Radiomic Analysis of Quantitative T2 Mapping and Conventional MRI in Predicting Histologic Grade of Bladder Cancer
Abstract
:1. Introduction
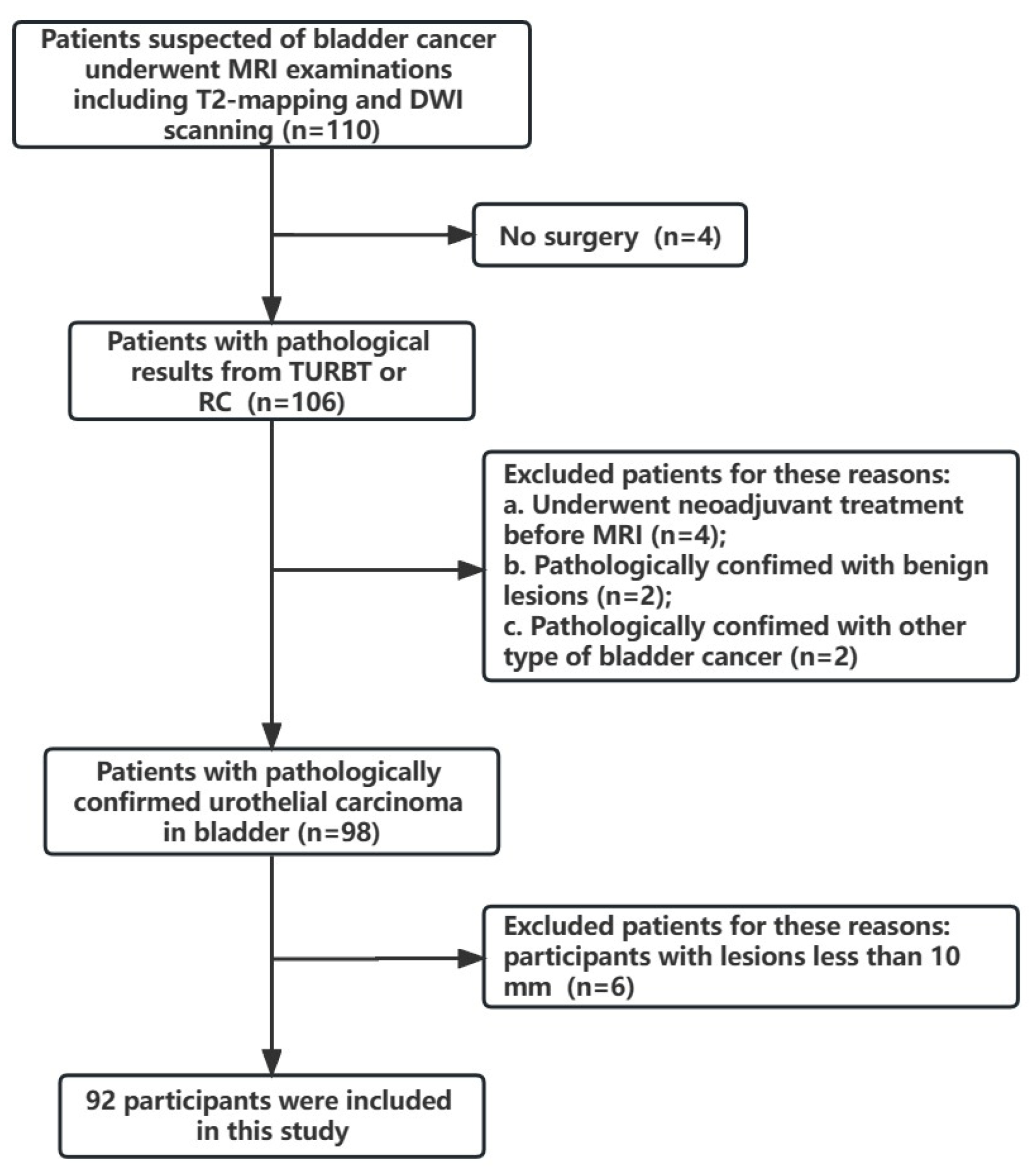
2. Materials and Methods
2.1. Image Acquisition
2.2. Image Recognition and Feature Extraction
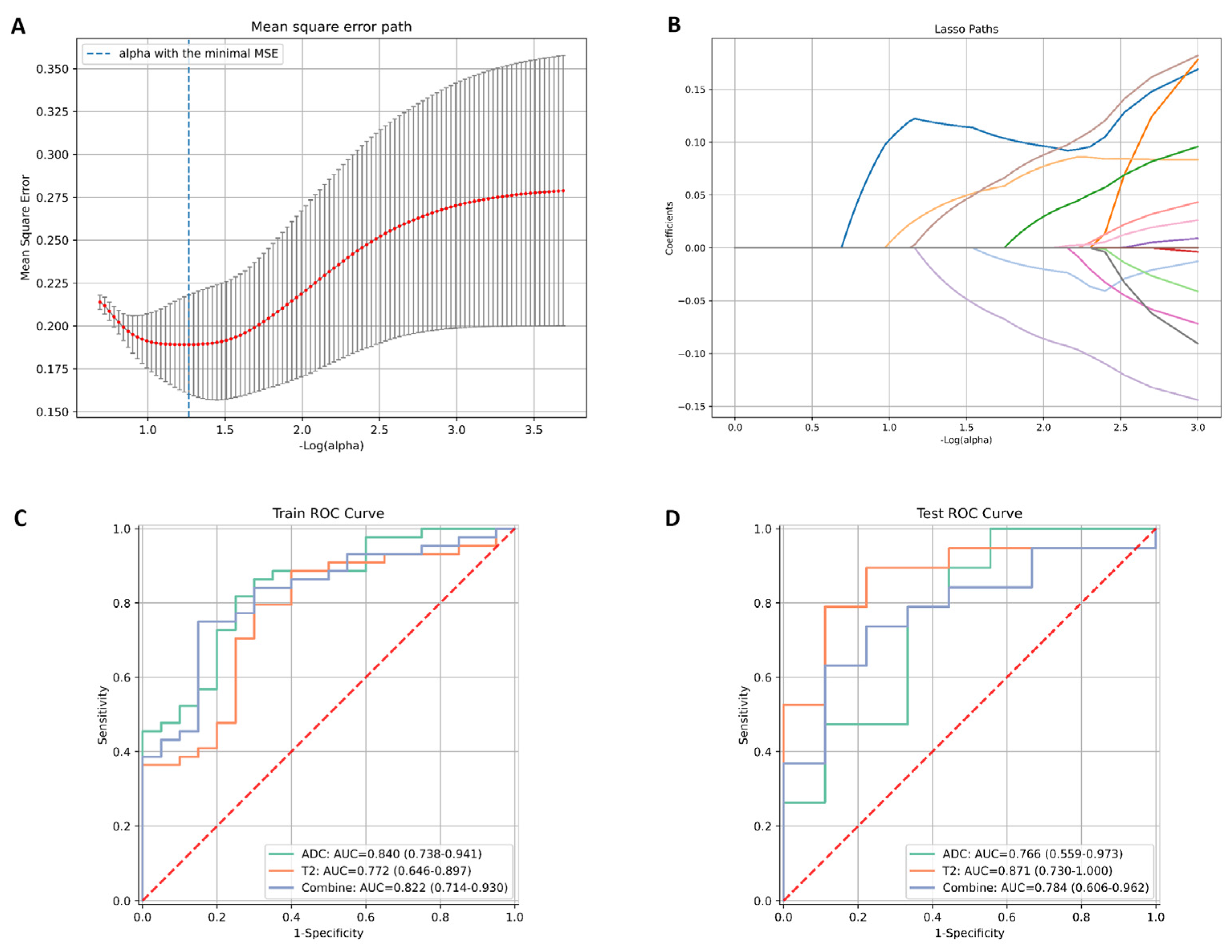
2.3. Feature Selection and Model Construction
2.4. Statistical Analysis
3. Results
3.1. Comparison of the Clinical Characteristics between the Training and Test Groups
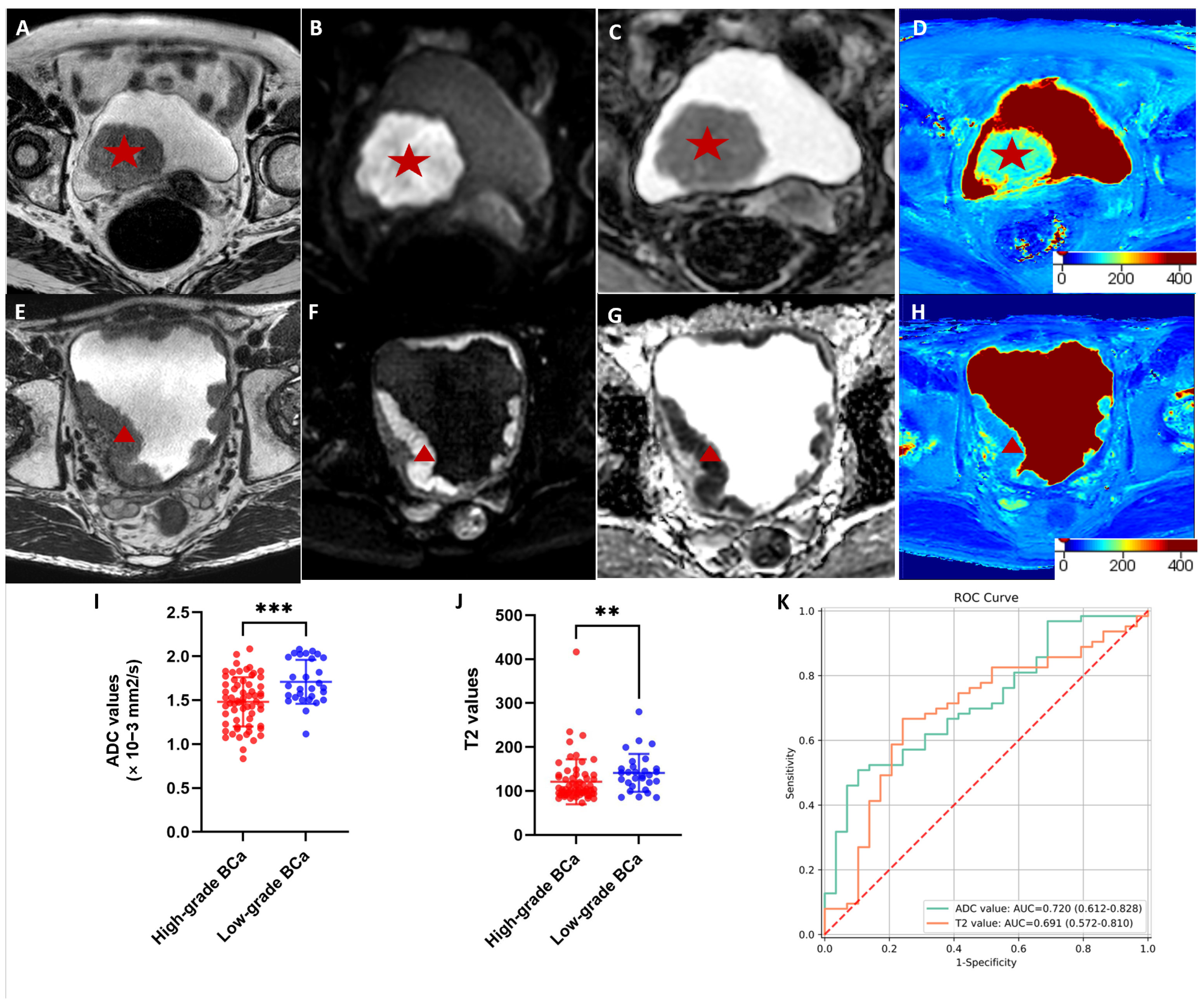
3.2. Comparisons of the T2 and ADC Values between High- and Low-Grade Cancer
3.3. Diagnostic Performance Outcomes of the Radiomic Models Based on T2 Mapping and ADC Images in Evaluating Histologic Grade
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Babjuk, M.; Burger, M.; Compérat, E.M.; Gontero, P.; Mostafid, A.H.; Palou, J.; van Rhijn, B.W.G.; Rouprêt, M.; Shariat, S.F.; Sylvester, R.; et al. European Association of Urology Guidelines on Non-Muscle-Invasive Bladder Cancer (TaT1 and Carcinoma In Situ)—2019 Update. Eur. Urol. 2019, 76, 639–657. [Google Scholar] [CrossRef] [PubMed]
- Flaig, T.W.; Spiess, P.E.; Agarwal, N.; Bangs, R.; Boorjian, S.A.; Buyyounouski, M.K.; Chang, S.; Downs, T.M.; Efstathiou, J.A.; Friedlander, T.; et al. Bladder Cancer, Version 3.2020, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2020, 18, 329–354. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.; Becher, E.; Steinberg, G.D. Update on the Guideline of Guidelines: Non-Muscle-Invasive Bladder Cancer. BJU Int. 2020, 125, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Soria, F.; Giordano, A.; Gontero, P. Transurethral Resection of Bladder Tumor and the Need for Re-Transurethral Resection of Bladder Tumor: Time to Change Our Practice? Curr. Opin. Urol. 2020, 30, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Naselli, A.; Hurle, R.; Paparella, S.; Buffi, N.M.; Lughezzani, G.; Lista, G.; Casale, P.; Saita, A.; Lazzeri, M.; Guazzoni, G. Role of Restaging Transurethral Resection for T1 Non–Muscle Invasive Bladder Cancer: A Systematic Review and Meta-Analysis. Eur. Urol. Focus 2018, 4, 558–567. [Google Scholar] [CrossRef]
- Ye, L.; Chen, Y.; Xu, H.; Xie, H.; Yao, J.; Liu, J.; Song, B. Biparametric Magnetic Resonance Imaging Assessment for Detection of Muscle-Invasive Bladder Cancer: A Systematic Review and Meta-Analysis. Eur. Radiol. 2022, 32, 6480–6492. [Google Scholar]
- Kozikowski, M.; Suarez-Ibarrola, R.; Osiecki, R.; Bilski, K.; Gratzke, C.; Shariat, S.F.; Miernik, A.; Dobruch, J. Role of Radiomics in the Prediction of Muscle-Invasive Bladder Cancer: A Systematic Review and Meta-Analysis. Eur. Urol. Focus 2022, 8, 728–738. [Google Scholar] [CrossRef]
- Smits, M. MRI Biomarkers in Neuro-Oncology. Nat. Rev. Neurol. 2021, 17, 486–500. [Google Scholar] [CrossRef]
- Van Baelen, K.; Geukens, T.; Maetens, M.; Tjan-Heijnen, V.; Lord, C.J.; Linn, S.; Bidard, F.-C.; Richard, F.; Yang, W.W.; Steele, R.E.; et al. Current and Future Diagnostic and Treatment Strategies for Patients with Invasive Lobular Breast Cancer. Ann. Oncol. 2022, 33, 769–785. [Google Scholar] [CrossRef]
- Roussel, E.; Capitanio, U.; Kutikov, A.; Oosterwijk, E.; Pedrosa, I.; Rowe, S.P.; Gorin, M.A. Novel Imaging Methods for Renal Mass Characterization: A Collaborative Review. Eur. Urol. 2022, 81, 476–488. [Google Scholar] [CrossRef] [PubMed]
- Selby, N.M.; Blankestijn, P.J.; Boor, P.; Combe, C.; Eckardt, K.-U.; Eikefjord, E.; Garcia-Fernandez, N.; Golay, X.; Gordon, I.; Grenier, N.; et al. Magnetic Resonance Imaging Biomarkers for Chronic Kidney Disease: A Position Paper from the European Cooperation in Science and Technology Action PARENCHIMA. Nephrol. Dial. Transplant. 2018, 33, ii4–ii14. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Aty, H.; Schulz-Menger, J. Cardiovascular Magnetic Resonance T2-Weighted Imaging of Myocardial Edema in Acute Myocardial Infarction. Recent. Pat. Cardiovasc. Drug Discov. 2007, 2, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, X.; Wang, J.; Wang, Y.; Qi, H.; Kong, X.; Liu, D.; Liu, J.; Zheng, H.; Xiong, F.; et al. Simultaneous T1, T2, and T2* Mapping of Carotid Plaque: The SIMPLE* Technique. Radiology 2023, 307, e222061. [Google Scholar] [CrossRef] [PubMed]
- Luetkens, J.A.; Klein, S.; Träber, F.; Schmeel, F.C.; Sprinkart, A.M.; Kuetting, D.L.R.; Block, W.; Uschner, F.E.; Schierwagen, R.; Hittatiya, K.; et al. Quantification of Liver Fibrosis at T1 and T2 Mapping with Extracellular Volume Fraction MRI: Preclinical Results. Radiology 2018, 288, 748–754. [Google Scholar] [CrossRef] [PubMed]
- Compérat, E.M.; Burger, M.; Gontero, P.; Mostafid, A.H.; Palou, J.; Rouprêt, M.; van Rhijn, B.W.G.; Shariat, S.F.; Sylvester, R.J.; Zigeuner, R.; et al. Grading of Urothelial Carcinoma and The New “World Health Organisation Classification of Tumours of the Urinary System and Male Genital Organs 2016”. Eur. Urol. Focus 2019, 5, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Xu, L.; Zhao, L.; Mao, L.; Li, X.; Jin, Z.; Sun, H. CT-Based Radiomics to Predict the Pathological Grade of Bladder Cancer. Eur. Radiol. 2020, 30, 6749–6756. [Google Scholar] [CrossRef] [PubMed]
- Hou, L.; Zhou, W.; Ren, J.; Du, X.; Xin, L.; Zhao, X.; Cui, Y.; Zhang, R. Radiomics Analysis of Multiparametric MRI for the Preoperative Prediction of Lymph Node Metastasis in Cervical Cancer. Front. Oncol. 2020, 10, 1393. [Google Scholar] [CrossRef] [PubMed]
- Sathiadoss, P.; Schieda, N.; Haroon, M.; Osman, H.; Alrasheed, S.; Flood, T.A.; Melkus, G. Utility of Quantitative T2-Mapping Compared to Conventional and Advanced Diffusion Weighted Imaging Techniques for Multiparametric Prostate MRI in Men with Hip Prosthesis. J. Magn. Reson. Imaging 2022, 55, 265–274. [Google Scholar] [CrossRef]
- Adams, L.C.; Bressem, K.K.; Jurmeister, P.; Fahlenkamp, U.L.; Ralla, B.; Engel, G.; Hamm, B.; Busch, J.; Makowski, M.R. Use of Quantitative T2 Mapping for the Assessment of Renal Cell Carcinomas: First Results. Cancer Imaging 2019, 19, 35. [Google Scholar] [CrossRef]
- Gmeiner, J.; Garstka, N.; Helbich, T.H.; Shariat, S.F.; Baltzer, P.A. Vesical Imaging Reporting and Data System (VI-RADS): Are the Individual MRI Sequences Equivalent in Diagnostic Performance of High Grade NMIBC and MIBC? Eur. J. Radiol. 2021, 142, 109829. [Google Scholar] [CrossRef] [PubMed]
- Arita, Y.; Yoshida, S.; Shigeta, K.; Kwee, T.C.; Edo, H.; Okawara, N.; Hashimoto, M.; Ishii, R.; Ueda, R.; Mikami, S.; et al. Diagnostic Value of the Vesical Imaging-Reporting and Data System in Bladder Urothelial Carcinoma with Variant Histology. Eur. Urol. Oncol. 2023, 6, 99–102. [Google Scholar] [CrossRef] [PubMed]
- Lobo, N.; Shariat, S.F.; Guo, C.C.; Fernandez, M.I.; Kassouf, W.; Choudhury, A.; Gao, J.; Williams, S.B.; Galsky, M.D.; Taylor, J.A.; et al. What Is the Significance of Variant Histology in Urothelial Carcinoma? Eur. Urol. Focus 2020, 6, 653–663. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xu, X.; Tian, Q.; Li, B.; Wu, Y.; Yang, Z.; Liang, Z.; Liu, Y.; Cui, G.; Lu, H. Radiomics Assessment of Bladder Cancer Grade Using Texture Features from Diffusion-Weighted Imaging. J. Magn. Reson. Imaging JMRI 2017, 46, 1281–1288. [Google Scholar] [CrossRef] [PubMed]
- Meng, Z.; Guo, R.; Li, Y.; Guan, Y.; Wang, T.; Zhao, Y.; Sutton, B.; Li, Y.; Liang, Z.-P. Accelerating T2 Mapping of the Brain by Integrating Deep Learning Priors with Low-Rank and Sparse Modeling. Magn. Reson. Med. 2021, 85, 1455–1467. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Hu, S.; Wang, Z.; Guan, R.; Zhou, X.; Gao, Q.; Yan, G. Feasibility and Reproducibility of T2 Mapping and DWI for Identifying Malignant Lymph Nodes in Rectal Cancer. Eur. Radiol. 2021, 31, 3347–3354. [Google Scholar] [CrossRef] [PubMed]
- Kitajima, K.; Takahashi, S.; Ueno, Y.; Yoshikawa, T.; Ohno, Y.; Obara, M.; Miyake, H.; Fujisawa, M.; Sugimura, K. Clinical Utility of Apparent Diffusion Coefficient Values Obtained Using High B-Value When Diagnosing Prostate Cancer Using 3 Tesla MRI: Comparison between Ultra-High b-Value (2000 s/Mm2) and Standard High b-Value (1000 s/Mm2). J. Magn. Reson. Imaging 2012, 36, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Heidemeier, A.; Thurner, A.; Metz, C.; Pabst, T.; Heidemeier, H.; Rasche, L.; Kortüm, K.M.; Einsele, H.; Grimm, R.; Weiland, E.; et al. Whole-Body MRI with an Ultrahigh b-Value of 2000 s/Mm2 Improves the Specificity of Diffusion-Weighted Imaging in Patients with Plasma Cell Dyscrasias. Acad Radiol 2022, 29, e1–e8. [Google Scholar] [CrossRef]
- Cieszanowski, A.; Anysz-Grodzicka, A.; Szeszkowski, W.; Kaczynski, B.; Maj, E.; Gornicka, B.; Grodzicki, M.; Grudzinski, I.P.; Stadnik, A.; Krawczyk, M.; et al. Characterization of Focal Liver Lesions Using Quantitative Techniques: Comparison of Apparent Diffusion Coefficient Values and T2 Relaxation Times. Eur. Radiol. 2012, 22, 2514–2524. [Google Scholar] [CrossRef]
- van Rhijn, B.W.G.; Hentschel, A.E.; Bründl, J.; Compérat, E.M.; Hernández, V.; Čapoun, O.; Bruins, H.M.; Cohen, D.; Rouprêt, M.; Shariat, S.F.; et al. Prognostic Value of the WHO1973 and WHO2004/2016 Classification Systems for Grade in Primary Ta/T1 Non-Muscle-Invasive Bladder Cancer: A Multicenter European Association of Urology Non-Muscle-Invasive Bladder Cancer Guidelines Panel Study. Eur. Urol. Oncol. 2021, 4, 182–191. [Google Scholar] [CrossRef]
| Variables | Training Group (n = 64) | Testing Group (n = 28) | p |
|---|---|---|---|
| Age (years, mean ± SD) | 66.1 ± 11.2 | 67.3 ± 12.0 | 0.264 |
| Gender | 0.505 | ||
| Male | 54 (15.6) | 23 (82.1) | |
| Female | 10 (84.4) | 5 (17.9) | |
| Number of lesion | 0.332 | ||
| Single | 39 (60.9) | 15 (53.6) | |
| Multiple | 25 (39.1) | 13 (46.4) | |
| Tumor size (mm, mean ± SD) | 26.5 ± 15.0 | 30.6 ± 17.6 | 0.663 |
| Stage | 0.475 | ||
| <T2 | 39 (60.9) | 18 (64.3) | |
| ≥T2 | 25 (39.1) | 10 (35.7) | |
| Histologic grade | 0.810 | ||
| High | 45 (70.3) | 18 (64.3) | |
| Low | 19 (29.7) | 10 (35.7) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, L.; Wang, Y.; Xiang, W.; Yao, J.; Liu, J.; Song, B. Radiomic Analysis of Quantitative T2 Mapping and Conventional MRI in Predicting Histologic Grade of Bladder Cancer. J. Clin. Med. 2023, 12, 5900. https://doi.org/10.3390/jcm12185900
Ye L, Wang Y, Xiang W, Yao J, Liu J, Song B. Radiomic Analysis of Quantitative T2 Mapping and Conventional MRI in Predicting Histologic Grade of Bladder Cancer. Journal of Clinical Medicine. 2023; 12(18):5900. https://doi.org/10.3390/jcm12185900
Chicago/Turabian StyleYe, Lei, Yayi Wang, Wanxin Xiang, Jin Yao, Jiaming Liu, and Bin Song. 2023. "Radiomic Analysis of Quantitative T2 Mapping and Conventional MRI in Predicting Histologic Grade of Bladder Cancer" Journal of Clinical Medicine 12, no. 18: 5900. https://doi.org/10.3390/jcm12185900





