Similarities and Differences in the Management of Patients with Osteoporotic Vertebral Fractures and Those with Rebound-Associated Vertebral Fractures Following Discontinuation of Denosumab
Abstract
:1. Introduction
2. Methods
Literature Search
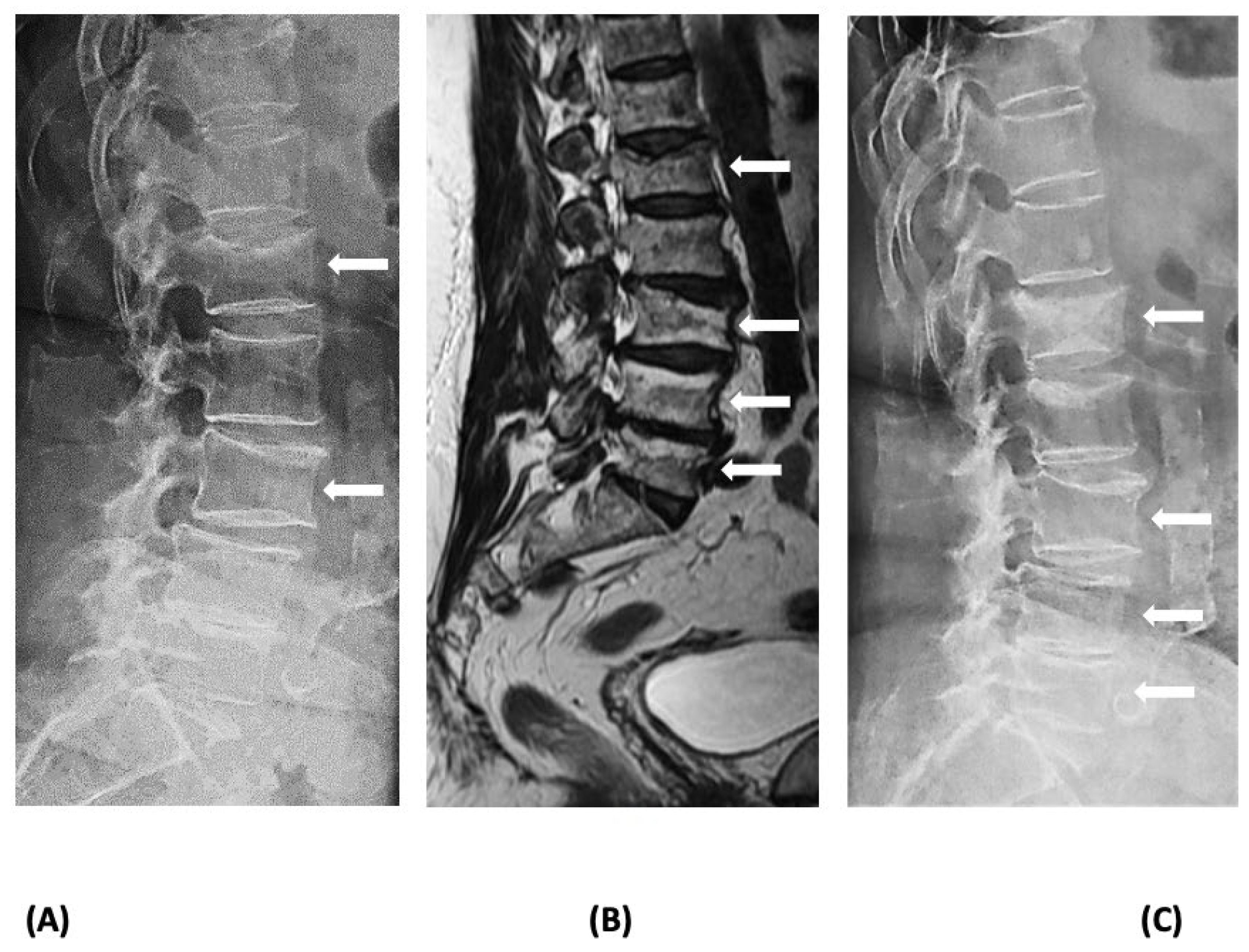
3. Diagnosis
4. Management
4.1. Pain Relief
4.1.1. Pharmacological Interventions
4.1.2. Non-Pharmacological Interventions
Bracing
Vertebroplasty/Kyphoplasty
Exercise
4.2. Antiosteoporotic Medication
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bekker, P.J.; Holloway, D.L.; Rasmussen, A.S.; Murphy, R.; Martin, S.W.; Leese, P.T.; Holmes, G.B.; Dunstan, C.R.; DePaoli, A.M. A single-dose placebo-controlled study of AMG 162, a fully human monoclonal antibody to RANKL, in postmenopausal women. J. Bone Miner. Res. 2004, 19, 1059–1066. [Google Scholar] [CrossRef]
- Bone, H.G.; Wagman, R.B.; Brandi, M.L.; Brown, J.P.; Chapurlat, R.; Cummings, S.R.; Czerwinski, E.; Fahrleitner-Pammer, A.; Kendler, D.L.; Lippuner, K.; et al. 10 years of denosumab treatment in postmenopausal women with osteoporosis: Results from the phase 3 randomised FREEDOM trial and open-label extension. Lancet Diabetes Endocrinol. 2017, 5, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Popp, A.W.; Zysset, P.K.; Lippuner, K. Rebound-associated vertebral fractures after discontinuation of denosumab-from clinic and biomechanics. Osteoporos. Int. 2016, 27, 1917–1921. [Google Scholar] [CrossRef]
- Anastasilakis, A.D.; Makras, P. Multiple clinical vertebral fractures following denosumab discontinuation. Osteoporos. Int. 2016, 27, 1929–1930. [Google Scholar] [CrossRef] [PubMed]
- Aubry-Rozier, B.; Gonzalez-Rodriguez, E.; Stoll, D.; Lamy, O. Severe spontaneous vertebral fractures after denosumab discontinuation: Three case reports. Osteoporos. Int. 2016, 27, 1923–1925. [Google Scholar] [CrossRef]
- Anastasilakis, A.D.; Polyzos, S.A.; Makras, P.; Aubry-Rozier, B.; Kaouri, S.; Lamy, O. Clinical Features of 24 Patients With Rebound-Associated Vertebral Fractures After Denosumab Discontinuation: Systematic Review and Additional Cases. J. Bone Miner. Res. 2017, 32, 1291–1296. [Google Scholar] [CrossRef]
- Bone, H.G.; Bolognese, M.A.; Yuen, C.K.; Kendler, D.L.; Miller, P.D.; Yang, Y.C.; Grazette, L.; San Martin, J.; Gallagher, J.C. Effects of denosumab treatment and discontinuation on bone mineral density and bone turnover markers in postmenopausal women with low bone mass. J. Clin. Endocrinol. Metab. 2011, 96, 972–980. [Google Scholar] [CrossRef]
- Cosman, F.; Huang, S.; McDermott, M.; Cummings, S.R. Multiple Vertebral Fractures After Denosumab Discontinuation: FREEDOM and FREEDOM Extension Trials Additional Post Hoc Analyses. J. Bone Miner. Res. 2022, 37, 2112–2120. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, S.; Langdahl, B. Mechanisms underlying the long-term and withdrawal effects of denosumab therapy on Bone. Nat. Rev. Rheumatol. 2023, 19, 307–317. [Google Scholar] [CrossRef]
- Anastasilakis, A.D.; Yavropoulou, M.P.; Makras, P.; Sakellariou, G.T.; Papadopoulou, F.; Gerou, S.; Papapoulos, S.E. Increased osteoclastogenesis in patients with vertebral fractures following discontinuation of denosumab treatment. Eur. J. Endocrinol. 2017, 176, 677–683. [Google Scholar] [CrossRef]
- Jähn-Rickert, K.; Wölfel, E.M.; Jobke, B.; Riedel, C.; Hellmich, M.; Werner, M.; McDonald, M.M.; Busse, B. Elevated Bone Hardness Under Denosumab Treatment, With Persisting Lower Osteocyte Viability During Discontinuation. Front. Endocrinol. 2020, 11, 250. [Google Scholar] [CrossRef]
- Palacios, S.; Neyro, J.L.; Fernández de Cabo, S.; Chaves, J.; Rejas, J. Impact of osteoporosis and bone fracture on health-related quality of life in postmenopausal women. Climacteric 2014, 17, 60–70. [Google Scholar] [CrossRef]
- Kendler, D.L.; Bauer, D.C.; Davison, K.S.; Dian, L.; Hanley, D.A.; Harris, S.T.; McClung, M.R.; Miller, P.D.; Schousboe, J.T.; Yuen, C.K.; et al. Vertebral Fractures: Clinical Importance and Management. Am. J. Med. 2016, 129, 221.e1–221.e10. [Google Scholar] [CrossRef]
- Fink, H.A.; Litwack-Harrison, S.; Ensrud, K.E.; Shen, J.; Schousboe, J.T.; Cawthon, P.M.; Cauley, J.A.; Lane, N.E.; Taylor, B.C.; Barrett-Connor, E.; et al. Association of Incident, Clinically Undiagnosed Radiographic Vertebral Fractures With Follow-Up Back Pain Symptoms in Older Men: The Osteoporotic Fractures in Men (MrOS) Study. J. Bone Miner. Res. 2017, 32, 2263–2268. [Google Scholar] [CrossRef]
- Fink, H.A.; Milavetz, D.L.; Palermo, L.; Nevitt, M.C.; Cauley, J.A.; Genant, H.K.; Black, D.M.; Ensrud, K.E. What proportion of incident radiographic vertebral deformities is clinically diagnosed and vice versa? J. Bone Miner. Res. 2005, 20, 1216–1222. [Google Scholar] [CrossRef] [PubMed]
- Nevitt, M.C.; Ettinger, B.; Black, D.M.; Stone, K.; Jamal, S.A.; Ensrud, K.; Segal, M.; Genant, H.K.; Cummings, S.R. The association of radiographically detected vertebral fractures with back pain and function: A prospective study. Ann. Intern. Med. 1998, 128, 793–800. [Google Scholar] [CrossRef] [PubMed]
- Anastasilakis, A.D.; Evangelatos, G.; Makras, P.; Iliopoulos, A. Magnetic resonance imaging has an advantage over conventional spine X-rays in the evaluation of rebound-associated vertebral fractures following denosumab discontinuation. Endocrine 2020, 69, 516–518. [Google Scholar] [CrossRef] [PubMed]
- Papaioannou, A.; Watts, N.B.; Kendler, D.L.; Yuen, C.K.; Adachi, J.D.; Ferko, N. Diagnosis and management of vertebral fractures in elderly adults. Am. J. Med. 2002, 113, 220–228. [Google Scholar] [CrossRef]
- Vogt, T.M.; Ross, P.D.; Palermo, L.; Musliner, T.; Genant, H.K.; Black, D.; Thompson, D.E. Vertebral fracture prevalence among women screened for the Fracture Intervention Trial and a simple clinical tool to screen for undiagnosed vertebral fractures. Fracture Intervention Trial Research Group. Mayo Clin. Proc. 2000, 75, 888–896. [Google Scholar] [CrossRef]
- Adams, J.E. Opportunistic Identification of Vertebral Fractures. J. Clin. Densitom 2016, 19, 54–62. [Google Scholar] [CrossRef]
- Miller, P.D. Clinical Management of Vertebral Compression Fractures. J. Clin. Densitom 2016, 19, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Koester, M.C.; Spindler, K.P. Pharmacologic agents in fracture healing. Clin. Sports Med. 2006, 25, 63–73, viii. [Google Scholar] [CrossRef]
- Beebe, F.A.; Barkin, R.L.; Barkin, S. A clinical and pharmacologic review of skeletal muscle relaxants for musculoskeletal conditions. Am. J. Ther. 2005, 12, 151–171. [Google Scholar] [CrossRef]
- Cashin, A.G.; Folly, T.; Bagg, M.K.; Wewege, M.A.; Jones, M.D.; Ferraro, M.C.; Leake, H.B.; Rizzo, R.R.N.; Schabrun, S.M.; Gustin, S.M.; et al. Efficacy, acceptability, and safety of muscle relaxants for adults with non-specific low back pain: Systematic review and meta-analysis. BMJ 2021, 374, n1446. [Google Scholar] [CrossRef]
- van Tulder, M.W.; Touray, T.; Furlan, A.D.; Solway, S.; Bouter, L.M. Muscle relaxants for non-specific low back pain. Cochrane Database Syst. Rev. 2003, 2003, Cd004252. [Google Scholar] [CrossRef]
- Friedman, B.W.; Dym, A.A.; Davitt, M.; Holden, L.; Solorzano, C.; Esses, D.; Bijur, P.E.; Gallagher, E.J. Naproxen with Cyclobenzaprine, Oxycodone/Acetaminophen, or Placebo for Treating Acute Low Back Pain: A Randomized Clinical Trial. JAMA 2015, 314, 1572–1580. [Google Scholar] [CrossRef] [PubMed]
- Friedman, B.W.; Irizarry, E.; Solorzano, C.; Zias, E.; Pearlman, S.; Wollowitz, A.; Jones, M.P.; Shah, P.D.; Gallagher, E.J. A Randomized, Placebo-Controlled Trial of Ibuprofen Plus Metaxalone, Tizanidine, or Baclofen for Acute Low Back Pain. Ann. Emerg. Med. 2019, 74, 512–520. [Google Scholar] [CrossRef] [PubMed]
- Pareek, A.; Chandurkar, N.; Chandanwale, A.S.; Ambade, R.; Gupta, A.; Bartakke, G. Aceclofenac-tizanidine in the treatment of acute low back pain: A double-blind, double-dummy, randomized, multicentric, comparative study against aceclofenac alone. Eur. Spine J. 2009, 18, 1836–1842. [Google Scholar] [CrossRef]
- Maugars, Y.; Guillot, P.; Glémarec, J.; Berthelot, J.M.; Le Goff, B.; Darrieutort-Laffite, C. Long-term follow up after denosumab treatment for osteoporosis—Rebound associated with hypercalcemia, parathyroid hyperplasia, severe bone mineral density loss, and multiple fractures: A case report. J. Med. Case Rep. 2020, 14, 130. [Google Scholar] [CrossRef]
- Yeung, M.; Ho, K.; Fornier, M.N.; Farooki, A. Vertebral Fractures after Denosumab Discontinuation in Breast Cancer Survivors: A Single Institution Experience. HSS J. 2021, 17, 185–191. [Google Scholar] [CrossRef]
- Armingeat, T.; Brondino, R.; Pham, T.; Legré, V.; Lafforgue, P. Intravenous pamidronate for pain relief in recent osteoporotic vertebral compression fracture: A randomized double-blind controlled study. Osteoporos. Int. 2006, 17, 1659–1665. [Google Scholar] [CrossRef] [PubMed]
- Laroche, M.; Cantogrel, S.; Jamard, B.; Constantin, A.; Zabraniecki, L.; Cantagrel, A.; Mazières, B. Comparison of the analgesic efficacy of pamidronate and synthetic human calcitonin in osteoporotic vertebral fractures: A double-blind controlled study. Clin. Rheumatol. 2006, 25, 683–686. [Google Scholar] [CrossRef] [PubMed]
- Tetsunaga, T.; Tetsunaga, T.; Nishida, K.; Tanaka, M.; Sugimoto, Y.; Takigawa, T.; Takei, Y.; Ozaki, T. Denosumab and alendronate treatment in patients with back pain due to fresh osteoporotic vertebral fractures. J. Orthop. Sci. 2017, 22, 230–236. [Google Scholar] [CrossRef]
- Mori, Y.; Izumiyama, T.; Mori, N.; Aizawa, T. The Effect of Teriparatide for the Treatment of Multiple Spontaneous Clinical Vertebral Fractures after Discontinuation of Denosumab in a Female Patient with Rheumatoid Arthritis: A Case Report. Tohoku J. Exp. Med. 2021, 254, 57–61. [Google Scholar] [CrossRef]
- Polyzos, S.A.; Terpos, E. Clinical vertebral fractures following denosumab discontinuation. Endocrine 2016, 54, 271–272. [Google Scholar] [CrossRef] [PubMed]
- Tsourdi, E.; Zillikens, M.C.; Meier, C.; Body, J.J.; Gonzalez Rodriguez, E.; Anastasilakis, A.D.; Abrahamsen, B.; McCloskey, E.; Hofbauer, L.C.; Guañabens, N.; et al. Fracture risk and management of discontinuation of denosumab therapy: A systematic review and position statement by ECTS. J. Clin. Endocrinol. Metab. 2020, 106, 264–281. [Google Scholar] [CrossRef]
- Broy, S.B. The Vertebral Fracture Cascade: Etiology and Clinical Implications. J. Clin. Densitom. 2016, 19, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Weber, A.; Huysmans, S.M.D.; van Kuijk, S.M.J.; Evers, S.; Jutten, E.M.C.; Senden, R.; Paulus, A.T.G.; van den Bergh, J.P.W.; de Bie, R.A.; Merk, J.M.R.; et al. Effectiveness and cost-effectiveness of dynamic bracing versus standard care alone in patients suffering from osteoporotic vertebral compression fractures: Protocol for a multicentre, two-armed, parallel-group randomised controlled trial with 12 months of follow-up. BMJ Open 2022, 12, e054315. [Google Scholar] [CrossRef]
- Bolton, K.; Wallis, J.A.; Taylor, N.F. Benefits and harms of non-surgical and non-pharmacological management of osteoporotic vertebral fractures: A systematic review and meta-analysis. Braz. J. Phys. Ther. 2022, 26, 100383. [Google Scholar] [CrossRef]
- Wong, C.C.; McGirt, M.J. Vertebral compression fractures: A review of current management and multimodal therapy. J. Multidiscip. Healthc. 2013, 6, 205–214. [Google Scholar] [CrossRef]
- Meccariello, L.; Muzii, V.F.; Falzarano, G.; Medici, A.; Carta, S.; Fortina, M.; Ferrata, P. Dynamic corset versus three-point brace in the treatment of osteoporotic compression fractures of the thoracic and lumbar spine: A prospective, comparative study. Aging Clin. Exp. Res. 2017, 29, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.M.; Park, S.Y.; Lee, S.H.; Suh, S.W.; Hong, J.Y. Comparative analysis of clinical outcomes in patients with osteoporotic vertebral compression fractures (OVCFs): Conservative treatment versus balloon kyphoplasty. Spine J. 2012, 12, 998–1005. [Google Scholar] [CrossRef]
- Buchbinder, R.; Johnston, R.V.; Rischin, K.J.; Homik, J.; Jones, C.A.; Golmohammadi, K.; Kallmes, D.F. Percutaneous vertebroplasty for osteoporotic vertebral compression fracture. Cochrane Database Syst. Rev. 2018, 4, Cd006349. [Google Scholar] [CrossRef]
- Ebeling, P.R.; Akesson, K.; Bauer, D.C.; Buchbinder, R.; Eastell, R.; Fink, H.A.; Giangregorio, L.; Guanabens, N.; Kado, D.; Kallmes, D.; et al. The Efficacy and Safety of Vertebral Augmentation: A Second ASBMR Task Force Report. J. Bone Miner. Res. 2019, 34, 3–21. [Google Scholar] [CrossRef] [PubMed]
- Lou, S.; Shi, X.; Zhang, X.; Lyu, H.; Li, Z.; Wang, Y. Percutaneous vertebroplasty versus non-operative treatment for osteoporotic vertebral compression fractures: A meta-analysis of randomized controlled trials. Osteoporos. Int. 2019, 30, 2369–2380. [Google Scholar] [CrossRef] [PubMed]
- Halvachizadeh, S.; Stalder, A.L.; Bellut, D.; Hoppe, S.; Rossbach, P.; Cianfoni, A.; Schnake, K.J.; Mica, L.; Pfeifer, R.; Sprengel, K.; et al. Systematic Review and Meta-Analysis of 3 Treatment Arms for Vertebral Compression Fractures: A Comparison of Improvement in Pain, Adjacent-Level Fractures, and Quality of Life Between Vertebroplasty, Kyphoplasty, and Nonoperative Management. JBJS Rev. 2021, 9, e21. [Google Scholar] [CrossRef] [PubMed]
- Blasco, J.; Martinez-Ferrer, A.; Macho, J.; San Roman, L.; Pomés, J.; Carrasco, J.; Monegal, A.; Guañabens, N.; Peris, P. Effect of vertebroplasty on pain relief, quality of life, and the incidence of new vertebral fractures: A 12-month randomized follow-up, controlled trial. J. Bone Miner. Res. 2012, 27, 1159–1166. [Google Scholar] [CrossRef]
- Che, H.; Breuil, V.; Cortet, B.; Paccou, J.; Thomas, T.; Chapuis, L.; Debiais, F.; Mehsen-Cetre, N.; Javier, R.M.; Loiseau Peres, S.; et al. Vertebral fractures cascade: Potential causes and risk factors. Osteoporos. Int. 2019, 30, 555–563. [Google Scholar] [CrossRef]
- Mudano, A.S.; Bian, J.; Cope, J.U.; Curtis, J.R.; Gross, T.P.; Allison, J.J.; Kim, Y.; Briggs, D.; Melton, M.E.; Xi, J.; et al. Vertebroplasty and kyphoplasty are associated with an increased risk of secondary vertebral compression fractures: A population-based cohort study. Osteoporos. Int. 2009, 20, 819–826. [Google Scholar] [CrossRef]
- Saad, A.; Botchu, R.; James, S. The Rates of Cement Leakage Following Vertebroplasty in Osteoporotic versus Metastatic Disease. Indian J. Radiol. Imaging 2022, 32, 46–50. [Google Scholar] [CrossRef]
- Shapiro, S.; Abel, T.; Purvines, S. Surgical removal of epidural and intradural polymethylmethacrylate extravasation complicating percutaneous vertebroplasty for an osteoporotic lumbar compression fracture. Case report. J. Neurosurg. 2003, 98, 90–92. [Google Scholar] [CrossRef] [PubMed]
- Bernhard, J.; Heini, P.F.; Villiger, P.M. Asymptomatic diffuse pulmonary embolism caused by acrylic cement: An unusual complication of percutaneous vertebroplasty. Ann. Rheum. Dis. 2003, 62, 85–86. [Google Scholar] [CrossRef]
- Kim, Y.J.; Lee, J.W.; Park, K.W.; Yeom, J.S.; Jeong, H.S.; Park, J.M.; Kang, H.S. Pulmonary cement embolism after percutaneous vertebroplasty in osteoporotic vertebral compression fractures: Incidence, characteristics, and risk factors. Radiology 2009, 251, 250–259. [Google Scholar] [CrossRef]
- Dupont, J.; Laurent, M.R.; Dedeyne, L.; Luyten, F.P.; Gielen, E.; Dejaeger, M. Rebound-associated vertebral fractures after stopping denosumab: Report of four cases. Jt. Bone Spine 2020, 87, 171–173. [Google Scholar] [CrossRef] [PubMed]
- Fernández Fernández, E.; Benavent Núñez, D.; Bonilla Hernán, G.; Monjo Henry, I.; García Carazo, S.; Bernad Pineda, M.; Balsa Criado, A.; Aguado Acín, P. Multiple vertebral fractures following discontinuation of denosumab treatment: Ten clinical cases report. Reumatol. Clin. Engl. Ed. 2020, 16, 480–484. [Google Scholar] [CrossRef]
- Gonzalez-Rodriguez, E.; Aubry-Rozier, B.; Stoll, D.; Zaman, K.; Lamy, O. Sixty spontaneous vertebral fractures after denosumab discontinuation in 15 women with early-stage breast cancer under aromatase inhibitors. Breast Cancer Res. Treat. 2020, 179, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Tripto-Shkolnik, L.; Rouach, V.; Marcus, Y.; Rotman-Pikielny, P.; Benbassat, C.; Vered, I. Vertebral Fractures Following Denosumab Discontinuation in Patients with Prolonged Exposure to Bisphosphonates. Calcif. Tissue Int. 2018, 103, 44–49. [Google Scholar] [CrossRef]
- Gibbs, J.C.; MacIntyre, N.J.; Ponzano, M.; Templeton, J.A.; Thabane, L.; Papaioannou, A.; Giangregorio, L.M. Exercise for improving outcomes after osteoporotic vertebral fracture. Cochrane Database Syst. Rev. 2019, 7, Cd008618. [Google Scholar] [CrossRef]
- Ponzano, M.; Tibert, N.; Brien, S.; Funnell, L.; Gibbs, J.C.; Keller, H.; Laprade, J.; Morin, S.N.; Papaioannou, A.; Weston, Z.; et al. International consensus on the non-pharmacological and non-surgical management of osteoporotic vertebral fractures. Osteoporos. Int. 2023, 34, 1065–1074. [Google Scholar] [CrossRef]
- Briggs, A.M.; Greig, A.M.; Wark, J.D. The vertebral fracture cascade in osteoporosis: A review of aetiopathogenesis. Osteoporos. Int. 2007, 18, 575–584. [Google Scholar] [CrossRef]
- Sinaki, M.; Mikkelsen, B.A. Postmenopausal spinal osteoporosis: Flexion versus extension exercises. Arch. Phys. Med. Rehabil. 1984, 65, 593–596. [Google Scholar]
- Cauley, J.A.; Hochberg, M.C.; Lui, L.Y.; Palermo, L.; Ensrud, K.E.; Hillier, T.A.; Nevitt, M.C.; Cummings, S.R. Long-term risk of incident vertebral fractures. JAMA 2007, 298, 2761–2767. [Google Scholar] [CrossRef]
- Johansson, H.; Siggeirsdóttir, K.; Harvey, N.C.; Odén, A.; Gudnason, V.; McCloskey, E.; Sigurdsson, G.; Kanis, J.A. Imminent risk of fracture after fracture. Osteoporos. Int. 2017, 28, 775–780. [Google Scholar] [CrossRef] [PubMed]
- Kanis, J.A.; Harvey, N.C.; McCloskey, E.; Bruyère, O.; Veronese, N.; Lorentzon, M.; Cooper, C.; Rizzoli, R.; Adib, G.; Al-Daghri, N.; et al. Algorithm for the management of patients at low, high and very high risk of osteoporotic fractures. Osteoporos. Int. 2020, 31, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Anastasilakis, A.D.; Evangelatos, G.; Makras, P.; Iliopoulos, A. Rebound-associated vertebral fractures may occur in sequential time points following denosumab discontinuation: Need for prompt treatment re-initiation. Bone Rep. 2020, 12, 100267. [Google Scholar] [CrossRef]
- Anastasilakis, A.D.; Papapoulos, S.E.; Polyzos, S.A.; Appelman-Dijkstra, N.M.; Makras, P. Zoledronate for the Prevention of Bone Loss in Women Discontinuing Denosumab Treatment. A Prospective 2-Year Clinical Trial. J. Bone Miner. Res. 2019, 34, 2220–2228. [Google Scholar] [CrossRef] [PubMed]
- Everts-Graber, J.; Reichenbach, S.; Gahl, B.; Häuselmann, H.; Ziswiler, H.R.; Studer, U.; Lehmann, T. Effects of zoledronate on bone mineral density and bone turnover after long-term denosumab therapy: Observations in a real-world setting. Bone 2022, 163, 116498. [Google Scholar] [CrossRef] [PubMed]
- Everts-Graber, J.; Reichenbach, S.; Gahl, B.; Ziswiler, H.R.; Studer, U.; Lehmann, T. Risk factors for vertebral fractures and bone loss after denosumab discontinuation: A real-world observational study. Bone 2021, 144, 115830. [Google Scholar] [CrossRef] [PubMed]
- Everts-Graber, J.; Reichenbach, S.; Ziswiler, H.R.; Studer, U.; Lehmann, T. A Single Infusion of Zoledronate in Postmenopausal Women Following Denosumab Discontinuation Results in Partial Conservation of Bone Mass Gains. J. Bone Miner. Res. 2020, 35, 1207–1215. [Google Scholar] [CrossRef]
- Kendler, D.; Chines, A.; Clark, P.; Ebeling, P.R.; McClung, M.; Rhee, Y.; Huang, S.; Stad, R.K. Bone Mineral Density After Transitioning From Denosumab to Alendronate. J. Clin. Endocrinol. Metab. 2020, 105, e255–e264. [Google Scholar] [CrossRef]
- Sølling, A.S.; Harsløf, T.; Brockstedt, H.K.; Langdahl, B. Discontinuation of denosumab in men with prostate cancer. Osteoporos. Int. 2023, 34, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Sølling, A.S.; Harsløf, T.; Langdahl, B. Treatment with Zoledronate Subsequent to Denosumab in Osteoporosis: A 2-Year Randomized Study. J. Bone Miner. Res. 2021, 36, 1245–1254. [Google Scholar] [CrossRef] [PubMed]
- Laroche, M.; Couture, G.; Ruyssen-Witrand, A.; Constantin, A.; Degboé, Y. Effect of risedronate on bone loss at discontinuation of denosumab. Bone Rep. 2020, 13, 100290. [Google Scholar] [CrossRef]
- Tutaworn, T.; Nieves, J.W.; Wang, Z.; Levin, J.E.; Yoo, J.E.; Lane, J.M. Bone loss after denosumab discontinuation is prevented by alendronate and zoledronic acid but not risedronate: A retrospective study. Osteoporos. Int. 2023, 34, 573–584. [Google Scholar] [CrossRef]
- Ha, J.; Kim, J.; Jeong, C.; Lim, Y.; Kim, M.K.; Kwon, H.S.; Song, K.H.; Kang, M.I.; Baek, K.H. Effect of follow-up raloxifene therapy after denosumab discontinuation in postmenopausal women. Osteoporos. Int. 2022, 33, 1591–1599. [Google Scholar] [CrossRef] [PubMed]
- Makras, P.; Appelman-Dijkstra, N.M.; Papapoulos, S.E.; van Wissen, S.; Winter, E.M.; Polyzos, S.A.; Yavropoulou, M.P.; Anastasilakis, A.D. The Duration of Denosumab Treatment and the Efficacy of Zoledronate to Preserve Bone Mineral Density After Its Discontinuation. J. Clin. Endocrinol. Metab. 2021, 106, e4155–e4162. [Google Scholar] [CrossRef]
- Anastasilakis, A.D.; Polyzos, S.A.; Makras, P.; Trovas, G.; Yavropoulou, M.P.; Tournis, S. Efficacy of Antiosteoporotic Medications in Patients With Rebound-Associated Fractures after Denosumab Discontinuation. J. Clin. Densitom. 2021, 24, 591–596. [Google Scholar] [CrossRef]
- Anastasilakis, A.D.; Trovas, G.; Balanika, A.; Polyzos, S.A.; Makras, P.; Tournis, S. Progression of Rebound-Associated Vertebral Fractures Following Denosumab Discontinuation Despite Reinstitution of Treatment: Suppressing Increased Bone Turnover May Not Be Enough. J. Clin. Densitom. 2021, 24, 338–340. [Google Scholar] [CrossRef]
- Niimi, R.; Kono, T.; Nishihara, A.; Hasegawa, M.; Kono, T.; Sudo, A. Second rebound-associated vertebral fractures after denosumab discontinuation. Arch. Osteoporos. 2020, 15, 7. [Google Scholar] [CrossRef]
- Burckhardt, P.; Faouzi, M.; Buclin, T.; Lamy, O. Fractures after Denosumab Discontinuation: A Retrospective Study of 797 Cases. J. Bone Miner. Res. 2021, 36, 1717–1728. [Google Scholar] [CrossRef]
- Leder, B.Z.; Tsai, J.N.; Uihlein, A.V.; Wallace, P.M.; Lee, H.; Neer, R.M.; Burnett-Bowie, S.A. Denosumab and teriparatide transitions in postmenopausal osteoporosis (the DATA-Switch study): Extension of a randomised controlled trial. Lancet 2015, 386, 1147–1155. [Google Scholar] [CrossRef] [PubMed]
- Cosman, F.; Crittenden, D.B.; Adachi, J.D.; Binkley, N.; Czerwinski, E.; Ferrari, S.; Hofbauer, L.C.; Lau, E.; Lewiecki, E.M.; Miyauchi, A.; et al. Romosozumab Treatment in Postmenopausal Women with Osteoporosis. N. Engl. J. Med. 2016, 375, 1532–1543. [Google Scholar] [CrossRef] [PubMed]
- McClung, M.R.; Bolognese, M.A.; Brown, J.P.; Reginster, J.Y.; Langdahl, B.L.; Shi, Y.; Timoshanko, J.; Libanati, C.; Chines, A.; Oates, M.K. Skeletal responses to romosozumab after 12 months of denosumab. JBMR Plus 2021, 5, e10512. [Google Scholar] [CrossRef] [PubMed]
- Kashii, M.; Ebina, K.; Kitaguchi, K.; Yoshikawa, H. Romosozumab was not effective in preventing multiple spontaneous clinical vertebral fractures after denosumab discontinuation: A case report. Bone Rep. 2020, 13, 100288. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anastasilakis, A.D.; Makras, P.; Paccou, J.; Bisbinas, I.; Polyzos, S.A.; Papapoulos, S.E. Similarities and Differences in the Management of Patients with Osteoporotic Vertebral Fractures and Those with Rebound-Associated Vertebral Fractures Following Discontinuation of Denosumab. J. Clin. Med. 2023, 12, 5874. https://doi.org/10.3390/jcm12185874
Anastasilakis AD, Makras P, Paccou J, Bisbinas I, Polyzos SA, Papapoulos SE. Similarities and Differences in the Management of Patients with Osteoporotic Vertebral Fractures and Those with Rebound-Associated Vertebral Fractures Following Discontinuation of Denosumab. Journal of Clinical Medicine. 2023; 12(18):5874. https://doi.org/10.3390/jcm12185874
Chicago/Turabian StyleAnastasilakis, Athanasios D., Polyzois Makras, Julien Paccou, Ilias Bisbinas, Stergios A. Polyzos, and Socrates E. Papapoulos. 2023. "Similarities and Differences in the Management of Patients with Osteoporotic Vertebral Fractures and Those with Rebound-Associated Vertebral Fractures Following Discontinuation of Denosumab" Journal of Clinical Medicine 12, no. 18: 5874. https://doi.org/10.3390/jcm12185874
APA StyleAnastasilakis, A. D., Makras, P., Paccou, J., Bisbinas, I., Polyzos, S. A., & Papapoulos, S. E. (2023). Similarities and Differences in the Management of Patients with Osteoporotic Vertebral Fractures and Those with Rebound-Associated Vertebral Fractures Following Discontinuation of Denosumab. Journal of Clinical Medicine, 12(18), 5874. https://doi.org/10.3390/jcm12185874







