Identification of Glaucoma in Diabetics Using the Laguna-ONhE Colourimetric Method and OCT Spectralis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Optic Disc Haemoglobin Measurements
2.2. Subjects
2.3. Inclusion and Exclusion Criteria
2.4. Examinations Carried Out
2.5. Statistical Analyses
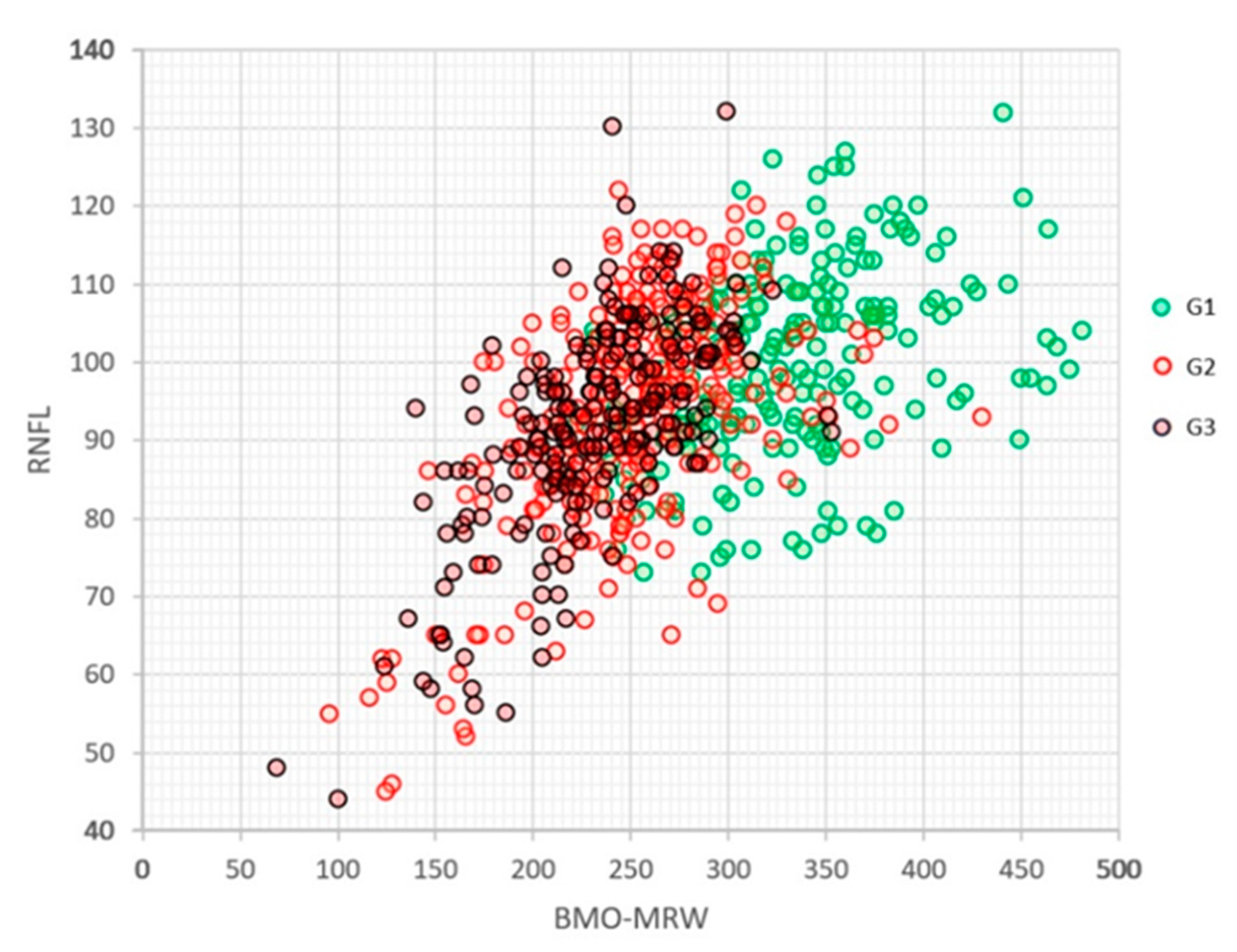
3. Results
3.1. Subjects and Tests Excluded
3.2. Description of the Three Groups (Table 1)
3.3. Results of Each Group
| AGE | DIABETES (Years) | IOP | PACHYMETRY | GDF | GIP | Vertical C/D Ratio | |
|---|---|---|---|---|---|---|---|
| G1 | 65.4 ± 8.5 | 11.8 ± 7.4 | 15.2 ± 3 | 549.5 ± 36.3 | 22.6 ± 9.3 | 48 ± 21.6 | 0.43 ± 0.11 |
| G2 (vs. G1) | 64.8 ± 8.1 (p = 0.2117) | 12.5 ± 8.3 (p = 0.1339) | 15.5 ± 3.3 (p = 0.2086) | 538 ± 36.3 (p = 0.0002) | −14.5 ± 14.4 (p < 0.0001) | −13 ± 26.9 (p < 0.0001) | 0.61 ± 0.09 (p < 0.0001) |
| G3 (vs. G1) | 64.5 ± 8.2 (p = 0.1419) | 11.2 ± 7.8 (p = 0.2191) | 15.8 ± 3.8 (p = 0.0349) | 541.7 ± 34.5 (p = 0.0106) | −34.3 ± 13.5 (p < 0.0001) | −37.4 ± 26.7 (p < 0.0001) | 0.66 ± 0.09 (p < 0.0001) |
| MRW G | MRW T | MRW ST | MRW SN | MRW N | MRW IN | MRW IT | |
| G1 | 332 ± 55 | 279 ± 81 | 336 ± 75 | 353 ± 81 | 333 ± 85 | 388 ± 76 | 361 ± 75 |
| G2 (vs. G1) | 252 ± 47 (p < 0.0001) | 213 ± 64 (p < 0.0001) | 257 ± 61 (p < 0.0001) | 262 ± 66 (p < 0.0001) | 262 ± 73 (p < 0.0001) | 309 ± 208 (p < 0.0001) | 274 ± 211 (p < 0.0001) |
| G3 (vs. G1) | 231 ± 44 (p < 0.0001) | 193 ± 58 (p < 0.0001) | 230 ± 52 (p < 0.0001) | 243 ± 65 (p < 0.0001) | 240 ± 67 (p < 0.0001) | 276 ± 64 (p < 0.0001) | 246 ± 68 (p < 0.0001) |
| RNFL G | RNFL T | RNFL ST | RNFL SN | RNFL N | RNFL IN | RNFL IT | |
| G1 | 100 ± 12 | 77 ± 18 | 126 ± 26 | 117 ± 29 | 84 ± 21 | 128 ± 25 | 135 ± 32 |
| G2 (vs. G1) | 94 ± 14 (p < 0.0001) | 81 ± 26 (p = 0.0402) | 120 ± 28 (p = 0.0039) | 103 ± 28 (p < 0.0001) | 89 ± 47 (p = 0.0534) | 117 ± 33 (p < 0.0001) | 113 ± 36 (p < 0.0001) |
| G3 (vs. G1) | 91 ± 14 (p < 0.0001) | 74 ± 22 (p = 0.0487) | 115 ± 25 (p < 0.0001) | 106 ± 27 (p < 0.0001) | 84 ± 59 (p = 0.4929) | 116 ± 30 (p < 0.0001) | 115 ± 35 (p < 0.0001) |
| DL glaucoma | Hb total | Hb cup | Cup area | Vessels | GIP slope (/year) | Cup area slope (/year) | |
| G1 | 0.95 ± 0.06 | 70.7 ± 3.6 | 65.0 ± 10.5 | 25.6% ± 10.0 | 32.2% ± 3.5 | −1.78 ± 4.75 | 0.57% ± 2.02 |
| G2 (vs. G1) | 0.55 ± 0.23 (p < 0.0001) | 65.2 ± 4.1 (p < 0.0001) | 55.6 ± 8.2 (p < 0.0001) | 43.3% ± 10.9 (p < 0.0001) | 30.2% ± 3.9 (p < 0.0001) | −3.51 ± 6.13 (p = 0.0023) | 0.97% ± 2.14 (p = 0.043) |
| G3 (vs. G1) | 0.32 ± 0.17 (p < 0.0001) | 64.18 ± 4.27 (p < 0.0001) | 55.8 ± 7.4 (p < 0.0001) | 49.4% ± 10.8 (p < 0.0001) | 30.2% ± 4.1 (p < 0.0001) | −4.26 ± 6.92 (p = 0.0002) | 1.02% ± 3.12 (p = 0.068) |
3.4. Normalised Data Result
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gonzalez de la Rosa, M.; de-la-Huerga-Moreno, S.; Alfonso-Lopez, F.; Cabrera-Lopez, F.; Pareja-Rios, A.; Gonzalez-Hernandez, D.; Gonzalez-Hernandez, M. Comparison of age-related vascular changes in the optic disc of patients with diabetes, with glaucomatous and non-glaucomatous features. BMJ Open Ophthalmol. 2022, 7, e001100. [Google Scholar] [CrossRef]
- Gonzalez de la Rosa, M.; Gonzalez-Hernandez, M.; Sigut, J.; Gonzalez de la Rosa, M. Measuring haemoglobin levels in the optic nerve head: Com-parisons with other structural and functional parameters of glaucoma. Investig. Ophthalmol. Vis. Sci. 2013, 54, 482–489. [Google Scholar] [CrossRef]
- Gonzalez-Hernandez, M.; Gonzalez-Hernandez, D.; Betancor-Caro, N.; Guedes-Guedes, I.; Guldager, M.K.; Gonzalez de la Rosa, M. Glaucoma Incidence and Progression in Diabetics: The Canary Islands Study Using the Laguna-ONhE Application. J. Clin. Med. 2022, 11, 7294. [Google Scholar] [CrossRef] [PubMed]
- Funk, R.O.; Hodge, D.O.; Kohli, D.; Roddy, G.W. Multiple Systemic Vascular Risk Factors Are Associated with Low-Tension Glaucoma. J. Glaucoma 2022, 31, 15–22. [Google Scholar] [CrossRef]
- Mitchell, P.; Smith, W.; Chey, T.; Healey, P.R. Open-angle glaucoma and diabetes: The Blue Mountains eye study, Australia. Ophthalmology 1997, 104, 712–718. [Google Scholar] [CrossRef]
- Bonovas, S.; Peponis, V.; Filioussi, K. Diabetes mellitus as a risk factor for primary open-angle glaucoma: A meta-analysis. Diabet. Med. 2004, 21, 609–614. [Google Scholar] [CrossRef]
- Zhou, M.; Wang, W.; Huang, W.; Zhang, X. Diabetes mellitus as a risk factor for open-angle glaucoma: A systematic review and meta-analysis. PLoS ONE. 2014, 9, e102972. [Google Scholar] [CrossRef]
- Gonzalez Hernandez, M.; Gonzalez Hernandez, D.; Perez Barbudo, D.; Rodriguez Esteve, P.; Betancor Caro, N.; Gonzalez de la Rosa, M. Fully Automated Colorimetric Analysis of the Optic Nerve Aided by Deep Learning and Its Association with Perimetry and OCT for the Study of Glaucoma. J. Clin. Med. 2021, 10, 3231. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez Hernandez, M.; Gonzalez Hernandez, D.; Rodriguez Esteve, P.; Gonzalez de la Rosa, M. New GIP index for progression analysis, using the Laguna-ONhE method for the optic nerve head hemoglobin evaluation. Investig. Ophthal. Vis. Sci. 2021, 62, 1828. [Google Scholar]
- Chauhan, B.C.; Danthurebandara, V.M.; Sharpe, G.P.; Demirel, S.; Girkin, C.A.; Mardin, C.Y.; Burgoyne, C.F. Bruch’s Membrane Opening Minimum Rim Width and Retinal Nerve Fiber Layer Thickness in a Normal White Population: A Multicenter Study. Ophthalmology 2015, 122, 1786–1794. [Google Scholar] [CrossRef] [PubMed]
- Bambo, M.P.; Fuentemilla, E.; Cameo, B.; Fuertes, I.; Ferrandez, B.; Güerri, N.; Polo, V.; Larrosa, J.M.; Pablo, L.E.; Garcia-Martin, E. Diagnostic capability of a linear discriminant function applied to a novel Spectralis OCT glaucoma-detection protocol. BMC Ophthalmol. 2020, 20, 35. [Google Scholar] [CrossRef]
- Yusof, A.M.Z.; Othman, O.; Tang, S.F.; Hassan, M.R.; Din, N.M. Diagnostic evaluation of optical coherence tomography parameters in normal, preperimetric and perimetric glaucoma patients. Int. J. Ophthalmol. 2022, 15, 1782–1790. [Google Scholar] [CrossRef] [PubMed]
- Park, K.; Kim, J.; Lee, J. The Relationship Between Bruch’s Membrane Opening-Minimum Rim Width and Retinal Nerve Fiber Layer Thickness and a New Index Using a Neural Network. Transl. Vis. Sci. Technol. 2018, 7, 14. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.B.; Cho, H.K. Deep learning classification of early normal-tension glaucoma and glaucoma suspects using Bruch’s membrane opening-minimum rim width and RNFL. Sci. Rep. 2020, 10, 19042. [Google Scholar] [CrossRef] [PubMed]
- Stagg, B.C.; Medeiros, F.A. A Comparison of OCT Parameters in Identifying Glaucoma Damage in Eyes Suspected of Having Glaucoma. Ophthalmol. Glaucoma 2020, 3, 90–96. [Google Scholar] [CrossRef]
- Li, R.; Wang, X.; Wei, Y.; Fang, Y.; Tian, T.; Kang, L.; Pan, Y. Diagnostic capability of different morphological parameters for primary open-angle glaucoma in the Chinese population. BMC Ophthalmol. 2021, 21, 151. [Google Scholar] [CrossRef]
- Gmeiner, J.M.; Schrems, W.A.; Mardin, C.Y.; Laemmer, R.; Kruse, F.E.; Schrems-Hoesl, L.M. Comparison of Bruch’s Membrane Opening Minimum Rim Width and Peripapillary Retinal Nerve Fiber Layer Thickness in Early Glaucoma Assessment. Investig. Ophthalmol. Vis. Sci. 2016, 57, 575–584. [Google Scholar] [CrossRef]
- Awe, M.; Khalili-Amiri, S.; Volkmann, I.R.; Junker, B.; Framme, C.; Hufendiek, K. Die auf der Bruchschen Membranöffnung basierende Minimale Randsaumweite: Korrelation und diagnostische Genauigkeit im Vergleich zur peripapillären retinalen Nervenfaserschichtdicke. Ophthalmologe 2019, 116, 33–42. [Google Scholar] [CrossRef]
- Shi, L.; Mohammadi, M.; Mohammadzadeh, V.; Su, E.; Weiss, R.E.; Caprioli, J.; Nouri-Mahdavi, K. Comparing Rates of Change in Moderate to Advanced Glaucoma: Retinal Nerve Fiber Layer vs. Bruch’s Membrane Opening-Minimum Rim Width. Am. J. Ophthalmol. 2023, 253, 181–188. [Google Scholar] [CrossRef]
- Cho, H.K.; Kee, C. Rate of Change in Bruch’s Membrane Opening-Minimum Rim Width and Peripapillary RNFL in Early Normal Tension Glaucoma. J. Clin. Med. 2020, 9, 2321. [Google Scholar] [CrossRef]
- El-Nimri, N.W.; Moghimi, S.; Nishida, T.; Yarmohammadi, A.; Zangwill, L.M.; Hou, H.; Proudfoot, J.; Walker, E.; Fazio, M.A.; Girkin, C.A.; et al. Racial Differences in Detection of Glaucoma Using Retinal Nerve Fiber Layer Thickness and Bruch Membrane Opening Minimum Rim Width. Am. J. Ophthalmol. 2022, 246, 2233–2235. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.K.; Park, J.M.; Kee, C. Effect of optic disc size on correlation between Bruch’s membrane opening-minimum rim width and peripapillary retinal nerve fibre layer thickness. Eye 2019, 33, 1930–1938. [Google Scholar] [CrossRef]
- Gonzalez Hernandez, M.; Gonzalez Hernandez, D.; Perez Barbudo, D.; Gonzalez de la Rosa, M. Optic disc area frequency distribution in a large sample of retinographic images. BMJ Open Ophthalmol. 2022, 7, e000972. [Google Scholar] [CrossRef]
- Pena-Betancor, C.; Gonzalez-Hernandez, M.; Fumero-Batista, F.; Sigut, J.; Medina-Mesa, E.; Alayon, S.; de la Rosa, M.G. Estimation of the relative amount of hemoglobin in the cup and neuroretinal rim using stereoscopic color fundus images. Investig. Ophthalmol. Vis. Sci. 2015, 56, 1562–1568. [Google Scholar] [CrossRef] [PubMed]
- Medina-Mesa, E.; Gonzalez-Hernandez, M.; Sigut, J.; Fumero-Batista, F.; Pena-Betancor, C.; Alayon, S.; de la Rosa, M.G. Estimating the amount of hemoglobin in the neuroretinal rim using color images and OCT. Curr. Eye Res. 2016, 41, 798–805. [Google Scholar] [CrossRef] [PubMed]
- Meneses, L.S.; Ciarlini, L.R.; Ayub, G.; Vasconcellos, J.P.C.; Costa, V.P. Discrimination Between Healthy Eyes and Those with Mild Glaucoma Damage Using Hemoglobin Measurements of the Optic Nerve Head. J. Glaucoma 2022, 31, 567–573. [Google Scholar] [CrossRef]
- Mendez-Hernandez, C.; Rodriguez-Uña, I.; Gonzalez de la Rosa, M.; Arribas Pardo, P.; Garcia Feijoo, J. Glaucoma diagnostic capacity of optic nerve head haemoglobin measures compared with spectral domain OCT and HRT III confocal tomography. Acta Ophthalmol. 2016, 94, 697–704. [Google Scholar] [CrossRef]
- Rocha, J.A.G.; Dias, D.T.; Lemos, M.B.C.; Kanadani, F.N.; Paranhos, A.; Gracitelli, C.P.; Prata, T.S. Optic Nerve Head Hemoglobin Levels in Glaucoma: A Structural and Functional Correlation Study. J. Ophthalmol. 2021, 2021, 9916102. [Google Scholar] [CrossRef]
- Bao, Y.K.; Yan, Y.; Gordon, M.; McGill, J.B.; Kass, M.; Rajagopal, R. Visual Field Loss in Patients with Diabetes in the Absence of Clinically-Detectable Vascular Retinopathy in a Nationally Representative Survey. Investig. Ophthalmol. Vis. Sci. 2019, 60, 4711–4716. [Google Scholar] [CrossRef]
- Mendez-Hernandez, C.; Gutierrez-Diaz, E.; Pazos, M.; Gimenez-Gomez, R.; Pinazo-Duran, M.D. Agreement between Five Experts and the Laguna ONhE Automatic Colourimetric Application Interpreting the Glaucomatous Aspect of the Optic Nerve. J. Clin. Med. 2023, 12, 5485. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements.; opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas.; methods.; instructions or products referred to in the content. |





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonzalez-Hernandez, M.; Betancor-Caro, N.; Mesa-Lugo, F.; Rodriguez-Talavera, I.; Pareja-Rios, A.; Guedes-Guedes, I.; Estevez-Jorge, B.; Trujillo-Blanco, M.; Cordova-Villegas, R.; Espinoza-Gonzalez, J.; et al. Identification of Glaucoma in Diabetics Using the Laguna-ONhE Colourimetric Method and OCT Spectralis. J. Clin. Med. 2023, 12, 5876. https://doi.org/10.3390/jcm12185876
Gonzalez-Hernandez M, Betancor-Caro N, Mesa-Lugo F, Rodriguez-Talavera I, Pareja-Rios A, Guedes-Guedes I, Estevez-Jorge B, Trujillo-Blanco M, Cordova-Villegas R, Espinoza-Gonzalez J, et al. Identification of Glaucoma in Diabetics Using the Laguna-ONhE Colourimetric Method and OCT Spectralis. Journal of Clinical Medicine. 2023; 12(18):5876. https://doi.org/10.3390/jcm12185876
Chicago/Turabian StyleGonzalez-Hernandez, Marta, Nisamar Betancor-Caro, Fatima Mesa-Lugo, Ivan Rodriguez-Talavera, Alicia Pareja-Rios, Isabel Guedes-Guedes, Beatriz Estevez-Jorge, Maricela Trujillo-Blanco, Roberto Cordova-Villegas, Juan Espinoza-Gonzalez, and et al. 2023. "Identification of Glaucoma in Diabetics Using the Laguna-ONhE Colourimetric Method and OCT Spectralis" Journal of Clinical Medicine 12, no. 18: 5876. https://doi.org/10.3390/jcm12185876







