 11, 1267–1276. [Google Scholar] [CrossRef]
- Hanna, J.; Hubel, A. Preservation of stem cells. Organogenesis 2009, 5, 134–137. [Google Scholar] [CrossRef]
- Dadgar, N.; Altemus, J.; Li, Y.; Lightner, A.L. Effect of Crohn’s disease mesenteric mesenchymal stem cells and their extracellular vesicles on T-cell immunosuppressive capacity. J. Cell. Mol. Med. 2022, 26, 4924–4939. [Google Scholar] [CrossRef]
- Galleu, A.; Riffo-Vasquez, Y.; Trento, C.; Lomas, C.; Dolcetti, L.; Cheung, T.S.; von Bonin, M.; Barbieri, L.; Halai, K.; Ward, S.; et al. Apoptosis in mesenchymal stromal cells induces in vivo recipient-mediated immunomodulation. Sci. Transl. Med. 2017, 9, eaam7828. [Google Scholar] [CrossRef]
- Iqbal, N.; Constable, L.; Tozer, P.; Lung, P.; Hart, A.; Powell, N. P336 Can Mesenchymal Stem Cell (MSC) apoptosis be used as a biomarker for treatment success in perianal fistulising Crohn’s Disease (pCD)? Findings from a prospective pilot study. J. Crohn’s Colitis 2022, 16 (Supp. 1), i353. [Google Scholar] [CrossRef]
- Altemus, J.; Dadgar, N.; Li, Y.; Lightner, A.L. Adipose tissue-derived mesenchymal stem cells’ acellular product extracellular vesicles as a potential therapy for Crohn’s disease. J. Cell Physiol. 2022, 237, 3001–3011. [Google Scholar] [CrossRef]
- Yang, E.; Panaccione, N.; Whitmire, N.; Dulai, P.S.; Vande Casteele, N.; Singh, S.; Boland, B.S.; Collins, A.; Sandborn, W.J.; Panaccione, R.; et al. Efficacy and safety of simultaneous treatment with two biologic medications in refractory Crohn’s disease. Aliment. Pharmacol. Ther. 2020, 51, 1031–1038. [Google Scholar] [CrossRef] [PubMed]
- Adegbola, S.O.; Dibley, L.; Sahnan, K.; Wade, T.; Verjee, A.; Sawyer, R.; Mannick, S.; McCluskey, D.; Yassin, N.; Phillips, R.K.S.; et al. Burden of disease and adaptation to life in patients with Crohn’s perianal fistula: A qualitative exploration. Health Qual Life Outcomes 2020, 18, 370. [Google Scholar] [CrossRef] [PubMed]
- Mahadev, S.; Young, J.M.; Selby, W.; Solomon, M.J. Self-reported depressive symptoms and suicidal feelings in perianal Crohn’s disease. Color. Dis. 2012, 14, 331–335. [Google Scholar] [CrossRef] [PubMed]
- Boudiaf, R.; Bouchard, D.; Rivière, P.; Brochard, C.; Laharie, D.; Abramowitz, L.; Pigot, F.; GREP members (Groupe de Recherche en Proctologie). Assessment of sexual dysfunction in patients with perianal Crohn’s disease. Color. Dis. 2021, 23, 114–122. [Google Scholar] [CrossRef]
| Therapy | Evidence to Date in pCD |
|---|---|
| Anti-TNF | Present et al. showed 50% reduction in fistula drainage in 68% of patients in the Infliximab 5 mg/kg induction dose cohort compared to 26% of patients treated with the placebo (p = 0.002) [19]. The ACCENT II trial then went on to show that maintenance IFX for a period of 54 weeks was superior to the placebo in patients who responded to induction therapy [20]. At week 54, 36% of patients in the IFX maintenance group had a complete absence of draining fistulas compared with 19% in the placebo group (p = 0.009) [20]. Furthermore, multiple retrospective studies have assessed the benefit of Infliximab including higher serum levels (ranging from >7.2 to >20) [21,22,23] corresponding to improved clinical and radiological remission outcomes [21,22,23,24,25,26,27]. In the subgroup analysis of the CHARM study, fistula healing was seen in approximately 60% of patients after 2 years of Adalimumab therapy [28]. In the CHOICE trial, 39% of patients who had complete fistula healing to Adalimumab therapy were primary or secondary non-responders to Infliximab [29]. However, the ACCESS study suggested that fistula healing rates in Adalimumab-treated patients were much higher for Infliximab naïve patients than Infliximab-experienced (60% versus 28%, respectively; p < 0.01) [30]. Similar to studies of Infliximab in pCD, higher Adalimumab serum levels correlated with improved fistula outcomes [26,27]. Furthermore, local intra-fistula Anti-TNF injections have also been trialled in small pilot studies with mixed results and a lack of long-term data [31]. |
| Ustekinumab | A subgroup analysis of the pivotal studies of Ustekinumab in CD (UNITI-1, UNITI-2 and CERTIFI) showed complete fistula resolution in 25% of all pooled Ustekinumab patients at week 8 compared to only 14% of the pooled placebo group patients with active fistula (p = 0.073) [32]. In addition, a post-hoc pooled of the STARDUST and SEAVUE studies showed an overall 50% of patients receiving Ustekinumab had clinical fistula healing at the end of the study with no impact of trough levels or dosing intervals seen in the small numbers of patients studied (17 and 12, respectively) [33]. A Dutch nationwide prospective observational cohort study of 28 patients showed 36% complete clinical resolution after 24 weeks of treatment in patients who had prior anti-TNF exposure [34]. The BioLAP multicenter study from the GETAID group showed clinical success at 6 months (as assessed by the physician’s judgment without additional medical or surgical treatment) occurred in 38.5% (57/148) of patients. In this study, 33% of patients (29/88) with setons at Ustekinumab initiation had successful removal [35]. Finally, a recent retrospective analysis showed, in patients who had received at least 16 weeks of Ustekinumab therapy, clinical remission and response rates were 40.7% and 63.0%, respectively. The study also went on to show radiological healing observed in 44.8% [35]. An Ustekinumab trough concentration over 2.11 μg/mL was correlated with a higher likelihood of perianal fistula clinical remission [36]. |
| Vedolizumab | The GETAID BioLAP study also assessed 102 patients with active perianal disease treated with Vedolizumab, in whom success was reached in 23 patients (23%). Among patients with setons at initiation, 9/61 (15%) had a successful removal [37]. The ENTERPRISE study was a randomized trial evaluating the effectiveness of two different 22-week Vedolizumab treatment regimens in pCD with 78.6% of the patients having had previous Anti-TNF exposure [38]. Unfortunately, the study was ceased prematurely due to recruitment challenges resulting in low patient counts and as a result, all analyses were descriptive. In the standard dosing group, 9/14 patients (64%) showed ≥50% reduction from the baseline in the number of draining perianal fistulae at week 30 [38]. In contrast, only 6/14 patients (43%) in the group that received an additional week 10 dose achieved ≥50% reduction in drainage [38]. |
| Small molecules | Filgotinib, a selective JAK1 inhibitor, has shown good efficacy in pCD in the Phase II DIVERGENCE 2 study. The proportion of patients who achieved a combined fistula response at week 24 was numerically higher in the FIL 200 mg group (47.1%; 90% confidence interval [CI]: 26.0–68.9) than in the PBO group (25.0%; 90% CI: 7.2–52.7) [39]. Upadacitinib (UPA), another selective JAK1 inhibitor, has shown some promise in its pivotal studies. The subgroup analysis of U-EXCEL, U-EXCEED and U-ENDURE studies showed that 124 patients had perianal fistulas. Of these, the proportion of patients who achieved the complete resolution of draining and ≥50% reduction in draining was higher with UPA vs placebo at week 12 (47.7% vs. 9.1%; p = 0.002 and 50.0% vs. 13.6%; p = 0.004). However, the response was not statistically significant at week 52 [40]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anandabaskaran, S.; Hanna, L.; Iqbal, N.; Constable, L.; Tozer, P.; Hart, A. Where Are We and Where to Next?—The Future of Perianal Crohn’s Disease Management. J. Clin. Med. 2023, 12, 6379. https://doi.org/10.3390/jcm12196379
Anandabaskaran S, Hanna L, Iqbal N, Constable L, Tozer P, Hart A. Where Are We and Where to Next?—The Future of Perianal Crohn’s Disease Management. Journal of Clinical Medicine. 2023; 12(19):6379. https://doi.org/10.3390/jcm12196379
Chicago/Turabian StyleAnandabaskaran, Sulak, Luke Hanna, Nusrat Iqbal, Laura Constable, Phil Tozer, and Ailsa Hart. 2023. "Where Are We and Where to Next?—The Future of Perianal Crohn’s Disease Management" Journal of Clinical Medicine 12, no. 19: 6379. https://doi.org/10.3390/jcm12196379
APA StyleAnandabaskaran, S., Hanna, L., Iqbal, N., Constable, L., Tozer, P., & Hart, A. (2023). Where Are We and Where to Next?—The Future of Perianal Crohn’s Disease Management. Journal of Clinical Medicine, 12(19), 6379. https://doi.org/10.3390/jcm12196379







