Serum Inflammatory and Oxidative Stress Markers in Patients with Vitiligo
Abstract
1. Introduction
2. Material and Methods
2.1. Patients
2.2. Clinical Assessment
2.3. Blood Sampling
2.4. Assessment of Oxidative Stress Markers
2.5. Assessment of Inflammatory and Autoimmune Markers
2.6. Statistical Analysis
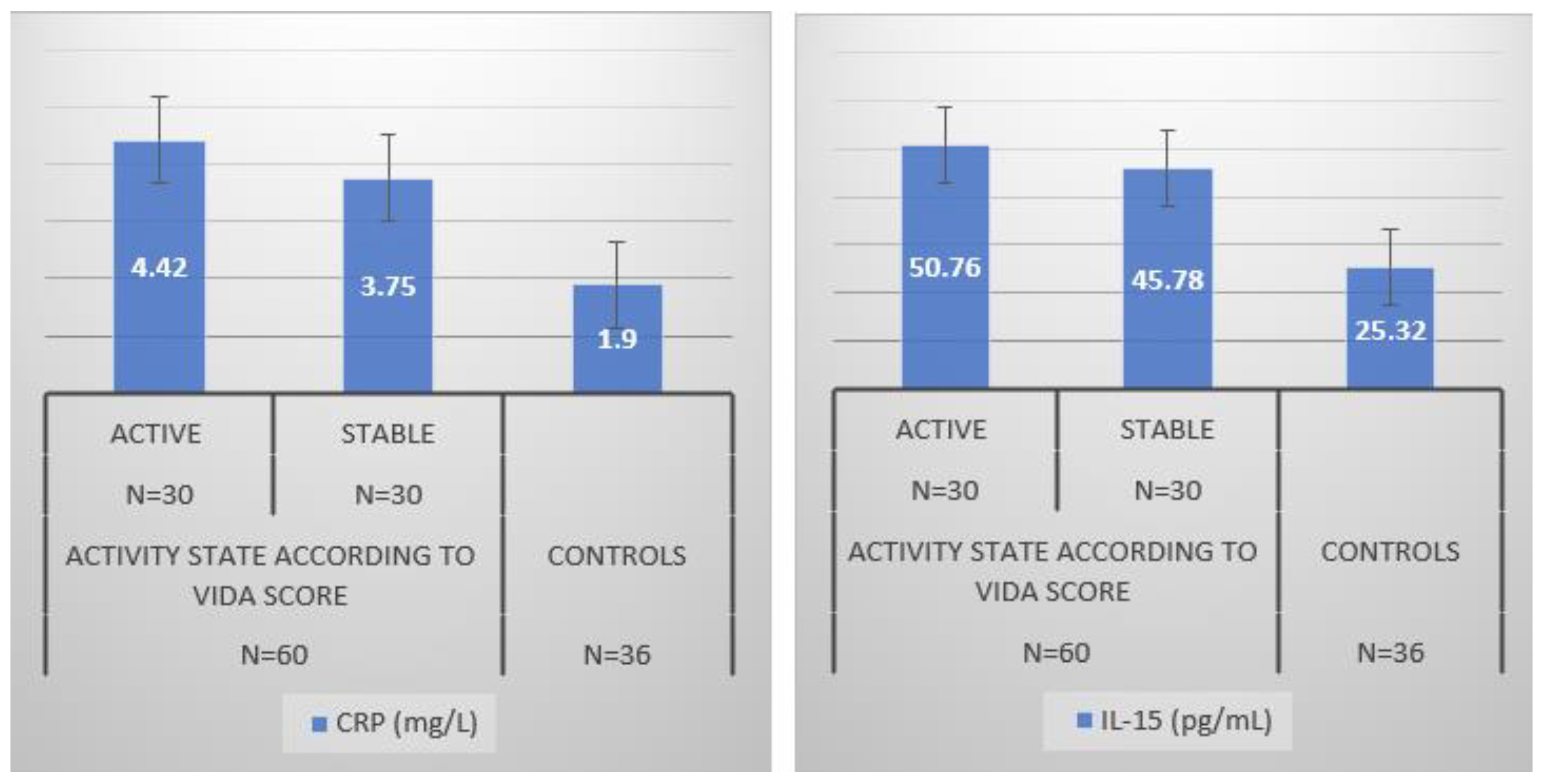
3. Results
Oxidative Stress Markers
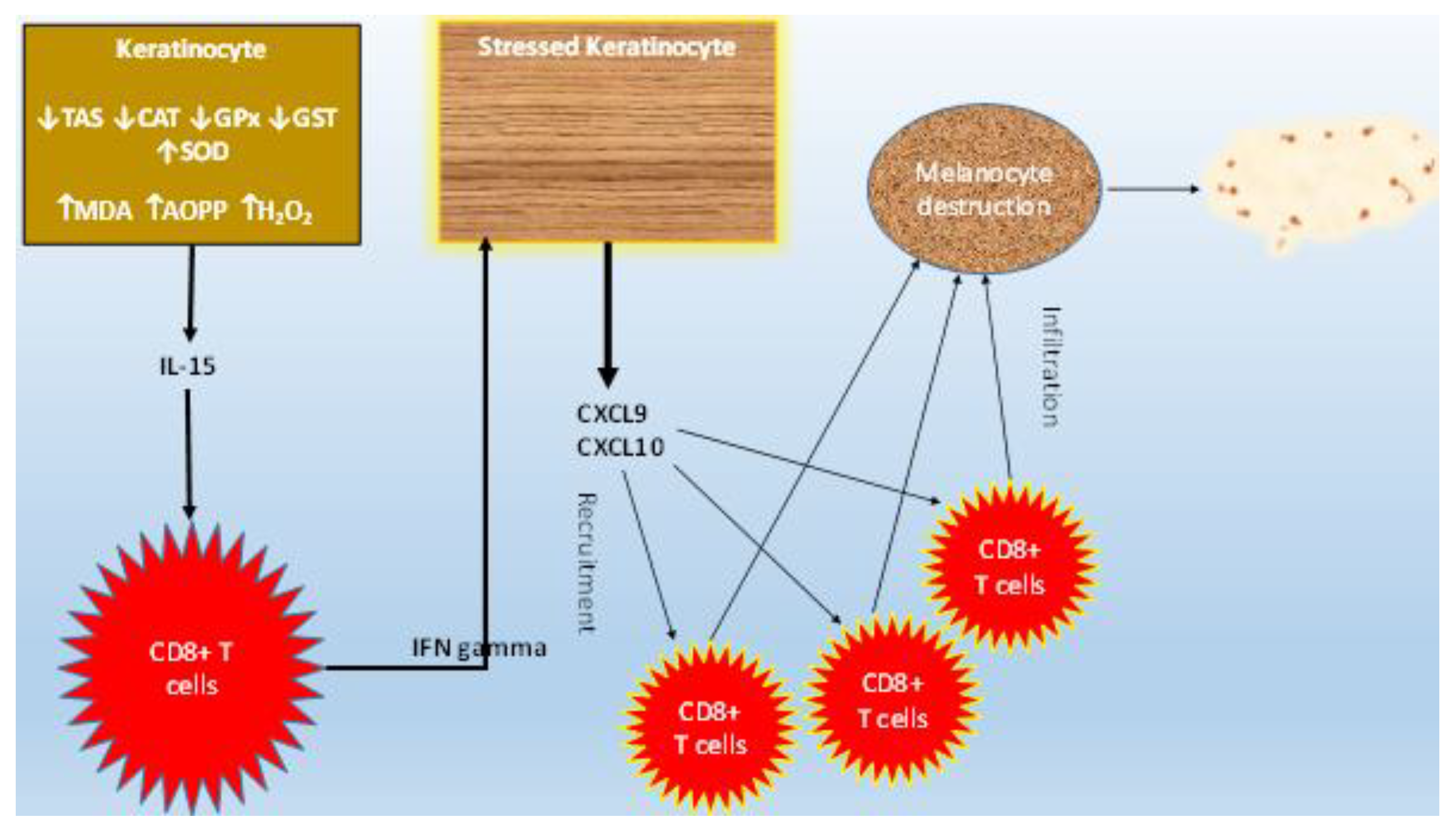
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bertolani, M.; Rodighiero, E.; de Felici Del Giudice, M.B.; Lotti, T.; Feliciani, C.; Satolli, F. Vitiligo: What’s old, what’s new. Dermatol. Rep. 2021, 13, 9142. [Google Scholar] [CrossRef] [PubMed]
- Krüger, C.; Schallreuter, K.U. A review of the worldwide prevalence of vitiligo in children/adolescents and adults. Int. J. Dermatol. 2012, 51, 1206–1212. [Google Scholar] [CrossRef]
- Ngolo, M.; Yassa, P.; Ndayazi, B. Vitiligo in the City of Bukavu (Democratic Republic of Congo). West Afr. J. Med. 2022, 39, 66–69. [Google Scholar] [CrossRef] [PubMed]
- Kiprono, S.; Chaula, B. Clinical epidemiological profile of vitiligo. East Afr. Med. J. 2012, 89, 278–281. [Google Scholar] [PubMed]
- He, Y.; Li, S.; Zhang, W.; Dai, W.; Cui, T.; Wang, G.; Gao, T.; Li, C. Dysregulated autophagy increased melanocyte sensitivity to H2O2-induced oxidative stress in vitiligo. Sci. Rep. 2017, 7, 42394. [Google Scholar] [CrossRef]
- Hlača, N.; Žagar, T.; Kaštelan, M.; Brajac, I.; Prpić-Massari, L. Current Concepts of Vitiligo Immunopathogenesis. Biomedicines 2022, 10, 1639. [Google Scholar] [CrossRef]
- He, S.; Xu, J.; Wu, J. The Promising Role of Chemokines in Vitiligo: From Oxidative Stress to the Autoimmune Response. Oxidative Med. Cell. Longev. 2022, 2022, 8796735. [Google Scholar] [CrossRef]
- Denat, L.; Kadekaro, A.L.; Marrot, L.; Leachman, S.A.; Abdel-Malek, Z.A. Melanocytes as instigators and victims of oxidative stress. J. Investigig. Dermatol. 2014, 134, 1512–1518. [Google Scholar] [CrossRef]
- Richmond, J.M.; Strassner, J.P.; Essien, K.I.; Harris, J.E. T-cell positioning by chemokines in autoimmune skin diseases. Immunol. Rev. 2019, 289, 186–204. [Google Scholar] [CrossRef]
- Li, S.; Zhu, G.; Yang, Y.; Jian, Z.; Guo, S.; Dai, W.; Shi, Q.; Ge, R.; Ma, J.; Liu, L.; et al. Oxidative stress drives CD8+ T-cell skin trafficking in patients with vitiligo through CXCL16 upregulation by activating the unfolded protein response in keratinocytes. J. Allergy Clin. Immunol. 2017, 140, 177–189.e9. [Google Scholar] [CrossRef]
- Gupta, S.; D’souza, P.; Dhali, T.K.; Arora, S. Serum Homocysteine and Total Antioxidant Status in Vitiligo: A Case Control Study in Indian Population. Indian J. Dermatol. 2016, 61, 131–136. [Google Scholar]
- Koca, R.; Armutcu, F.; Altinyazar, H.C.; Gürel, A. Oxidant-antioxidant enzymes and lipid peroxidation in generalized vitiligo. Clin. Exp. Dermatol. 2004, 29, 406–409. [Google Scholar] [CrossRef]
- Khan, R.; Satyam, A.; Gupta, S.; Sharma, V.K.; Sharma, A. Circulatory levels of antioxidants and lipid peroxidation in Indian patients with generalized and localized vitiligo. Arch. Dermatol. Res. 2009, 301, 731–737. [Google Scholar] [CrossRef]
- Shi, M.H.; Wu, Y.; Li, L.; Cai, Y.F.; Liu, M.; Gao, X.H.; Chen, H.D. Meta-analysis of the association between vitiligo and the level of superoxide dismutase or malondialdehyde. Clin. Exp. Dermatol. 2017, 42, 21–29. [Google Scholar] [CrossRef]
- Custurone, P.; Di Bartolomeo, L.; Irrera, N.; Borgia, F.; Altavilla, D.; Bitto, A.; Pallio, G.; Squadrito, F.; Vaccaro, M. Role of Cytokines in Vitiligo: Pathogenesis and Possible Targets for Old and New Treatments. Int. J. Mol. Sci. 2021, 22, 11429. [Google Scholar] [CrossRef]
- Chen, C.Y.; Wang, W.M.; Chung, C.H.; Tsao, C.H.; Chien, W.C.; Hung, C.T. Increased risk of psychiatric disorders in adult patients with vitiligo: A nationwide, population-based cohort study in Taiwan. J. Dermatol. 2020, 47, 470–475. [Google Scholar] [CrossRef]
- Miller, N.J.; Rice-Evans, C.; Davies, M.J.; Gopinathan, V.; Milner, A. A novel method for measuring antioxidant capacity and Its application to monitoring the antioxidant status in premature neonates. Clin. Sci. 1993, 84, 407–412. [Google Scholar] [CrossRef]
- Arthur, J.; Boyne, R. Superoxide dismutase and glutathione peroxidase activities in neutrophils from selenium deficient and copper deficient cattle. Life Sci. 1985, 36, 1569–1575. [Google Scholar] [CrossRef] [PubMed]
- Paglia, D.E.; Valentine, W.N. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J. Lab. Clin. Med. 1967, 70, 158–169. [Google Scholar] [PubMed]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [PubMed]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, T.; Kawada, K.; Shimada, T.; Mori, M. Lipid peroxidation in maternal and cord blood and protective mechanism against activated-oxygen toxicity in the blood. Am. J. Obstet. Gynecol. 1979, 135, 372–376. [Google Scholar] [CrossRef] [PubMed]
- Witko-Sarsat, V.; Gausson, V.; Descamps-Latscha, B. Are advanced oxidation protein products potential uremic toxins? Kidney Int. 2003, 63, S11–S14. [Google Scholar] [CrossRef] [PubMed]
- O’hagan, R.; Kamat, S.; Wieder, S.; Perl, M.; Silverberg, N. Association between BMI and vitiligo distribution: An observational cohort study. Br. J. Dermatol. 2023, 188, ljac106.011. [Google Scholar] [CrossRef]
- Dragoni, F.; Conti, R.; Cazzaniga, S.; Colucci, R.; Pisaneschi, L.; Naldi, L.; Moretti, S. No Association between Vitiligo and Obesity: A Case-Control Study. Med. Princ. Pract. 2017, 26, 421–426. [Google Scholar] [CrossRef]
- Xia, J.; Melian, C.; Guo, W.; Usmani, H.; Clark, R.; Lozeau, D. Vitiligo and Metabolic Syndrome: Systematic Review and Meta-Analysis. JMIR Dermatol. 2022, 5, e34772. [Google Scholar] [CrossRef]
- Schallreuter, K.U.; Wood, J.M.; Berger, J. Low catalase levels in the epidermis of patients with vitiligo. J. Investig. Dermatol. 1991, 97, 1081–1085. [Google Scholar] [CrossRef]
- Zedan, H.; Abdel-Motaleb, A.A.; Kassem, N.M.; Hafeez, H.A.; Hussein, M.R. Low glutathione peroxidase activity levels in patients with vitiligo. J. Cutan. Med. Surg. 2015, 19, 144–148. [Google Scholar] [CrossRef]
- Kostyuk, V.A.; Potapovich, A.I.; Cesareo, E.; Brescia, S.; Guerra, L.; Valacchi, G.; Pecorelli, A.; Deeva, I.B.; Raskovic, D.; De Luca, C.; et al. Dysfunction of glutathione S-transferase leads to excess 4-hydroxy-2-nonenal and H2O2 and impaired cytokine pattern in cultured keratinocytes and blood of vitiligo patients. Antioxid. Redox Signal. 2010, 13, 607–620. [Google Scholar] [CrossRef]
- Jain, A.; Mal, J.; Mehndiratta, V.; Chander, R.; Patra, S.K. Study of oxidative stress in vitiligo. Indian J. Clin. Biochem. 2011, 26, 78–81. [Google Scholar] [CrossRef]
- Jiménez-Cervantes, C.; Martínez-Esparza, M.; Pérez, C.; Daum, N.; Solano, F.; García-Borrón, J.C. Inhibition of melanogenesis in response to oxidative stress: Transient downregulation of melanocyte differentiation markers and possible involvement of microphthalmia transcription factor. J. Cell Sci. 2001, 114, 2335–2344. [Google Scholar] [CrossRef]
- Karsli, N.; Akcali, C.; Ozgoztasi, O.; Kirtak, N.; Inaloz, S. Role of oxidative stress in the pathogenesis of vitiligo with special emphasis on the antioxidant action of narrowband ultraviolet B phototherapy. J. Int. Med. Res. 2014, 42, 799–805. [Google Scholar] [CrossRef] [PubMed]
- Passeron, T.; Ortonne, J.P. Activation of the unfolded protein response in vitiligo: The missing link? J. Investig. Dermatol. 2012, 132, 2502–2504. [Google Scholar] [CrossRef]
- Yasmin, T.M.; Aya, B.Y.; Amal, H.; Amira, K.A.; Ahmed, G.S. Serum interleukin-22 and C-reactive protein in patients with vitiligo: A case–control study on 35 Egyptian patients. Egypt J. Dermatol. Venerol. 2021, 4, 32–37. [Google Scholar]
- Tokura, Y.; Phadungsaksawasdi, P.; Kurihara, K.; Fujiyama, T.; Honda, T. Pathophysiology of Skin Resident Memory T Cells. Front. Immunol. 2021, 11, 618897. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Guo, W.; Chang, Y.; Chen, J.; Kang, P.; Yi, X.; Cui, T.; Guo, S.; Xiao, Q.; Jian, Z.; et al. Oxidative stress-induced IL-15 trans-presentation in keratinocytes contributes to CD8+ T cells activation via JAK-STAT pathway in vitiligo. Free. Radic. Biol. Med. 2019, 139, 80–91. [Google Scholar] [CrossRef] [PubMed]
- Richmond, J.M.; Strassner, J.P.; Zapata, L., Jr.; Garg, M.; Riding, R.L.; Refat, M.A.; Fan, X.; Azzolino, V.; Tovar-Garza, A.; Tsurushita, N.; et al. Antibody blockade of IL-15 signaling has the potential to durably reverse vitiligo. Sci. Transl. Med. 2018, 10, eaam7710. [Google Scholar] [CrossRef]
- Feng, Y.; Lu, Y. Advances in vitiligo: Update on therapeutic targets. Front. Immunol. 2022, 13, 986918. [Google Scholar] [CrossRef] [PubMed]
- Bertolotti, A.; Boniface, K.; Vergier, B.; Mossalayi, D.; Taieb, A.; Ezzedine, K.; Seneschal, J. Type I interferon signature in the initiation of the immune response in vitiligo. Pigment. Cell Melanoma Res. 2014, 27, 398–407. [Google Scholar] [CrossRef]
- Marchioro, H.Z.; Silva de Castro, C.C.; Fava, V.M.; Sakiyama, P.H.; Dellatorre, G.; Miot, H.A. Update on the pathogenesis of vitiligo. An. Bras. Dermatol. 2022, 97, 478–490. [Google Scholar] [CrossRef]
- Yang, L.; Yang, S.; Lei, J.; Hu, W.; Chen, R.; Lin, F.; Xu, A.E. Role of chemokines and the corresponding receptors in vitiligo: A pilot study. J. Dermatol. 2018, 45, 31–38. [Google Scholar] [CrossRef]
- Richmond, J.M.; Bangari, D.S.; Essien, K.I.; Currimbhoy, S.D.; Groom, J.R.; Pandya, A.G.; Youd, M.E.; Luster, A.D.; Harris, J.E. Keratinocyte-Derived Chemokines Orchestrate T-Cell Positioning in the Epidermis during Vitiligo and May Serve as Biomarkers of Disease. J. Investig. Dermatol. 2017, 137, 350–358. [Google Scholar] [CrossRef]
- Jacquemin, C.; Rambert, J.; Guillet, S.; Thiolat, D.; Boukhedouni, N.; Doutre, M.S.; Darrigade, A.S.; Ezzedine, K.; Blanco, P.; Taieb, A.; et al. Heat shock protein 70 potentiates interferon alpha production by plasmacytoid dendritic cells: Relevance for cutaneous lupus and vitiligo pathogenesis. Br. J. Dermatol. 2017, 177, 1367–1375. [Google Scholar] [CrossRef] [PubMed]
- van den Boorn, J.G.; Konijnenberg, D.; Dellemijn, T.A.; van der Veen, J.P.; Bos, J.D.; Melief, C.J.; Vyth-Dreese, F.A.; Luiten, R.M. Autoimmune destruction of skin melanocytes by perilesional T cells from vitiligo patients. J. Investig. Dermatol. 2009, 129, 2220–2232. [Google Scholar] [CrossRef] [PubMed]
- Rashighi, M.; Agarwal, P.; Richmond, J.M.; Harris, T.H.; Dresser, K.; Su, M.W.; Zhou, Y.; Deng, A.; Hunter, C.A.; Luster, A.D.; et al. CXCL10 is critical for the progression and maintenance of depigmentation in a mouse model of vitiligo. Sci. Transl. Med. 2014, 6, 223ra23. [Google Scholar] [CrossRef] [PubMed]
- Richmond, J.M.; Masterjohn, E.; Chu, R.; Tedstone, J.; Youd, M.E.; Harris, J.E. CXCR3 Depleting Antibodies Prevent and Reverse Vitiligo in Mice. J. Investig. Dermatol. 2017, 137, 982–985. [Google Scholar] [CrossRef] [PubMed]


| Units | Clinical Interpretation |
|---|---|
| 100% | Complete depigmentation |
| 90% | Pigment specks |
| 75% | Depigmented areas exceed pigmented areas |
| 50% | Pigmented and depigmented areas are equal |
| 25% | Pigmented areas exceed depigmented areas |
| 10% | Only depigmentation specks are present |
| Grade | Disease Activity | Stage |
|---|---|---|
| +4 | ≤6 weeks | Active |
| +3 | 6 weeks–3 months | Active |
| +2 | 3–6 months | Active |
| +1 | 6–12 months | Active |
| 0 | Stable for ≥one year | Stable |
| −1 | Stable with spontaneous repigmentation | Stable |
| Vitiligo Patients n = 60 | Controls n = 36 | |
|---|---|---|
| Age (mean ± SD, years) | 34.5 ± 10 | 34.1 ± 13 |
| Gender (female/male) | 33/27 | 20/16 |
| BMI (mean ± SD, Kg/m2) | 25.7 ± 4.5 | 26.0 ± 3.5 |
| Disease duration (Mean ± SD, years) | 5.6 ± 4.3 | - |
| Median [min–max] | 6 [1–12] | |
| VASI scores (%) | ||
| Mean ± SD | 9.40 ± 2.4 | |
| Median [min–max] | 5.5 [2–12.5] | |
| VIDA score | ||
| Mean ± SD | 1.71 ± 1.2 | |
| Median [min–max] | 3 [−1–4] | |
| VIDA scale, n (%) | ||
| +4 | 24 (40%) | |
| +3 | 2 (3.33%) | |
| +2 | 2 (3.33%) | |
| +1 | 2 (3.33%) | |
| 0 | 25 (41.66%) | |
| −1 | 5 (8.33%) |
| Clinical Stage | Controls n = 36 | P1 | P2 | P3 | ||
|---|---|---|---|---|---|---|
| Active (n = 30) | Stable (n = 30) | |||||
| VASI Score | NS | |||||
| Mean ± SD | 9.50 ± 2.5 | 8.80 ± 3.40 | ||||
| Median [Range] | 7.5 [3–12.2] | 7 [2–12.5] | ||||
| Antioxidant parameters | ||||||
| TAS (mmol/L) | 0.005 | 0.040 | 0.050 | |||
| Mean ± SD | 1.70 ± 0.45 | 1.97 ± 0.19 | 2.05 ± 0.22 | |||
| Median [range] | 1.56 [1.15–2.25] | 1.80 [1.70–2.35] | 1.90 [1.80–2.35] | |||
| SOD (U/mg) | 0.001 | 0.020 | 0.030 | |||
| Mean ± SD | 3.70 ± 0.75 | 3.57 ± 1.12 | 3.15 ± 1.02 | |||
| Median [range] | 4 [2.50–4.5] | 4.20 [2.40–4.70] | 3 [2.10–4.20] | |||
| CAT (U/mg) | 0.001 | 0.020 | 0.040 | |||
| Mean ± SD | 34.75 ± 3.5 | 36.33 ± 4.02 | 38.5 ± 3.1 | |||
| Median [range] | 35 [31–38.40] | 37 [32.25–40.4] | 38 [35–41.60] | |||
| GPx (U/mg) | 0.001 | 0.020 | 0.040 | |||
| Mean ± SD | 1.72 ± 0.85 | 1.89 ± 0.90 | 2.20 ± 0.44 | |||
| Median [range] | 1.75 [0.85–2.70] | 1.80 [0.95–2.80] | 2.08 [1.76–2.70] | |||
| GST (UI/mL) | 0.001 | 0.001 | 0.020 | |||
| Mean ± SD | 18.12 ± 4.1 | 18.94 ± 3.5 | 20.00 ± 1.23 | |||
| Median [range] | 18 [14–22] | 19 [15–23] | 19.5 [18–22] | |||
| Oxidant damage parameters | ||||||
| MDA (mmol/mL) | 0.000 | 0.000 | NS | |||
| Mean ± SD | 5.30 ± 1.00 | 4.90 ± 2.10 | 4.50 ± 1.20 | |||
| Median [range] | 4.50 [3.90–8.50] | 4.50 [3.10–8.50] | 4.10 [3.00–7.00] | |||
| AOPP (umol/L) | ||||||
| Mean ± SD | 250.15 ± 200.20 | 205.36 ± 200.10 | 100.45 ± 50.18 | 0.000 | 0.000 | NS |
| Median [range] | 215.30 [70.80–845.30] | 217.90 [65.65–850.40] | 95.55 [70.20–250.30] | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kassab, A.; Khalij, Y.; Ayed, Y.; Dar-Odeh, N.; Kokandi, A.A.; Denguezli, M.; Youssef, M. Serum Inflammatory and Oxidative Stress Markers in Patients with Vitiligo. J. Clin. Med. 2023, 12, 5861. https://doi.org/10.3390/jcm12185861
Kassab A, Khalij Y, Ayed Y, Dar-Odeh N, Kokandi AA, Denguezli M, Youssef M. Serum Inflammatory and Oxidative Stress Markers in Patients with Vitiligo. Journal of Clinical Medicine. 2023; 12(18):5861. https://doi.org/10.3390/jcm12185861
Chicago/Turabian StyleKassab, Asma, Yassine Khalij, Yosra Ayed, Najla Dar-Odeh, Amal A. Kokandi, Meriam Denguezli, and Monia Youssef. 2023. "Serum Inflammatory and Oxidative Stress Markers in Patients with Vitiligo" Journal of Clinical Medicine 12, no. 18: 5861. https://doi.org/10.3390/jcm12185861
APA StyleKassab, A., Khalij, Y., Ayed, Y., Dar-Odeh, N., Kokandi, A. A., Denguezli, M., & Youssef, M. (2023). Serum Inflammatory and Oxidative Stress Markers in Patients with Vitiligo. Journal of Clinical Medicine, 12(18), 5861. https://doi.org/10.3390/jcm12185861







