Dynamic Hyperinflation While Exercising—A Potential Predictor of Pulmonary Deterioration in Cystic Fibrosis
Abstract
:1. Introduction
2. Methods
2.1. Patients
2.2. Pulmonary Function Tests
2.3. Parameters Evaluated
2.4. Statistics
3. Results
3.1. Baseline Characteristics of the Study Groups
3.1.1. Clinical Parameters—DH vs. Non-DH
3.1.2. Cardiopulmonary Measures—DH vs. Non-DH
3.2. Prognostic Values of CPET Parameters
3.2.1. DH as a Prognostic Factor
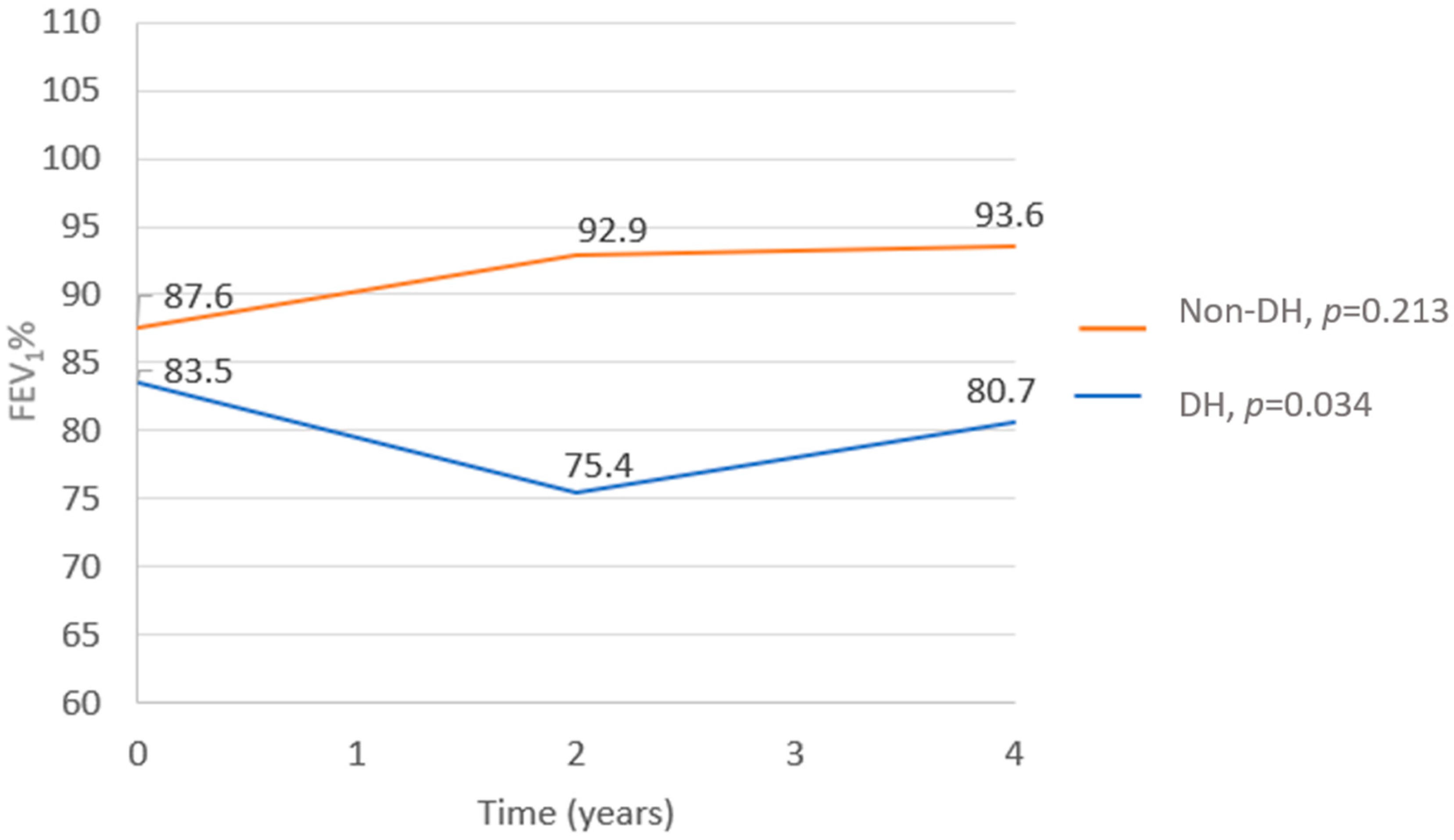
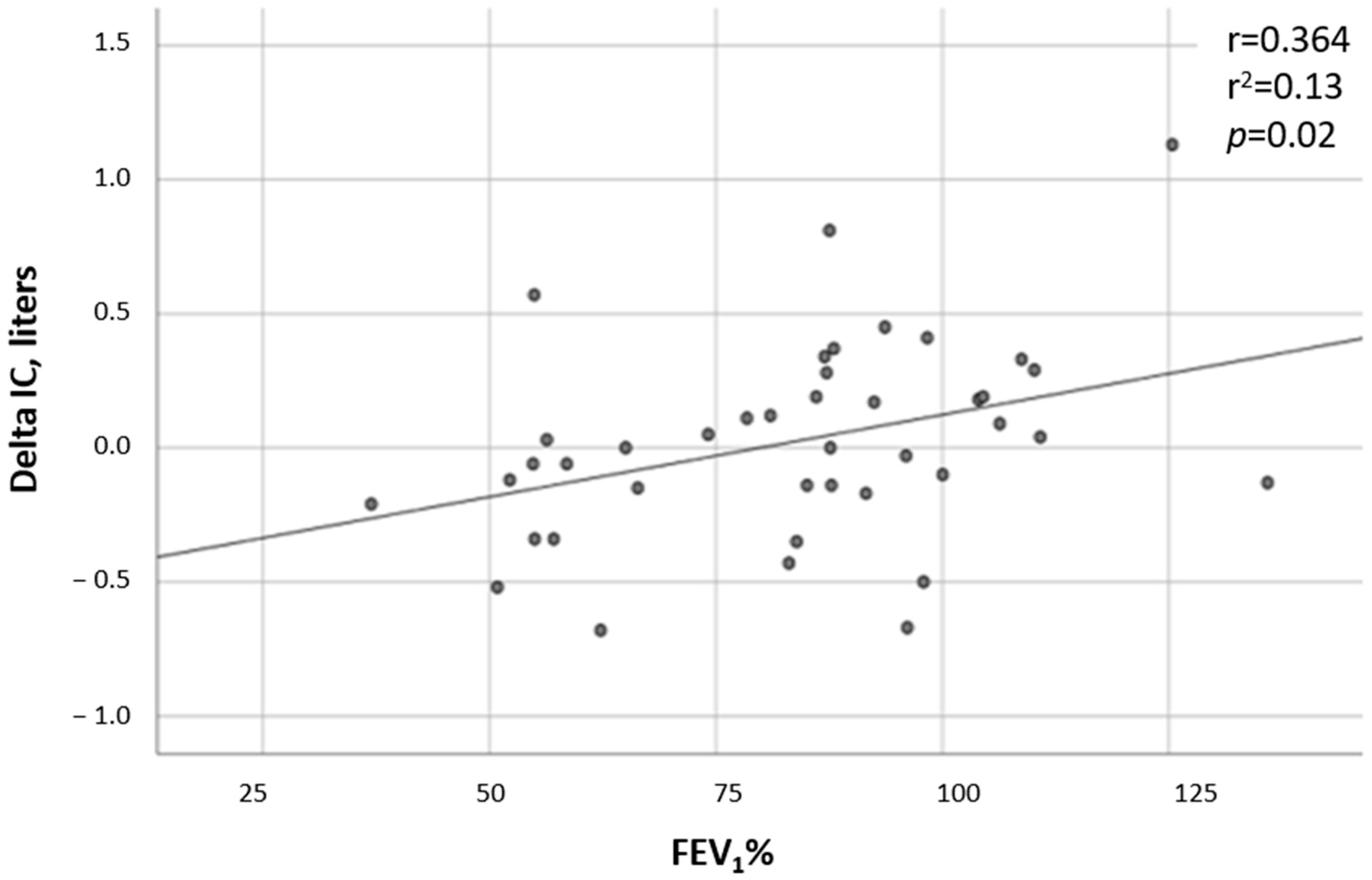
- FEV1% decline—Data regarding the FEV1% at the time of the CPET and in the following 2 and 4 years after the test were available in all but one test, which was missing 4 years after the CPET. While the FEV1% did not change in the non-DH group (p = 0.213), a significant deterioration was observed in the DH group (p = 0.0034). In comparing the two groups, the FEV1% trend from the time of the CPET to 4 years after the test showed a substantial decline in the DH group compared to the non-DH group (p = 0.009, Figure 1). A correlation was found between the severity of DH and the FEV1%, with patients having lower ∆ICs also having lower FEV1% values (r = 0.36 and p = 0.019; Figure 2).
- DH and LCI—LCI measurements were available for 26 tests. Significantly higher LCI values were found in the DH group, with a mean (SD) value of 17.45 (4.41) in the DH group versus 10.6 (4.40) in the non-DH group (p = 0.024, Figure 3). A negative correlation was found between the ∆IC and the LCI (r = −0.55 and p = 0.004; Figure 4).
- DH and IV antibiotic courses—IV antibiotic courses due to pulmonary exacerbations during the four years following the CPET were documented in 13/25 (52%) in the non-DH group and in 12/16 (75%) in the DH group (p = 0.006). The total number of IV antibiotic courses was higher in the DH group—totaling 52 courses versus 35, respectively, with a p = 0.046. Furthermore, a correlation was found between the degree of DH and the frequency of pulmonary exacerbations (r = −0.43 and p = 0.005; Figure 5).
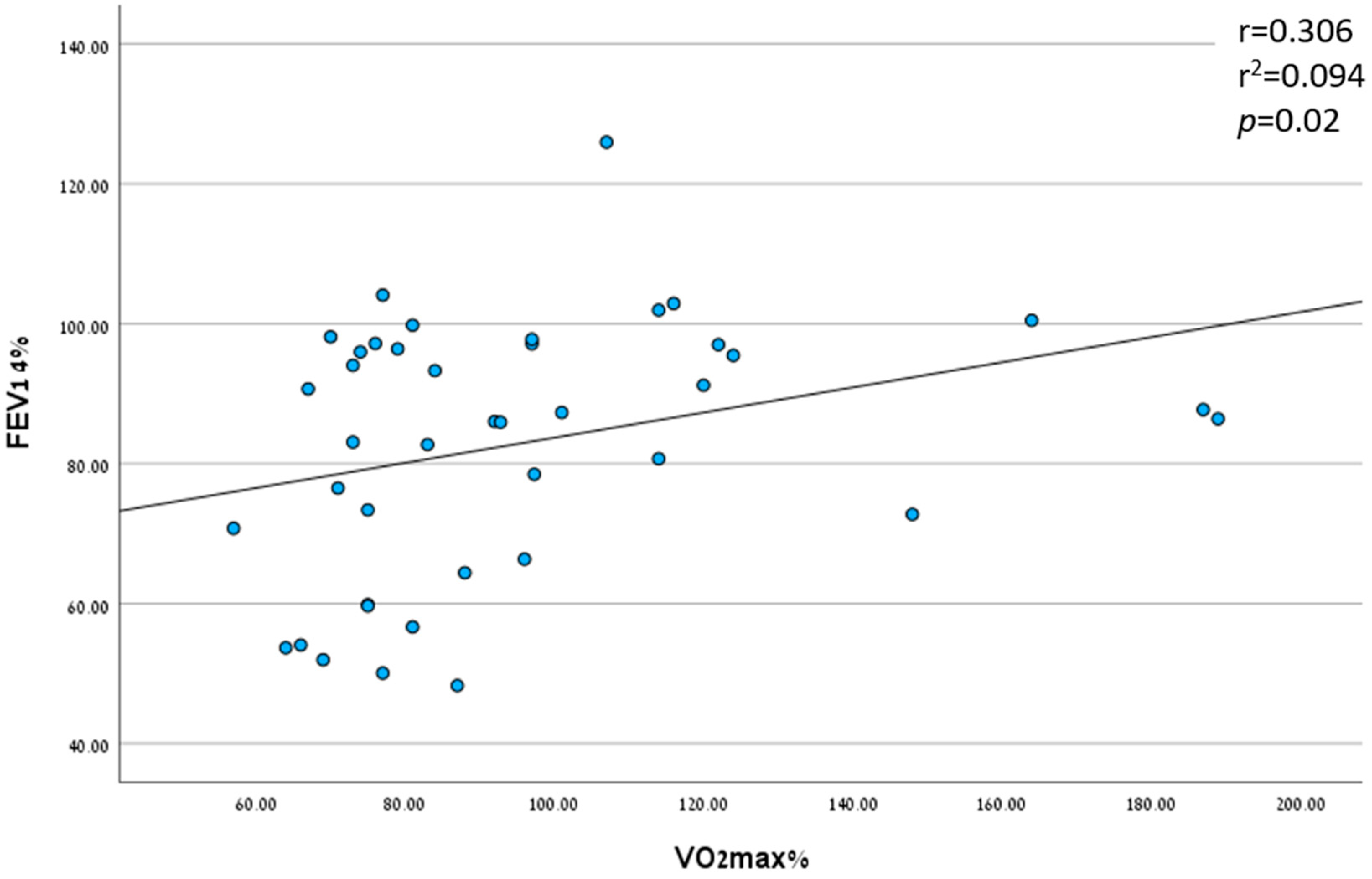
3.2.2. Peak VO2 as a Prognostic Factor
3.2.3. End Tidal CO2 (EtCO2) and Tidal Volume (TV)/IC Ratio as Prognostic Factors
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Szczesniak, R.; Heltshe, S.L.; Stanojevic, S.; Mayer-Hamblett, N. Use of FEV1 in cystic fibrosis epidemiologic studies and clinical trials: A statistical perspective for the clinical researcher. J. Cyst. Fibros. 2017, 16, 318–326. [Google Scholar] [CrossRef] [PubMed]
- Elborn, J.S. Cystic fibrosis. Lancet 2016, 388, 2519–2531. [Google Scholar] [CrossRef]
- Urquhart, D.S.; Saynor, Z.L. Exercise testing in cystic fibrosis: Who and why? Paediatr. Respir. Rev. 2018, 27, 28–32. [Google Scholar] [CrossRef]
- Hebestreit, H.; Hulzebos, E.H.; Schneiderman, J.E.; Karila, C.; Boas, S.R.; Kriemler, S.; Dwyer, T.; Sahlberg, M.; Urquhart, D.S.; Lands, L.C.; et al. Cardiopulmonary exercise testing provides additional prognostic information in cystic fibrosis. Am. J. Respir. Crit. Care Med. 2019, 199, 987–995. [Google Scholar] [CrossRef]
- Nixon, P.A.; Orenstein, D.M.; Kelsey, S.F.; Doershuk, C.F. The prognostic value of exercise testing in patients with cystic fibrosis. N. Engl. J. Med. 1992, 327, 1785–1788. [Google Scholar] [CrossRef]
- Moorcroft, A.J.; Dodd, M.E.; Webb, A.K. Exercise testing and prognosis in adult cystic fibrosis. Thorax 1997, 52, 291–293. [Google Scholar] [CrossRef] [PubMed]
- Vendrusculo, F.M.; Heinzmann-Filho, J.P.; da Silva, J.S.; Perez Ruiz, M.; Donadio, M.V.F. Peak oxygen uptake and mortality in cystic fibrosis: Systematic review and meta-analysis. Respir. Care 2019, 64, 91–98. [Google Scholar] [CrossRef]
- Kominami, K.; Noda, K.; Minagawa, N.; Yonezawa, K.; Akino, M. The concept of detection of dynamic lung hyperinflation using cardiopulmonary exercise testing. Medicine 2023, 102, E33356. [Google Scholar] [CrossRef]
- Karapanagiotis, S.; Gambazza, S.; Brivio, A.; D’Abrosca, F.; Colombo, C. Ventilatory limitation and dynamic hyperinflation during exercise testing in Cystic Fibrosis. Pediatr. Pulmonol. 2017, 52, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Stevens, D.; Stephenson, A.; Faughnan, M.E.; Leek, E.; Tullis, E. Prognostic relevance of dynamic hyperinflation during cardiopulmonary exercise testing in adult patients with cystic fibrosis. J. Cyst. Fibros. 2013, 12, 655–661. [Google Scholar] [CrossRef] [PubMed]
- Reuveny, R.; Vilozni, D.; Dagan, A.; Ashkenazi, M.; Velner, A.; Segel, M.J. The role of inspiratory capacity and tidal flow in diagnosing exercise ventilatory limitation in Cystic Fibrosis. Respir. Med. 2022, 192, 106713. [Google Scholar] [CrossRef] [PubMed]
- Graham, B.L.; Steenbruggen, I.; Miller, M.R.; Barjaktarevic, I.Z.; Cooper, B.G.; Hall, G.L.; Hallstrand, T.S.; Kaminsky, D.A.; McCarthy, K.; McCormack, M.C.; et al. Standardization of spirometry 2019 update an official American Thoracic Society and European Respiratory Society technical statement. Am. J. Respir. Crit. Care Med. 2019, 200, E70–E88. [Google Scholar] [CrossRef]
- O’donnell, D.E.; Revill, S.M.; Webb, K.A. Dynamic Hyperinflation and Exercise Intolerance in Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2001, 164, 770–777. [Google Scholar] [CrossRef] [PubMed]
- Hebestreit, H.; Arets, H.G.; Aurora, P.; Boas, S.; Cerny, F.; Hulzebos, E.H.; Karila, C.; Lands, L.C.; Lowman, J.D.; Swisher, A.; et al. Statement on exercise testing in cystic fibrosis. Respiration 2015, 90, 332–351. [Google Scholar] [CrossRef] [PubMed]
- Rowland, T.W. Developmental Exercise Physiology; Human Kinetics: Champaign, IL, USA, 1996. [Google Scholar]
- ATS/ACCP Statement on cardiopulmonary exercise testing. Am. J. Respir. Crit. Care Med. 2003, 167, 211–277. [CrossRef]
- Vilozni, D.; Dagan, A.; Lavie, M.; Sarouk, I.; Bar-Aluma, B.E.; Ashkenazi, M.; Mendelovich, S.L.; Betzalel, Y.; Efrati, O. The value of measuring inspiratory capacity in subjects with cystic fibrosis. Respir. Care 2018, 63, 981–987. [Google Scholar] [CrossRef]
- Stevens, D. Static hyperinflation is associated with ventilatory limitation and exercise tolerance in adult cystic fibrosis. Clin. Respir. J. 2018, 12, 1949–1957. [Google Scholar] [CrossRef]
- Di Paolo, M.; Teopompi, E.; Savi, D.; Crisafulli, E.; Longo, C.; Tzani, P.; Longo, F.; Ielpo, A.; Pisi, G.; Cimino, G.; et al. Reduced exercise ventilatory efficiency in adults with cystic fibrosis and normal to moderately impaired lung function. J. Appl. Physiol. 2019, 127, 501–512. [Google Scholar] [CrossRef] [PubMed]
- Savi, D.; di Paolo, M.; Simmonds, N.J.; Pascucci, C.; Quattrucci, S.; Palange, P. Is daily physical activity affected by dynamic hyperinflation in adults with cystic fibrosis? BMC Pulm. Med. 2018, 18, 60. [Google Scholar] [CrossRef] [PubMed]
- Chelabi, R.; Soumagne, T.; Guillien, A.; Puyraveau, M.; Degano, B. In cystic fibrosis, lung clearance index is sensitive to detecting abnormalities appearing at exercise in children with normal spirometry. Respir. Physiol. Neurobiol. 2018, 247, 9–11. [Google Scholar] [CrossRef]
- Varga, J. Mechanisms to dyspnoea and dynamic hyperinflation related exercise intolerance in COPD (Review). Acta Physiol. Hung. 2015, 102, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Berton, D.C.; Reis, M.; Siqueira, A.C.B.; Barroco, A.C.; Takara, L.S.; Bravo, D.M.; Andreoni, S.; Neder, J.A. Effects of tiotropium and formoterol on dynamic hyperinflation and exercise endurance in COPD. Respir. Med. 2010, 104, 1288–1296. [Google Scholar] [CrossRef] [PubMed]
- O’donnell, D.E.; Flüge, T.; Gerken, F.; Hamilton, A.; Webb, K.; Aguilaniu, B.; Make, B.; Magnussen, H. Effects of tiotropium on lung hyperinflation, dyspnoea and exercise tolerance in COPD. Eur. Respir. J. 2004, 23, 832–840. [Google Scholar] [CrossRef] [PubMed]
- Levine, H.; Cohen-Cymberknoh, M.; Klein, N.; Hoshen, M.; Mussaffi, H.; Stafler, P.; Breuer, O.; Kerem, E.; Blau, H. Reversible airway obstruction in cystic fibrosis: Common, but not associated with characteristics of asthma. J. Cyst. Fibros. 2016, 15, 652–659. [Google Scholar] [CrossRef] [PubMed]



| DH, n = 16 | Non-DH, n = 25 | p | |
|---|---|---|---|
| Age—mean (SD) * | 25.9 (14.8) | 23.9 (10.2) | 0.520 |
| Female, n (%) # | 11 (69%) | 8 (32%) | 0.030 |
| BMI * | 21 (3.76) | 21.8 (3.51) | 0.490 |
| Genotype # Both allele minimal function At least one allele residual function | 10 (62.5%) 6 (37.5%) | 15 (60%) 10 (40%) | 0.873 |
| Pancreatic insufficiency, n (%) # | 11 (69%) | 17 (68%) | 0.618 |
| CF-related diabetes $ | 3 (18%) | 2 (8%) | 0.362 |
| CF-related liver disease $ | 1 (6%) | 6 (24%) | 0.215 |
| Chronic PSA # | 12 (75%) | 14 (56%) | 0.322 |
| CFTR modulators tx, n (%) # | 5 (31%) | 4 (16%) | 0.276 |
| FEV1%, median (SD) * | 83.5 (25) | 87.6 (19.2) | 0.174 |
| DH, n = 16 | Non-DH, n = 25 | p | |
|---|---|---|---|
| Breathing frequency % of predicted breaths per minute, mean (SD) * | 143.8 (34.6) | 121.4 (30.6) | 0.043 |
| Breathing reserve %, mean (SD) * | 25.8 (15.9) | 28.6 (14.3) | 0.580 |
| Peak VO2%, mean (SD) * | 97.3 (33.6) | 92.8 (31.1) | 0.668 |
| VO2% predicted at the anaerobic threshold, mean (SD) | 59.7 (22.4) | 49.2 (19.1) | 0.137 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shmueli, E.; Gendler, Y.; Stafler, P.; Levine, H.; Steuer, G.; Bar-On, O.; Blau, H.; Prais, D.; Mei-Zahav, M. Dynamic Hyperinflation While Exercising—A Potential Predictor of Pulmonary Deterioration in Cystic Fibrosis. J. Clin. Med. 2023, 12, 5834. https://doi.org/10.3390/jcm12185834
Shmueli E, Gendler Y, Stafler P, Levine H, Steuer G, Bar-On O, Blau H, Prais D, Mei-Zahav M. Dynamic Hyperinflation While Exercising—A Potential Predictor of Pulmonary Deterioration in Cystic Fibrosis. Journal of Clinical Medicine. 2023; 12(18):5834. https://doi.org/10.3390/jcm12185834
Chicago/Turabian StyleShmueli, Einat, Yulia Gendler, Patrick Stafler, Hagit Levine, Guy Steuer, Ophir Bar-On, Hannah Blau, Dario Prais, and Meir Mei-Zahav. 2023. "Dynamic Hyperinflation While Exercising—A Potential Predictor of Pulmonary Deterioration in Cystic Fibrosis" Journal of Clinical Medicine 12, no. 18: 5834. https://doi.org/10.3390/jcm12185834
APA StyleShmueli, E., Gendler, Y., Stafler, P., Levine, H., Steuer, G., Bar-On, O., Blau, H., Prais, D., & Mei-Zahav, M. (2023). Dynamic Hyperinflation While Exercising—A Potential Predictor of Pulmonary Deterioration in Cystic Fibrosis. Journal of Clinical Medicine, 12(18), 5834. https://doi.org/10.3390/jcm12185834








