REM-OSA as a Tool to Understand Both the Architecture of Sleep and Pathogenesis of Sleep Apnea—Literature Review
Abstract
:1. Introduction
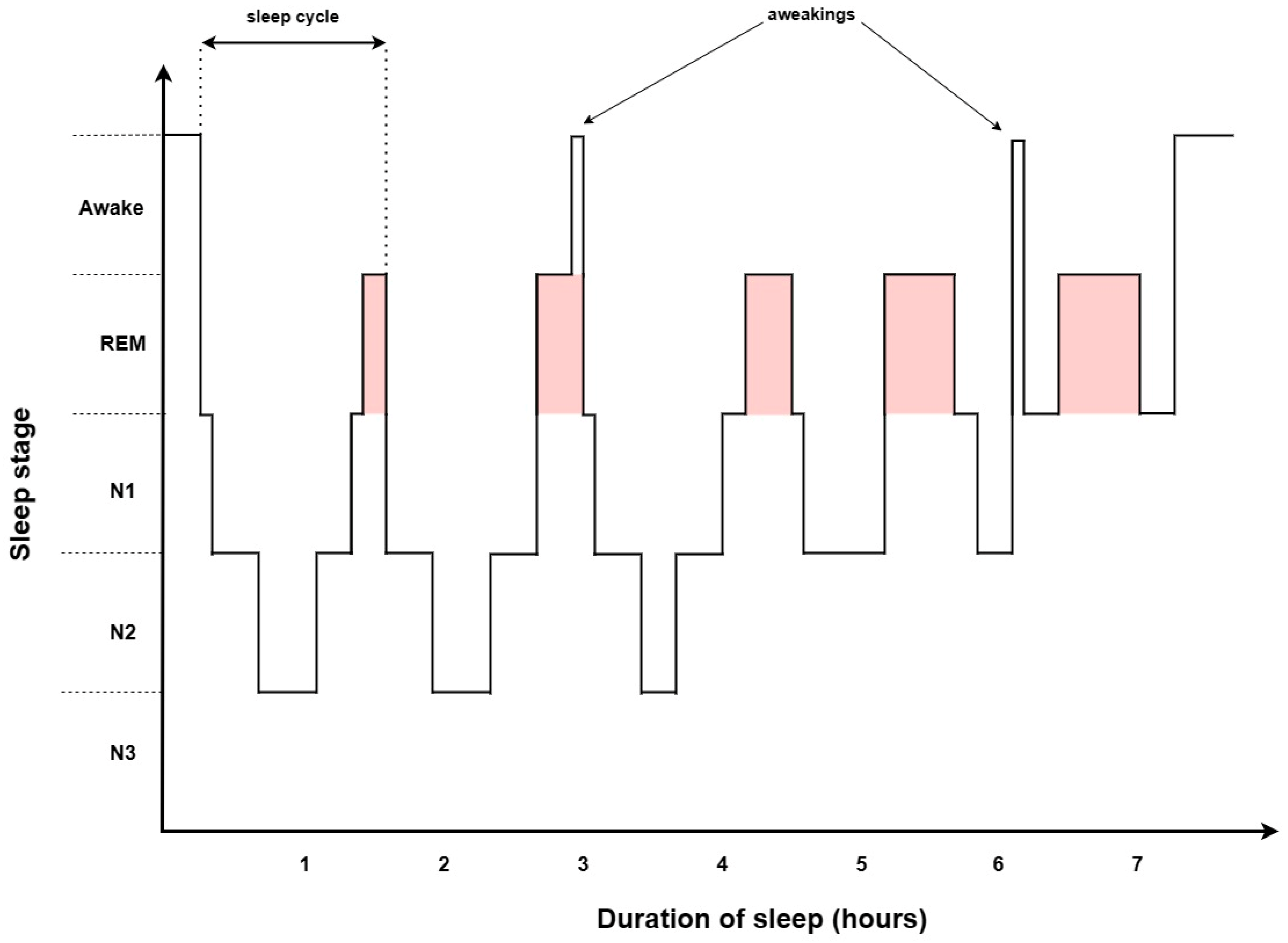
2. Sleep Architecture
2.1. NREM
2.2. REM
2.3. Polysomnography
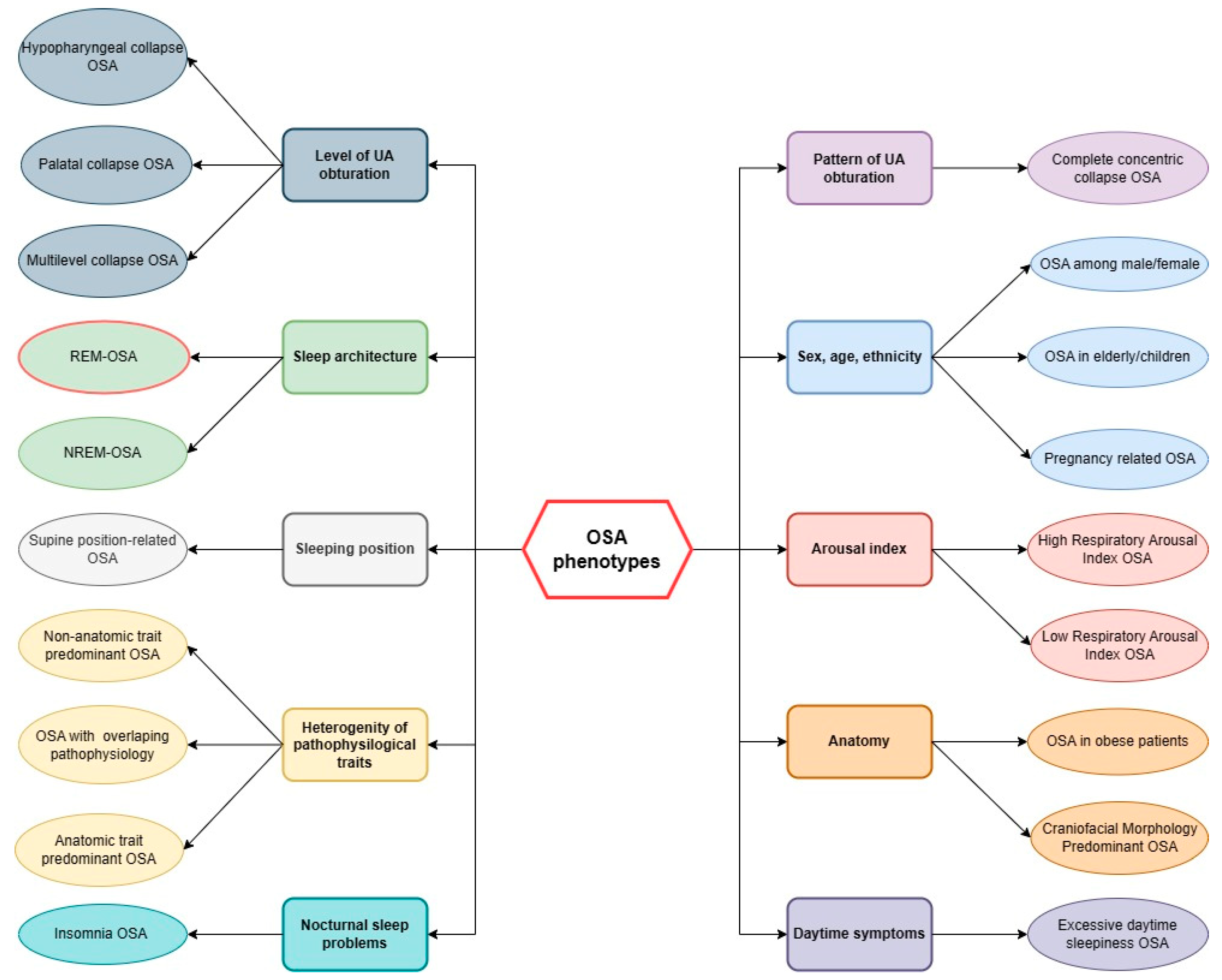
3. REM-OSA Phenotype
4. Non-Stage Specific OSA Phenotype
5. Clinical Significance of REM-OSA Phenotype
6. Treatment Adjustments in REM-OSA Phenotype
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Robbins, R.; Affouf, M.; Seixas, A.; Beaugris, L.; Avirappattu, G.; Jean-Louis, G. Four-Year Trends in Sleep Duration and Quality: A Longitudinal Study Using Data from a Commercially Available Sleep Tracker. J. Med. Internet Res. 2020, 22, e14735. [Google Scholar] [CrossRef]
- Svensson, T.; Inoue, M.; Saito, E.; Sawada, N.; Iso, H.; Mizoue, T.; Goto, A.; Yamaji, T.; Shimazu, T.; Iwasaki, M.; et al. The Association Between Habitual Sleep Duration and Mortality According to Sex and Age: The Japan Public Health Center-Based Prospective Study. J. Epidemiol. 2021, 31, 109–118. [Google Scholar] [CrossRef]
- Garfield, V. The Association Between Body Mass Index (BMI) and Sleep Duration: Where Are We after Nearly Two Decades of Epidemiological Research? Int. J. Environ. Res. Public Health 2019, 16, 4327. [Google Scholar] [CrossRef]
- Besedovsky, L.; Lange, T.; Haack, M. The Sleep-Immune Crosstalk in Health and Disease. Physiol. Rev. 2019, 99, 1325–1380. [Google Scholar] [CrossRef]
- Kocevska, D.; Barclay, N.L.; Bramer, W.M.; Gehrman, P.R.; Van Someren, E.J.W. Heritability of Sleep Duration and Quality: A Systematic Review and Meta-Analysis. Sleep Med. Rev. 2021, 59, 101448. [Google Scholar] [CrossRef]
- Schwartz, W.J.; Klerman, E.B. Circadian Neurobiology and the Physiologic Regulation of Sleep and Wakefulness. Neurol. Clin. 2019, 37, 475–486. [Google Scholar] [CrossRef]
- Medic, G.; Wille, M.; Hemels, M.E.H. Short- and Long-Term Health Consequences of Sleep Disruption. Nat. Sci. Sleep 2017, 9, 151–161. [Google Scholar] [CrossRef]
- Cowie, M.R. Sleep Apnea: State of the Art. Trends Cardiovasc. Med. 2017, 27, 280–289. [Google Scholar] [CrossRef]
- Malhotra, A.; Ayappa, I.; Ayas, N.; Collop, N.; Kirsch, D.; Mcardle, N.; Mehra, R.; Pack, A.I.; Punjabi, N.; White, D.P.; et al. Metrics of Sleep Apnea Severity: Beyond the Apnea-Hypopnea Index. Sleep 2021, 44, zsab030. [Google Scholar] [CrossRef]
- Lechat, B.; Naik, G.; Reynolds, A.; Aishah, A.; Scott, H.; Loffler, K.A.; Vakulin, A.; Escourrou, P.; McEvoy, R.D.; Adams, R.J.; et al. Multinight Prevalence, Variability, and Diagnostic Misclassification of Obstructive Sleep Apnea. Am. J. Respir. Crit. Care Med. 2022, 205, 563–569. [Google Scholar] [CrossRef]
- Gabryelska, A.; Turkiewicz, S.; Karuga, F.F.; Sochal, M.; Strzelecki, D.; Białasiewicz, P. Disruption of Circadian Rhythm Genes in Obstructive Sleep Apnea Patients-Possible Mechanisms Involved and Clinical Implication. Int. J. Mol. Sci. 2022, 23, 709. [Google Scholar] [CrossRef] [PubMed]
- Young, T.; Skatrud, J.; Peppard, P.E. Risk Factors for Obstructive Sleep Apnea in Adults. JAMA 2004, 291, 2013–2016. [Google Scholar] [CrossRef] [PubMed]
- Iftikhar, I.H.; Bittencourt, L.; Youngstedt, S.D.; Ayas, N.; Cistulli, P.; Schwab, R.; Durkin, M.W.; Magalang, U.J. Comparative Efficacy of CPAP, MADs, Exercise-Training, and Dietary Weight Loss for Sleep Apnea: A Network Meta-Analysis. Sleep Med. 2017, 30, 7–14. [Google Scholar] [CrossRef]
- Randerath, W.; Verbraecken, J.; De Raaff, C.A.L.; Hedner, J.; Herkenrath, S.; Hohenhorst, W.; Jakob, T.; Marrone, O.; Marklund, M.; McNicholas, W.T.; et al. European Respiratory Society Guideline on Non-CPAP Therapies for Obstructive Sleep Apnoea. Eur. Respir. Rev. 2021, 30, 210200. [Google Scholar] [CrossRef]
- Gabryelska, A.; Chrzanowski, J.; Sochal, M.; Kaczmarski, P.; Turkiewicz, S.; Ditmer, M.; Karuga, F.F.; Czupryniak, L.; Białasiewicz, P. Nocturnal Oxygen Saturation Parameters as Independent Risk Factors for Type 2 Diabetes Mellitus among Obstructive Sleep Apnea Patients. J. Clin. Med. 2021, 10, 3770. [Google Scholar] [CrossRef]
- Gabryelska, A.; Karuga, F.F.; Szmyd, B.; Białasiewicz, P. HIF-1α as a Mediator of Insulin Resistance, T2DM, and Its Complications: Potential Links With Obstructive Sleep Apnea. Front. Physiol. 2020, 11, 540381. [Google Scholar] [CrossRef]
- Qie, R.; Zhang, D.; Liu, L.; Ren, Y.; Zhao, Y.; Liu, D.; Liu, F.; Chen, X.; Cheng, C.; Guo, C.; et al. Obstructive Sleep Apnea and Risk of Type 2 Diabetes Mellitus: A Systematic Review and Dose-Response Meta-Analysis of Cohort Studies. J. Diabetes 2020, 12, 455–464. [Google Scholar] [CrossRef]
- Xu, S.; Wan, Y.; Xu, M.; Ming, J.; Xing, Y.; An, F.; Ji, Q. The Association between Obstructive Sleep Apnea and Metabolic Syndrome: A Systematic Review and Meta-Analysis. BMC Pulm. Med. 2015, 15, 105. [Google Scholar] [CrossRef]
- Salari, N.; Khazaie, H.; Abolfathi, M.; Ghasemi, H.; Shabani, S.; Rasoulpoor, S.; Mohammadi, M.; Rasoulpoor, S.; Khaledi-Paveh, B. The Effect of Obstructive Sleep Apnea on the Increased Risk of Cardiovascular Disease: A Systematic Review and Meta-Analysis. Neurol. Sci. 2022, 43, 219–231. [Google Scholar] [CrossRef]
- Zhou, L.; Shan, X.; Peng, Y.; Liu, G.; Guo, W.; Luo, H.; Li, H.; Zong, D.; Ouyang, R. Reduced Regional Homogeneity and Neurocognitive Impairment in Patients with Moderate-to-Severe Obstructive Sleep Apnea. Sleep Med. 2020, 75, 418–427. [Google Scholar] [CrossRef]
- Zinchuk, A.; Yaggi, H.K. Phenotypic Subtypes of OSA: A Challenge and Opportunity for Precision Medicine. Chest 2020, 157, 403–420. [Google Scholar] [CrossRef] [PubMed]
- Vyazovskiy, V.V.; Delogu, A. NREM and REM Sleep: Complementary Roles in Recovery after Wakefulness. Neuroscientist 2014, 20, 203–219. [Google Scholar] [CrossRef] [PubMed]
- Collop, N.A.; Salas, R.E.; Delayo, M.; Gamaldo, C. Normal Sleep and Circadian Processes. Crit. Care Clin. 2008, 24, 449–460. [Google Scholar] [CrossRef]
- Hori, T.; Sugita, Y.; Koga, E.; Shirakawa, S.; Inoue, K.; Uchida, S.; Kuwahara, H.; Kousaka, M.; Kobayashi, T.; Tsuji, Y.; et al. Proposed Supplements and Amendments to ‘A Manual of Standardized Terminology, Techniques and Scoring System for Sleep Stages of Human Subjects’, the Rechtschaffen & Kales (1968) Standard. Psychiatry Clin. Neurosci. 2001, 55, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Berry, R.B.; Brooks, R.; Gamaldo, C.E.; Harding, S.M.; Lloyd, R.M.; Marcus, C.L.; Vaughn, B.V. AASM|Scoring Manual Version 2.2 The AASM Manual for the Scoring of Sleep and Associated Events Rules, Terminology and Technical Specifications Version 2.2. 2015. Available online: https://learn.aasm.org/Listing/a1341000002XmQtAAK (accessed on 1 May 2023).
- Patel, A.K.; Reddy, V.; Shumway, K.R.; Araujo, J.F. Physiology, Sleep Stages. In StatPearls; StatPearls Publishing: St. Petersburg, FL, USA, 2022. [Google Scholar]
- Baker, F.C.; Willoughby, A.R.; De Zambotti, M.; Franzen, P.L.; Prouty, D.; Javitz, H.; Hasler, B.; Clark, D.B.; Colrain, I.M. Age-Related Differences in Sleep Architecture and Electroencephalogram in Adolescents in the National Consortium on Alcohol and Neurodevelopment in Adolescence Sample. Sleep 2016, 39, 1429–1439. [Google Scholar] [CrossRef]
- Gaudreau, H.; Carrier, J.; Montplaisir, J. Age-Related Modifications of NREM Sleep EEG: From Childhood to Middle Age. J. Sleep Res. 2001, 10, 165–172. [Google Scholar] [CrossRef]
- Guilleminault, C.; Do Kim, Y.; Chowdhuri, S.; Horita, M.; Ohayon, M.; Kushida, C. Sleep and Daytime Sleepiness in Upper Airway Resistance Syndrome Compared to Obstructive Sleep Apnoea Syndrome. Eur. Respir. J. 2001, 17, 838–847. [Google Scholar] [CrossRef]
- Vakulin, A.; D’Rozario, A.; Kim, J.W.; Watson, B.; Cross, N.; Wang, D.; Coeytaux, A.; Bartlett, D.; Wong, K.; Grunstein, R. Quantitative Sleep EEG and Polysomnographic Predictors of Driving Simulator Performance in Obstructive Sleep Apnea. Clin. Neurophysiol. 2016, 127, 1428–1435. [Google Scholar] [CrossRef]
- Appleton, S.L.; Vakulin, A.; D’Rozario, A.; Vincent, A.D.; Teare, A.; Martin, S.A.; Wittert, G.A.; McEvoy, R.D.; Catcheside, P.G.; Adams, R.J. Quantitative Electroencephalography Measures in Rapid Eye Movement and Nonrapid Eye Movement Sleep Are Associated with Apnea-Hypopnea Index and Nocturnal Hypoxemia in Men. Sleep 2019, 42, zsz092. [Google Scholar] [CrossRef]
- Carlson, D.M.; Önal, E.; Carley, D.W.; Lopata, M.; Basner, R.C.; Rood, A. Palatal Muscle Electromyogram Activity in Obstructive Sleep Apnea. Am. J. Respir. Crit. Care Med. 1995, 152, 1022–1027. [Google Scholar] [CrossRef]
- McSharry, D.G.; Saboisky, J.P.; DeYoung, P.; Matteis, P.; Jordan, A.S.; Trinder, J.; Smales, E.; Hess, L.; Guo, M.; Malhotra, A. A Mechanism for Upper Airway Stability during Slow Wave Sleep. Sleep 2013, 36, 555–563. [Google Scholar] [CrossRef] [PubMed]
- Berry, R.B.; Bonnet, M.H.; Light, R.W. Effect of Ethanol on the Arousal Response to Airway Occlusion during Sleep in Normal Subjects. Am. Rev. Respir. Dis. 1992, 145, 445–452. [Google Scholar] [CrossRef]
- Subramanian, S.; Hesselbacher, S.; Mattewal, A.; Surani, S. Gender and Age Influence the Effects of Slow-Wave Sleep on Respiration in Patients with Obstructive Sleep Apnea. Sleep Breath. 2013, 17, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Parekh, A.; Mullins, A.E.; Kam, K.; Varga, A.W.; Rapoport, D.M.; Ayappa, I. Slow-Wave Activity Surrounding Stage N2 K-Complexes and Daytime Function Measured by Psychomotor Vigilance Test in Obstructive Sleep Apnea. Sleep 2019, 42, zsy256. [Google Scholar] [CrossRef]
- Krieger, J.; Maglasiu, N.; Sforza, E.; Kurtz, D. Breathing during Sleep in Normal Middle-Aged Subjects. Sleep 1990, 13, 143–154. [Google Scholar] [CrossRef]
- Rostig, S.; Kantelhardt, J.W.; Penzel, T.; Cassel, W.; Peter, J.H.; Vogelmeier, C.; Becker, H.F.; Jerrentrup, A. Nonrandom Variability of Respiration during Sleep in Healthy Humans. Sleep 2005, 28, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Yokoba, M.; Hawes, H.G.; Kieser, T.M.; Katagiri, M.; Easton, P.A. Parasternal Intercostal and Diaphragm Function during Sleep. J. Appl. Physiol. 2016, 121, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Gaiduk, M.; Perea, J.J.; Seepold, R.; Madrid, N.M.; Penzel, T.; Glos, M.; Ortega, J.A. Estimation of Sleep Stages Analyzing Respiratory and Movement Signals. IEEE J. Biomed. Health Inform. 2022, 26, 505–514. [Google Scholar] [CrossRef] [PubMed]
- Long, X.; Yang, J.; Weysen, T.; Haakma, R.; Foussier, J.; Fonseca, P.; Aarts, R.M. Measuring Dissimilarity between Respiratory Effort Signals Based on Uniform Scaling for Sleep Staging. Physiol. Meas. 2014, 35, 2529–2542. [Google Scholar] [CrossRef] [PubMed]
- Long, X.; Foussier, J.; Fonseca, P.; Haakma, R.; Aarts, R.M. Respiration Amplitude Analysis for REM and NREM Sleep Classification. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2013, 2013, 5017–5020. [Google Scholar] [CrossRef]
- Campana, L.M.; Owens, R.L.; Butler, J.P.; Suki, B.; Malhotra, A. Variability of Respiratory Mechanics during Sleep in Overweight and Obese Subjects with and without Asthma. Respir. Physiol. Neurobiol. 2013, 186, 290–295. [Google Scholar] [CrossRef]
- Hicks, A.; Cori, J.M.; Jordan, A.S.; Nicholas, C.L.; Kubin, L.; Semmler, J.G.; Malhotra, A.; McSharry, D.G.P.; Trinder, J.A. Mechanisms of the Deep, Slow-Wave, Sleep-Related Increase of Upper Airway Muscle Tone in Healthy Humans. J. Appl. Physiol. 2017, 122, 1304–1312. [Google Scholar] [CrossRef]
- Rowley, J.A.; Williams, B.C.; Smith, P.L.; Schwartz, A.R. Neuromuscular Activity and Upper Airway Collapsibility. Mechanisms of Action in the Decerebrate Cat. Am. J. Respir. Crit. Care Med. 1997, 156, 515–521. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, A.R.; Thut, D.C.; Brower, R.G.; Gauda, E.B.; Roach, D.; Permutt, S.; Smith, P.L. Modulation of Maximal Inspiratory Airflow by Neuromuscular Activity: Effect of CO2. J. Appl. Physiol. 1993, 74, 1597–1605. [Google Scholar] [CrossRef] [PubMed]
- Pillar, G.; Malhotra, A.; Fogel, R.B.; Beauregard, J.; Slamowitz, D.I.; Shea, S.A.; White, D.P. Upper Airway Muscle Responsiveness to Rising PCO(2) during NREM Sleep. J. Appl. Physiol. 2000, 89, 1275–1282. [Google Scholar] [CrossRef]
- Malhotra, A.; Pillar, G.; Fogel, R.B.; Beauregard, J.; Edwards, J.K.; Slamowitz, D.I.; Shea, S.A.; White, D.P. Genioglossal but Not Palatal Muscle Activity Relates Closely to Pharyngeal Pressure. Am. J. Respir. Crit. Care Med. 2000, 162, 1058–1062. [Google Scholar] [CrossRef]
- Bangash, M.F.; Xie, A.; Skatrud, J.B.; Reichmuth, K.J.; Barczi, S.R.; Morgan, B.J. Cerebrovascular Response to Arousal from NREM and REM Sleep. Sleep 2008, 31, 321–327. [Google Scholar] [CrossRef]
- Zhou, X.S.; Shahabuddin, S.; Zahn, B.R.; Babcock, M.A.; Badr, M.S. Effect of Gender on the Development of Hypocapnic Apnea/Hypopnea during NREM Sleep. J. Appl. Physiol. 2000, 89, 192–199. [Google Scholar] [CrossRef]
- Pillar, G.; Malhotra, A.; Fogel, R.; Beauregard, J.; Schnall, R.; White, D.P. Airway Mechanics and Ventilation in Response to Resistive Loading during Sleep: Influence of Gender. Am. J. Respir. Crit. Care Med. 2000, 162, 1627–1632. [Google Scholar] [CrossRef]
- Chowdhuri, S.; Bascom, A.; Mohan, D.; Diamond, M.P.; Badr, M.S. Testosterone Conversion Blockade Increases Breathing Stability in Healthy Men during NREM Sleep. Sleep 2013, 36, 1793–1798. [Google Scholar] [CrossRef]
- Zhou, X.S.; Rowley, J.A.; Demirovic, F.; Diamond, M.P.; Badr, M.S. Effect of Testosterone on the Apneic Threshold in Women during NREM Sleep. J. Appl. Physiol. 2003, 94, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Morselli, L.L.; Temple, K.A.; Leproult, R.; Ehrmann, D.A.; Van Cauter, E.; Mokhlesi, B. Determinants of Slow-Wave Activity in Overweight and Obese Adults: Roles of Sex, Obstructive Sleep Apnea and Testosterone Levels. Front. Endocrinol. 2018, 9, 377. [Google Scholar] [CrossRef] [PubMed]
- Rowley, J.A.; Sanders, C.S.; Zahn, B.R.; Badr, M.S. Gender Differences in Upper Airway Compliance during NREM Sleep: Role of Neck Circumference. J. Appl. Physiol. 2002, 92, 2535–2541. [Google Scholar] [CrossRef]
- Rowley, J.A.; Zhou, X.S.; Diamond, M.P.; Badr, M.S. The Determinants of the Apnea Threshold during NREM Sleep in Normal Subjects. Sleep 2006, 29, 95–103. [Google Scholar] [CrossRef]
- Chowdhuri, S.; Pranathiageswaran, S.; Loomis-King, H.; Salloum, A.; Badr, M.S. Aging Is Associated with Increased Propensity for Central Apnea during NREM Sleep. J. Appl. Physiol. 2018, 124, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Chowdhuri, S.; Pranathiageswaran, S.; Franco-Elizondo, R.; Jayakar, A.; Hosni, A.; Nair, A.; Badr, M.S. Effect of Age on Long-Term Facilitation and Chemosensitivity during NREM Sleep. J. Appl. Physiol. 2015, 119, 1088–1096. [Google Scholar] [CrossRef]
- Yön, M.İ.; Köktürk, O. Is NREM-Predominant Obstructive Sleep Apnea Syndrome a Different Clinical Entity? Turk. J. Med. Sci. 2018, 48, 967–972. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Lahan, V.; Sindhwani, G. Sleep-Stage-Independent Obstructive Sleep Apnea: An Unidentified Group? Neurol. Sci. 2013, 34, 1543–1550. [Google Scholar] [CrossRef]
- Yamauchi, M.; Fujita, Y.; Kumamoto, M.; Yoshikawa, M.; Ohnishi, Y.; Nakano, H.; Strohl, K.P.; Kimura, H. Nonrapid Eye Movement-Predominant Obstructive Sleep Apnea: Detection and Mechanism. J. Clin. Sleep Med. 2015, 11, 987–993. [Google Scholar] [CrossRef]
- Al Oweidat, K.; Alryalat, S.A.; Al-Essa, M.; Obeidat, N. Comparing REM- and NREM-Related Obstructive Sleep Apnea in Jordan: A Cross-Sectional Study. Can. Respir. J. 2018, 2018, 9270329. [Google Scholar] [CrossRef]
- Joosten, S.A.; Landry, S.A.; Wong, A.M.; Mann, D.L.; Terrill, P.I.; Sands, S.A.; Turton, A.; Beatty, C.; Thomson, L.; Hamilton, G.S.; et al. Assessing the Physiologic Endotypes Responsible for REM- and NREM-Based OSA. Chest 2021, 159, 1998–2007. [Google Scholar] [CrossRef]
- Liu, Y.; Su, C.; Liu, R.; Lei, G.; Zhang, W.; Yang, T.; Miao, J.; Li, Z. NREM-AHI Greater than REM-AHI versus REM-AHI Greater than NREM-AHI in Patients with Obstructive Sleep Apnea: Clinical and Polysomnographic Features. Sleep Breath. 2011, 15, 463–470. [Google Scholar] [CrossRef]
- Bonnet, M.H.; Arand, D.L. Heart Rate Variability: Sleep Stage, Time of Night, and Arousal Influences. Electroencephalogr. Clin. Neurophysiol. 1997, 102, 390–396. [Google Scholar] [CrossRef]
- Somers, V.K.; Dyken, M.E.; Mark, A.L.; Abboud, F.M. Sympathetic-Nerve Activity during Sleep in Normal Subjects. N. Engl. J. Med. 1993, 328, 303–307. [Google Scholar] [CrossRef]
- Burgess, H.J.; Holmes, A.L.; Dawson, D. The Relationship between Slow-Wave Activity, Body Temperature, and Cardiac Activity during Nighttime Sleep. Sleep 2001, 24, 343–349. [Google Scholar] [CrossRef]
- Mikutta, C.; Wenke, M.; Spiegelhalder, K.; Hertenstein, E.; Maier, J.G.; Schneider, C.L.; Fehér, K.; Koenig, J.; Altorfer, A.; Riemann, D.; et al. Co-Ordination of Brain and Heart Oscillations during Non-Rapid Eye Movement Sleep. J. Sleep Res. 2022, 31, e13466. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.C.; Sattari, N.; Whitehurst, L.N.; Mednick, S.C. Age-Related Losses in Cardiac Autonomic Activity during a Daytime Nap. Psychophysiology 2021, 58, e13701. [Google Scholar] [CrossRef] [PubMed]
- Monti, A.; Medigue, C.; Nedelcoux, H.; Escourrou, P. Autonomic Control of the Cardiovascular System during Sleep in Normal Subjects. Eur. J. Appl. Physiol. 2002, 87, 174–181. [Google Scholar] [CrossRef]
- Letzen, J.E.; Robinson, M.L.; Saletin, J.M.; Sheinberg, R.B.; Smith, M.T.; Campbell, C.M. Racial Disparities in Sleep-Related Cardiac Function in Young, Healthy Adults: Implications for Cardiovascular-Related Health. Sleep 2021, 44, zsab164. [Google Scholar] [CrossRef]
- Hall, M.H.; Middleton, K.; Thayer, J.F.; Lewis, T.T.; Kline, C.E.; Matthews, K.A.; Kravitz, H.M.; Krafty, R.T.; Buysse, D.J. Racial Differences in Heart Rate Variability during Sleep in Women: The Study of Women across the Nation Sleep Study. Psychosom. Med. 2013, 75, 783–790. [Google Scholar] [CrossRef] [PubMed]
- Morgan, B.J.; Crabtree, D.C.; Puleo, D.S.; Badr, M.S.; Toiber, F.; Skatrud, J.B. Neurocirculatory Consequences of Abrupt Change in Sleep State in Humans. J. Appl. Physiol. 1996, 80, 1627–1636. [Google Scholar] [CrossRef]
- Meadows, G.E.; Dunroy, H.M.A.; Morrell, M.J.; Corfield, D.R. Hypercapnic Cerebral Vascular Reactivity Is Decreased, in Humans, during Sleep Compared with Wakefulness. J. Appl. Physiol. 2003, 94, 2197–2202. [Google Scholar] [CrossRef]
- Alqatari, A.A.; Alturki, J.A.; Abdulali, K.A.; Alhumud, D.A.; Alibrahim, M.A.; Alarab, Y.A.; Salem, A.M.; Yar, T.; Alqurashi, Y.D.; Alsunni, A.A.; et al. Changes in Heart Rate Variability and Baroreflex Sensitivity during Daytime Naps. Nat. Sci. Sleep 2020, 12, 661–669. [Google Scholar] [CrossRef]
- Cellini, N.; Whitehurst, L.N.; Mcdevitt, E.A.; Mednick, S.C. Heart Rate Variability during Daytime Naps in Healthy Adults: Autonomic Profile and Short-Term Reliability. Psychophysiology 2016, 53, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Stoohs, R.; Guilleminault, C. Cardiovascular Changes Associated with Obstructive Sleep Apnea Syndrome. J. Appl. Physiol. 1992, 72, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, B.Y.; Cao, J.; Guo, M.N.; Dong, L.X. The Relationship between Arousal and Nocturnal Heart Rate Variability in Patients with Obstructive Sleep Apnea-Hypopnea Syndrome. Zhonghua Jie He He Hu Xi Za Zhi 2006, 29, 233–235. [Google Scholar] [PubMed]
- Bonsignore, M.R.; Romano, S.; Marrone, O.; Chiodi, M.; Bonsignore, G. Different Heart Rate Patterns in Obstructive Apneas during NREM Sleep. Sleep 1997, 20, 1167–1174. [Google Scholar] [CrossRef]
- Lofaso, F.; Goldenberg, F.; D’Ortho, M.P.; Coste, A.; Harf, A. Arterial Blood Pressure Response to Transient Arousals from NREM Sleep in Nonapneic Snorers with Sleep Fragmentation. Chest 1998, 113, 985–991. [Google Scholar] [CrossRef] [PubMed]
- Viigimae, M.; Karai, D.; Pilt, K.; Pirn, P.; Huhtala, H.; Polo, O.; Meigas, K.; Kaik, J. QT Interval Variability Index and QT Interval Duration during Different Sleep Stages in Patients with Obstructive Sleep Apnea. Sleep Med. 2017, 37, 160–167. [Google Scholar] [CrossRef]
- Trinder, J.; Kleiman, J.; Carrington, M.; Smith, S.; Breen, S.; Tan, N.; Kim, Y. Autonomic Activity during Human Sleep as a Function of Time and Sleep Stage. J. Sleep Res. 2001, 10, 253–264. [Google Scholar] [CrossRef]
- Cerri, M.; Amici, R. Thermoregulation and Sleep: Functional Interaction and Central Nervous Control. Compr. Physiol. 2021, 11, 1591–1604. [Google Scholar] [CrossRef] [PubMed]
- Nissen, C.; Piosczyk, H.; Holz, J.; Maier, J.G.; Frase, L.; Sterr, A.; Riemann, D.; Feige, B. Sleep Is More than Rest for Plasticity in the Human Cortex. Sleep 2021, 44, zsaa216. [Google Scholar] [CrossRef] [PubMed]
- Tasali, E.; Leproult, R.; Ehrmann, D.A.; Van Cauter, E. Slow-Wave Sleep and the Risk of Type 2 Diabetes in Humans. Proc. Natl. Acad. Sci. USA 2008, 105, 1044–1049. [Google Scholar] [CrossRef]
- Lanfranco, F.; Motta, G.; Minetto, M.A.; Ghigo, E.; Maccario, M. Growth Hormone/Insulin-like Growth Factor-I Axis in Obstructive Sleep Apnea Syndrome: An Update. J. Endocrinol. Investig. 2010, 33, 192–196. [Google Scholar] [CrossRef]
- Xu, H.; Xia, Y.; Li, X.; Qian, Y.; Zou, J.; Fang, F.; Yi, H.; Wu, H.; Guan, J.; Yin, S. Association between Obstructive Sleep Apnea and Lipid Metabolism during REM and NREM Sleep. J. Clin. Sleep Med. 2020, 16, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Aserinsky, E.; Kleitman, N. Regularly Occurring Periods of Eye Motility, and Concomitant Phenomena, during Sleep. Science 1953, 118, 273–274. [Google Scholar] [CrossRef]
- Berger, A.J. What Causes Muscle Atonia in REM? Sleep 2008, 31, 1477–1478. [Google Scholar] [CrossRef]
- Dijkstra, F.; Van den Bossche, K.; de Bruyn, B.; Reyn, N.; Viaene, M.; De Volder, I.; Cras, P.; Crosiers, D. REM Sleep without Atonia and the Relation with Lewy Body Disease. Parkinsonism Relat. Disord. 2019, 67, 90–98. [Google Scholar] [CrossRef]
- Simor, P.; van der Wijk, G.; Nobili, L.; Peigneux, P. The Microstructure of REM Sleep: Why Phasic and Tonic? Sleep Med. Rev. 2020, 52, 101305. [Google Scholar] [CrossRef]
- Pivik, R.T. Tonic States and Phasic Events in Relation to Sleep Mentation. In The Mind in Sleep: Psychology and Psychophysiology; John Wiley & Sons: Oxford, UK, 1991. [Google Scholar]
- Siclari, F.; Bernardi, G.; Cataldi, J.; Tononi, G. Dreaming in NREM Sleep: A High-Density EEG Study of Slow Waves and Spindles. J. Neurosci. 2018, 38, 9175–9185. [Google Scholar] [CrossRef]
- Jiang, F. Sleep and Early Brain Development. Ann. Nutr. Metab. 2019, 75 (Suppl. S1), 44–53. [Google Scholar] [CrossRef]
- Blumberg, M.S.; Coleman, C.M.; Gerth, A.I.; McMurray, B. Spatiotemporal Structure of REM Sleep Twitching Reveals Developmental Origins of Motor Synergies. Curr. Biol. 2013, 23, 2100–2109. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Ma, L.; Yang, G.; Gan, W.B. REM Sleep Selectively Prunes and Maintains New Synapses in Development and Learning. Nat. Neurosci. 2017, 20, 427. [Google Scholar] [CrossRef]
- Hobson, J.A.; Pace-Schott, E.F. The Cognitive Neuroscience of Sleep: Neuronal Systems, Consciousness and Learning. Nat. Rev. Neurosci. 2002, 3, 679–693. [Google Scholar] [CrossRef]
- Schmidt, M.H. The Energy Allocation Function of Sleep: A Unifying Theory of Sleep, Torpor, and Continuous Wakefulness. Neurosci. Biobehav. Rev. 2014, 47, 122–153. [Google Scholar] [CrossRef] [PubMed]
- Peever, J.; Fuller, P.M. The Biology of REM Sleep. Curr. Biol. 2016, 26, R34. [Google Scholar] [CrossRef]
- Markun, L.C.; Sampat, A. Clinician-Focused Overview and Developments in Polysomnography. Curr. Sleep Med. Rep. 2020, 6, 309–321. [Google Scholar] [CrossRef]
- Mokhlesi, B.; Punjabi, N.M. “REM-Related” Obstructive Sleep Apnea: An Epiphenomenon or a Clinically Important Entity? Sleep 2012, 35, 5–7. [Google Scholar] [CrossRef] [PubMed]
- Djonlagic, I.; Guo, M.; Igue, M.; Malhotra, A.; Stickgold, R. REM-Related Obstructive Sleep Apnea: When Does It Matter? Effect on Motor Memory Consolidation versus Emotional Health. J. Clin. Sleep Med. 2020, 16, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Maquet, P. Positron Emission Tomography Studies of Sleep and Sleep Disorders. J. Neurol. 1997, 244. [Google Scholar] [CrossRef]
- Hoshino, T.; Sasanabe, R.; Murotani, K.; Hori, R.; Mano, M.; Nomura, A.; Konishi, N.; Baku, M.; Arita, A.; Kuczynski, W.; et al. Insomnia as a Symptom of Rapid Eye Movement-Related Obstructive Sleep Apnea. J. Clin. Med. 2020, 9, 1821. [Google Scholar] [CrossRef]
- Devita, M.; Peppard, P.E.; Mesas, A.E.; Mondini, S.; Rusconi, M.L.; Barnet, J.H.; Hagen, E.W. Associations between the Apnea-Hypopnea Index during REM and NREM Sleep and Cognitive Functioning in a Cohort of Middle-Aged Adults. J. Clin. Sleep Med. 2019, 15, 965–971. [Google Scholar] [CrossRef] [PubMed]
- Mattewal, A.; Casturi, L.; Subramanian, S. A Child with REM Sleep Disturbance. J. Clin. Sleep Med. 2010, 6, 97. [Google Scholar] [CrossRef]
- Stores, G. Aspects of Sleep Disorders in Children and Adolescents. Dialogues Clin. Neurosci. 2009, 11, 81. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.C.; Au, C.T.; Yu, M.W.; Wing, Y.K.; Li, A.M. Natural History of REM-OSA in Children and Its Associations with Adverse Blood Pressure Outcomes: A Longitudinal Follow-Up Study. Nat. Sci. Sleep 2021, 13, 1967–1984. [Google Scholar] [CrossRef]
- Palmer, L.J.; Redline, S. Genomic Approaches to Understanding Obstructive Sleep Apnea. Respir. Physiol. Neurobiol. 2003, 135, 187–205. [Google Scholar] [CrossRef] [PubMed]
- Leader, B.A.; Koritala, B.S.C.; Moore, C.A.; Grigg Dean, E.H.; Kottyan, L.C.; Smith, D.F. Epigenetics of Obstructive Sleep Apnea Syndrome: A Systematic Review. J. Clin. Sleep Med. 2021, 17, 2533–2541. [Google Scholar] [CrossRef]
- Wang, A.; Wei, Z.; Yuan, H.; Zhu, Y.; Peng, Y.; Gao, Z.; Liu, Y.; Shen, J.; Xu, H.; Guan, J.; et al. FKBP5 Genetic Variants Are Associated with Respiratory- and Sleep-Related Parameters in Chinese Patients with Obstructive Sleep Apnea. Front. Neurosci. 2023, 17, 1170889. [Google Scholar] [CrossRef]
- Bartolucci, M.L.; Berteotti, C.; Alvente, S.; Bastianini, S.; Guidi, S.; Lo Martire, V.; Matteoli, G.; Silvani, A.; Stagni, F.; Bosi, M.; et al. Obstructive Sleep Apneas Naturally Occur in Mice during REM Sleep and Are Highly Prevalent in a Mouse Model of Down Syndrome. Neurobiol. Dis. 2021, 159, 105508. [Google Scholar] [CrossRef]
- Krohn, L.; Heilbron, K.; Blauwendraat, C.; Reynolds, R.H.; Yu, E.; Senkevich, K.; Rudakou, U.; Estiar, M.A.; Gustavsson, E.K.; Brolin, K.; et al. Genome-Wide Association Study of REM Sleep Behavior Disorder Identifies Polygenic Risk and Brain Expression Effects. Nat. Commun. 2022, 13, 7496. [Google Scholar] [CrossRef]
- Duce, B.; Kulkas, A.; Langton, C.; Töyräs, J.; Hukins, C. The Prevalence of REM-Related Obstructive Sleep Apnoea Is Reduced by the AASM 2012 Hypopnoea Criteria. Sleep Breath. 2018, 22, 57–64. [Google Scholar] [CrossRef]
- Chiu, H.Y.; Liu, Y.Y.; Shiao, T.H.; Su, K.C.; Chou, K.T.; Chen, Y.M. Clinical Characteristics of Rapid Eye Movement-Related Obstructive Sleep Apnea: An Experience in a Tertiary Medical Center of Taiwan. Nat. Sci. Sleep 2022, 14, 1521. [Google Scholar] [CrossRef] [PubMed]
- Subramani, Y.; Singh, M.; Wong, J.; Kushida, C.A.; Malhotra, A.; Chung, F. Understanding Phenotypes of Obstructive Sleep Apnea: Applications in Anesthesia, Surgery, and Perioperative Medicine. Anesth. Analg. 2017, 124, 179. [Google Scholar] [CrossRef]
- Mokhlesi, B.; Finn, L.A.; Hagen, E.W.; Young, T.; Hla, K.M.; Van Cauter, E.; Peppard, P.E. Obstructive Sleep Apnea during REM Sleep and Hypertension: Results of the Wisconsin Sleep Cohort. Am. J. Respir. Crit. Care Med. 2014, 190, 1158–1167. [Google Scholar] [CrossRef]
- Appleton, S.L.; Vakulin, A.; Martin, S.A.; Lang, C.J.; Wittert, G.A.; Taylor, A.W.; McEvoy, R.D.; Antic, N.A.; Catcheside, P.G.; Adams, R.J. Hypertension Is Associated with Undiagnosed OSA during Rapid Eye Movement Sleep. Chest 2016, 150, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Mansukhani, M.P.; Kara, T.; Caples, S.M.; Somers, V.K. Chemoreflexes, Sleep Apnea, and Sympathetic Dysregulation. Curr. Hypertens. Rep. 2014, 16, 476. [Google Scholar] [CrossRef] [PubMed]
- Grassi, G.; Seravalle, G.; Mancia, G. Sympathetic Activation in Cardiovascular Disease: Evidence, Clinical Impact and Therapeutic Implications. Eur. J. Clin. Investig. 2015, 45, 1367–1375. [Google Scholar] [CrossRef] [PubMed]
- Varga, A.W.; Mokhlesi, B. REM Obstructive Sleep Apnea: Risk for Adverse Health Outcomes and Novel Treatments. Sleep Breath. 2019, 23, 413. [Google Scholar] [CrossRef]
- Nagaoka, M.; Goda, A.; Takeuchi, K.; Kikuchi, H.; Finger, M.; Inami, T.; Soejima, K.; Satoh, T. Nocturnal Hypoxemia, But Not Sleep Apnea, Is Associated with a Poor Prognosis in Patients with Pulmonary Arterial Hypertension. Circ. J. 2018, 82, 3076–3081. [Google Scholar] [CrossRef]
- Martínez-García, M.Á.; Capote, F.; Campos-Rodríguez, F.; Lloberes, P.; Díaz De Atauri, M.J.; Somoza, M.; Masa, J.F.; González, M.; Sacristán, L.; Barbé, F.; et al. Effect of CPAP on Blood Pressure in Patients with Obstructive Sleep Apnea and Resistant Hypertension: The HIPARCO Randomized Clinical Trial. JAMA 2013, 310, 2407–2415. [Google Scholar] [CrossRef]
- Grimaldi, D.; Beccuti, G.; Touma, C.; Van Cauter, E.; Mokhlesi, B. Association of Obstructive Sleep Apnea in Rapid Eye Movement Sleep with Reduced Glycemic Control in Type 2 Diabetes: Therapeutic Implications. Diabetes Care 2014, 37, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Bialasiewicz, P.; Czupryniak, L.; Pawlowski, M.; Nowak, D. Sleep Disordered Breathing in REM Sleep Reverses the Downward Trend in Glucose Concentration. Sleep Med. 2011, 12, 76–82. [Google Scholar] [CrossRef]
- Chami, H.A.; Gottlieb, D.J.; Redline, S.; Punjabi, N.M. Association between Glucose Metabolism and Sleep-Disordered Breathing during REM Sleep. Am. J. Respir. Crit. Care Med. 2015, 192, 1118–1126. [Google Scholar] [CrossRef] [PubMed]
- Ljunggren, M.; Theorell-Haglöw, J.; Freyhult, E.; Sahlin, C.; Franklin, K.A.; Malinovschi, A.; Janson, C.; Lindberg, E. Association between Proteomics and Obstructive Sleep Apnea Phenotypes in a Community-Based Cohort of Women. J. Sleep Res. 2020, 29, e13041. [Google Scholar] [CrossRef] [PubMed]
- Lemos, V.; de Oliveira, R.M.; Naia, L.; Szegö, É.; Ramos, E.; Pinho, S.; Magro, F.; Cavadas, C.; Cristina Rego, A.; Costa, V.; et al. The NAD+-Dependent Deacetylase SIRT2 Attenuates Oxidative Stress and Mitochondrial Dysfunction and Improves Insulin Sensitivity in Hepatocytes. Hum. Mol. Genet. 2017, 26, 4105–4117. [Google Scholar] [CrossRef]
- Wang, F.; Tong, Q. SIRT2 Suppresses Adipocyte Differentiation by Deacetylating FOXO1 and Enhancing FOXO1’s Repressive Interaction with PPARγ. Mol. Biol. Cell. 2009, 20, 801–808. [Google Scholar] [CrossRef]
- Chami, H.A.; Baldwin, C.M.; Silverman, A.; Zhang, Y.; Rapoport, D.; Punjabi, N.M.; Gottlieb, D.J. Sleepiness, Quality of Life, and Sleep Maintenance in REM versus Non-REM Sleep-Disordered Breathing. Am. J. Respir. Crit. Care Med. 2012, 181, 997–1002. [Google Scholar] [CrossRef]
- Punjabi, N.M.; Bandeen-Roche, K.; Marx, J.J.; Neubauer, D.N.; Smith, P.L.; Schwartz, A.R. The Association between Daytime Sleepiness and Sleep-Disordered Breathing in NREM and REM Sleep. Sleep 2002, 25, 307–314. [Google Scholar] [CrossRef]
- Gabryelska, A.; Białasiewicz, P. Association between Excessive Daytime Sleepiness, REM Phenotype and Severity of Obstructive Sleep Apnea. Sci. Rep. 2020, 10, 34. [Google Scholar] [CrossRef]
- Smith, C.T.; Conway, J.M.; Rose, G.M. Brief Paradoxical Sleep Deprivation Impairs Reference, but Not Working, Memory in the Radial Arm Maze Task. Neurobiol. Learn Mem. 1998, 69, 211–217. [Google Scholar] [CrossRef]
- Varga, A.W.; Kishi, A.; Mantua, J.; Lim, J.; Koushyk, V.; Leibert, D.P.; Osorio, R.S.; Rapoport, D.M.; Ayappa, I. Apnea-Induced Rapid Eye Movement Sleep Disruption Impairs Human Spatial Navigational Memory. J. Neurosci. 2014, 34, 14571–14577. [Google Scholar] [CrossRef]
- Ackermann, S.; Rasch, B. Differential Effects of Non-REM and REM Sleep on Memory Consolidation? Curr. Neurol. Neurosci. Rep. 2014, 14, 430. [Google Scholar] [CrossRef] [PubMed]
- Igue, M.; Guo, M.; Malhotra, A.; Stickgold, R.; Djonlagic, I. Consequences of Rem-Predominant and Rem-Exclusive Sleep Apnea on Motor Memory Consolidation during Sleep. Sleep Med. 2013, 14, e107–e108. [Google Scholar] [CrossRef]
- Turan, I.; Sayan Ozacmak, H.; Ozacmak, V.H.; Ergenc, M.; Bayraktaroğlu, T. The Effects of Glucagon-like Peptide 1 Receptor Agonist (Exenatide) on Memory Impairment, and Anxiety- and Depression-like Behavior Induced by REM Sleep Deprivation. Brain Res. Bull. 2021, 174, 194–202. [Google Scholar] [CrossRef]
- Geckil, A.A.; Ermis, H. The Relationship between Anxiety, Depression, Daytime Sleepiness in the REM-Related Mild OSAS and the NREM-Related Mild OSAS. Sleep Breath. 2020, 24, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.A.; Paek, J.H.; Han, S.H. REM-Related Sleep-Disordered Breathing Is Associated with Depressive Symptoms in Men but Not in Women. Sleep Breath. 2016, 20, 995–1002. [Google Scholar] [CrossRef]
- Palagini, L.; Baglioni, C.; Ciapparelli, A.; Gemignani, A.; Riemann, D. REM Sleep Dysregulation in Depression: State of the Art. Sleep Med. Rev. 2013, 17, 377–390. [Google Scholar] [CrossRef]
- Marotta, A.M.; Borel, J.C.; Galerneau, L.M.; Tamisier, R.; Bonsignore, M.R.; Pépin, J.L. Cardiovascular Events in Moderately to Severely Obese Obstructive Sleep Apnea Patients on Positive Airway Pressure Therapy. Respiration 2017, 93, 179–188. [Google Scholar] [CrossRef]
- Ou, Q.; Chen, Y.C.; Zhuo, S.Q.; Tian, X.T.; He, C.H.; Lu, X.L.; Gao, X.L. Continuous Positive Airway Pressure Treatment Reduces Mortality in Elderly Patients with Moderate to Severe Obstructive Severe Sleep Apnea: A Cohort Study. PLoS ONE 2015, 10, e0127775. [Google Scholar] [CrossRef]
- López-Padilla, D.; Alonso-Moralejo, R.; Martínez-García, M.Á.; De la Torre Carazo, S.; Díaz de Atauri, M.J. Continuous Positive Airway Pressure and Survival of Very Elderly Persons with Moderate to Severe Obstructive Sleep Apnea. Sleep Med. 2016, 19, 23–29. [Google Scholar] [CrossRef]
- Aalaei, S.; Rezaeitalab, F.; Tabesh, H.; Amini, M.; Afsharisaleh, L.; Mostafavi, S.M.; Asadpour, H.; Eslami, S. Factors Affecting Patients’ Adherence to Continuous Positive Airway Pressure Therapy for Obstructive Sleep Apnea Disorder: A Multi-Method Approach. Iran. J. Med. Sci. 2020, 45, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Sawyer, A.M.; Gooneratne, N.S.; Marcus, C.L.; Ofer, D.; Richards, K.C.; Weaver, T.E. A Systematic Review of CPAP Adherence across Age Groups: Clinical and Empiric Insights for Developing CPAP Adherence Interventions. Sleep Med. Rev. 2011, 15, 343–356. [Google Scholar] [CrossRef] [PubMed]
- Berry, R.B.; Kryger, M.H.; Massie, C.A. A Novel Nasal Expiratory Positive Airway Pressure (EPAP) Device for the Treatment of Obstructive Sleep Apnea: A Randomized Controlled Trial. Sleep 2011, 34, 479–485. [Google Scholar] [CrossRef]
- Sutherland, K.; Takaya, H.; Qian, J.; Petocz, P.; Ng, A.T.; Cistulli, P.A. Oral Appliance Treatment Response and Polysomnographic Phenotypes of Obstructive Sleep Apnea. J. Clin. Sleep Med. 2015, 11, 861–868. [Google Scholar] [CrossRef] [PubMed]
- Tong, B.K.; Tran, C.; Ricciardiello, A.; Chiang, A.; Donegan, M.; Murray, N.; Szollosi, I.; Amatoury, J.; Carberry, J.C.; Eckert, D.J. Efficacy of a Novel Oral Appliance and the Role of Posture on Nasal Resistance in Obstructive Sleep Apnea. J. Clin. Sleep Med. 2020, 16, 483–492. [Google Scholar] [CrossRef]
- Strollo, P.J.; Soose, R.J.; Maurer, J.T.; de Vries, N.; Cornelius, J.; Froymovich, O.; Hanson, R.D.; Padhya, T.A.; Steward, D.L.; Gillespie, M.B.; et al. Upper-Airway Stimulation for Obstructive Sleep Apnea. N. Engl. J. Med. 2014, 370, 139–149. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karuga, F.F.; Kaczmarski, P.; Białasiewicz, P.; Szmyd, B.; Jaromirska, J.; Grzybowski, F.; Gebuza, P.; Sochal, M.; Gabryelska, A. REM-OSA as a Tool to Understand Both the Architecture of Sleep and Pathogenesis of Sleep Apnea—Literature Review. J. Clin. Med. 2023, 12, 5907. https://doi.org/10.3390/jcm12185907
Karuga FF, Kaczmarski P, Białasiewicz P, Szmyd B, Jaromirska J, Grzybowski F, Gebuza P, Sochal M, Gabryelska A. REM-OSA as a Tool to Understand Both the Architecture of Sleep and Pathogenesis of Sleep Apnea—Literature Review. Journal of Clinical Medicine. 2023; 12(18):5907. https://doi.org/10.3390/jcm12185907
Chicago/Turabian StyleKaruga, Filip Franciszek, Piotr Kaczmarski, Piotr Białasiewicz, Bartosz Szmyd, Julia Jaromirska, Filip Grzybowski, Piotr Gebuza, Marcin Sochal, and Agata Gabryelska. 2023. "REM-OSA as a Tool to Understand Both the Architecture of Sleep and Pathogenesis of Sleep Apnea—Literature Review" Journal of Clinical Medicine 12, no. 18: 5907. https://doi.org/10.3390/jcm12185907






