Prevalence of Taurodontism in Contemporary and Historical Populations from Radom: A Biometric Analysis of Radiological Data
Abstract
:1. Introduction
2. Materials and Methods
2.1. Contemporary Population
2.2. Historical Population
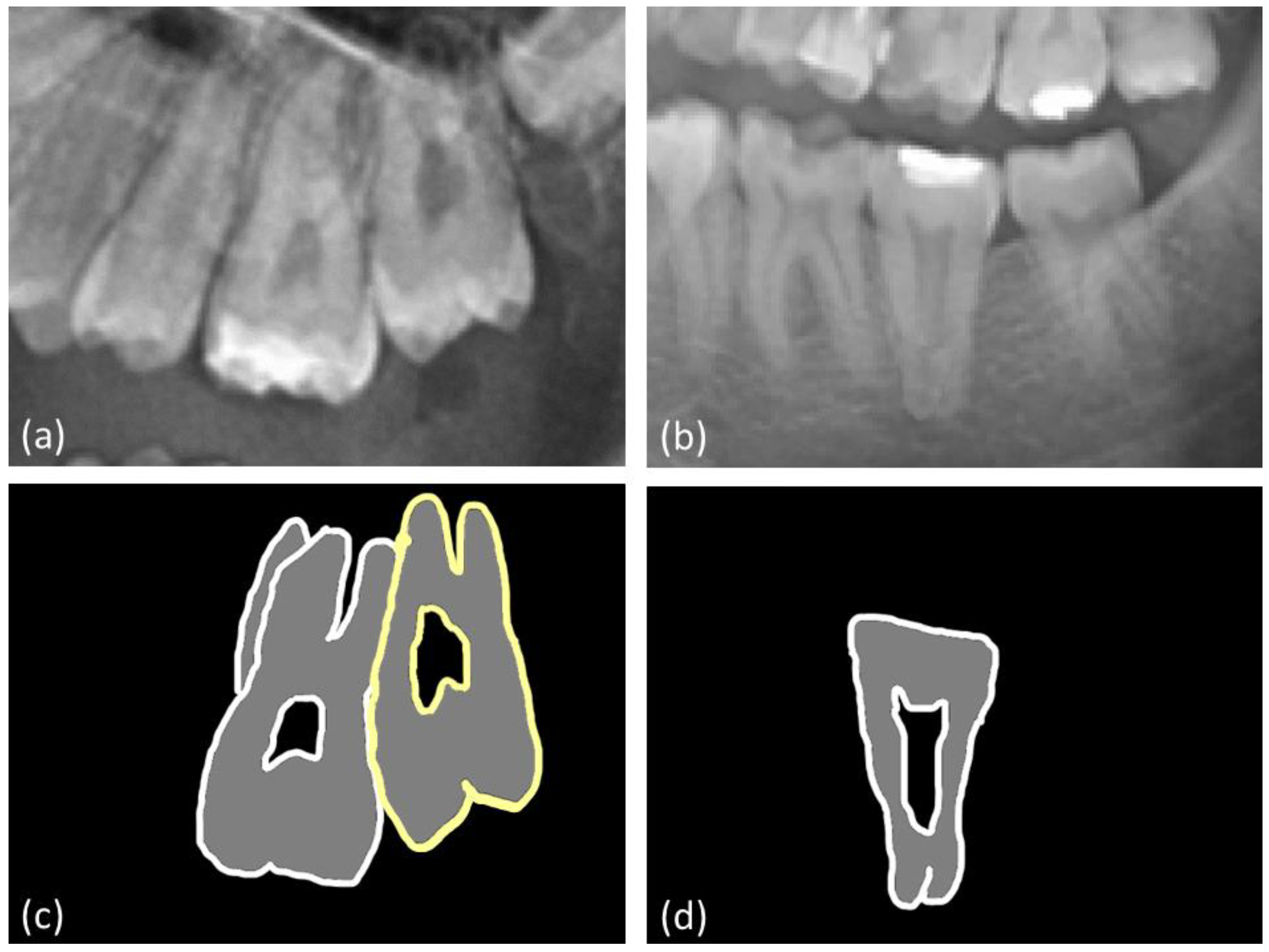
2.3. Measurements
2.4. Statistical Analysis
3. Results
3.1. Contemporary Population
3.2. Historical Population
3.3. Comparison of Prevalence of Taurodontism between Populations
4. Discussion
4.1. Limitations
4.2. Future Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bafna, Y.; Kambalimath, H.V.; Khandelwal, V.; Nayak, P. Taurodontism in deciduous molars. BMJ Case Rep. 2013, 2013, bcr2013010079. [Google Scholar] [CrossRef] [PubMed]
- Mohan, R.P.; Verma, S.; Agarwal, N.; Singh, U. Taurodontism. BMJ Case Rep. 2013, 2013, bcr2012008490. [Google Scholar] [CrossRef] [PubMed]
- Pach, J.; Regulski, P.A.; Tomczyk, J.; Strużycka, I. Clinical implications of a diagnosis of taurodontism: A literature review. Adv. Clin. Exp. Med. 2022, 31, 1385–1389. [Google Scholar] [CrossRef]
- Kalina, A.; Rożniatowski, P.; Regulski, P.; Turska-Szybka, A. The occurrence and intensity of taurodontism among patients in the hospital of the infant Jesus. Biometric analysis of panoramic radiographs. Dent. Med. Probl. 2015, 52, 455–461. [Google Scholar] [CrossRef]
- Jogendra, S.S.A.; Sreedevi, E.; Gopal, A.S.; Lakshmi, M.N. A rare condition of bimaxillary primary molar taurodontism. J. Dent. 2017, 18, 153–156. [Google Scholar]
- Prakash, R.; Vishnu, C.; Suma, B.; Velmurugan, N.; Kandaswamy, D. Endodontic management of taurodontic teeth. Indian J. Dent. Res. 2005, 16, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Dineshshankar, J.; Sivakumar, M.; Balasubramanium, A.M.; Kesavan, G.; Karthikeyan, M.; Prasad, V.S. Taurodontism. J. Pharm. Bioallied Sci. 2014, 6, S13–S15. [Google Scholar] [CrossRef]
- Patil, S.; Doni, B.; Kaswan, S.; Rahman, F. Prevalence of taurodontism in the North Indian population. J. Clin. Exp. Dent. 2013, 5, e179–e182. [Google Scholar] [CrossRef]
- Vashisth, P.; Dwivedi, S.; Arora, S.; Mayall, S. Multiple bilateral taurodontic teeth in primary dentition: A case report. Int. J. Clin. Pediatr. Dent. 2013, 6, 132–133. [Google Scholar] [CrossRef]
- Hegde, V.; Anegundi, R.T.; Pravinchandra, K.R. Biometric analysis—A reliable indicator for diagnosing taurodontism using panoramic radiographs. J. Clin. Diagn. Res. 2013, 7, 1779–1781. [Google Scholar] [CrossRef]
- Jamshidi, D.; Adl, A.; Sobhnamayan, F.; Bolurian, M. Root canal treatment of a hypertaurodont mandibular second molar: A case report. J. Dent. Res. Dent. Clin. Dent. Prospects 2015, 9, 57–59. [Google Scholar] [CrossRef] [PubMed]
- Chetty, M.; Roomaney, I.A.; Beighton, P. Taurodontism in dental genetics. BDJ Open 2021, 7, 25. [Google Scholar] [CrossRef] [PubMed]
- Jayashankara, C.; Shivanna, A.K.; Sridhara, K.; Kumar, P.S. Taurodontism: A dental rarity. J. Oral Maxillofac. Pathol. 2013, 17, 478. [Google Scholar] [CrossRef] [PubMed]
- Puttalingaiah, V.D.; Agarwal, P.; Miglani, R.; Gupta, P.; Sankaran, A.; Dube, G. Assessing the association of taurodontism with numeric dentition anomalies in an adult central Indian population. J. Nat. Sci. Biol. Med. 2014, 5, 429–433. [Google Scholar] [CrossRef] [PubMed]
- Bronoosh, P.; Haghnegahdar, A.; Dehbozorgi, M. Prevalence of taurodontism in premolars and molars in the South of Iran. J. Dent. Res. Dent. Clin. Dent. Prospects 2012, 6, 21–24. [Google Scholar] [CrossRef] [PubMed]
- Aricioğlu, B.; Tomrukçu, D.N.; Köse, T.E. Taurodontism and C-shaped anatomy: Is there an association? Oral Radiol. 2021, 37, 443–451. [Google Scholar] [CrossRef]
- Sears, J. Taurodontism in modern populations. Dent. Anthropol. J. 2018, 14, 14–19. [Google Scholar] [CrossRef]
- Einy, S.; Yitzhaki, I.H.; Cohen, O.; Smidt, A.; Zilberman, U. Taurodontism—Prevalence, extent, and clinical challenge in Ashkelon, Israel—A retrospective study. Appl. Sci. 2022, 12, 1062. [Google Scholar] [CrossRef]
- Colak, H.; Tan, E.; Bayraktar, Y.; Hamidi, M.M.; Colak, T. Taurodontism in a central anatolian population. Dent. Res. J. 2013, 10, 260–263. [Google Scholar] [CrossRef]
- Bürklein, S.; Breuer, D.; Schäfer, E. Prevalence of taurodont and pyramidal molars in a German population. J. Endod. 2011, 37, 158–162. [Google Scholar] [CrossRef]
- Jabali, A.H.; Chourasia, H.R.; Wasli, A.S.; Alkhayrat, A.M.; Mahnashi, H.M.; Kamly, M.J.; Varadarajan, S.; Patil, S. Taurodontism in maxillary and mandibular molars using cone beam computed tomography in a dental center in Saudi Arabia. Ann. Saudi Med. 2021, 41, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Balija, N.D.; Aurer, B.; Meštrović, S.; Varga, M.L. Prevalence of dental anomalies in orthodontic patients. Acta Stomatol. Croat. 2022, 56, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Pach, J.; Regulski, P.A.; Strużycka, I.; Tomczyk, J. Frequency of occurrence of taurodontism in the historical population of Radom (11th–19th centuries). Arch. Oral Biol. 2023, 147, 105638. [Google Scholar] [CrossRef] [PubMed]
- Przesmycka, A.; Jędrychowska-Dańska, K.; Masłowska, A.; Witas, H.; Regulski, P.; Tomczyk, J. Root and root canal diversity in human permanent maxillary first premolars and upper/lower first molars from a 14th–17th and 18th–19th century Radom population. Arch. Oral Biol. 2020, 110, 104603. [Google Scholar] [CrossRef] [PubMed]
- Parupalli, K.; Solomon, R.V.; Karteek, B.S.; Polasa, S. Application of cone-beam computed tomography in the analysis and management of intricate internal anatomy of hyper- and mesotaurodontic teeth. J. Conserv. Dent. 2020, 23, 211–214. [Google Scholar] [CrossRef] [PubMed]
- Jamshidi, D.; Tofangchiha, M.; Jafari Pozve, N.; Mohammadpour, M.; Nouri, B.; Hosseinzadeh, K. Prevalence of taurodont molars in a selected Iranian adult population. Iran Endod. J. 2017, 12, 282–287. [Google Scholar] [CrossRef] [PubMed]
- Janani, M.; Rahimi, S.; Shahi, S.; Aghbali, A.; Zand, V. Endodontic treatment of a hypertaurodont mandibular second molar: A case report. Iran Endod. J. 2011, 6, 133–135. [Google Scholar]




| Sex | Taurodontic Teeth | Hypotaurodontic Teeth | Mesotaurodontic Teeth | Hypertaurodontic Teeth | p Value (Taurodontism to Sex) |
|---|---|---|---|---|---|
| F | 350/923 (38%) | 272/350 (78%) | 59/350 (17%) | 19/350 (5%) | 0.0014 |
| M | 400/1275 (31%) | 307/400 (77%) | 73/400 (18%) | 20/400 (5%) |
| Tooth Group | Taurodontic Teeth | Cynodontic Teeth | p Value |
|---|---|---|---|
| Upper molars | 583 | 539 | <0.0001 |
| Lower molars | 167 | 909 | |
| Right molars | 396 | 726 | 0.2366 |
| Left molars | 354 | 722 |
| Tooth Group | Taurodontic Teeth | Hypotaurodontic Teeth | Mesotaurodontic teeth | Hypertaurodontic Teeth | p Value (Affected Teeth in Each Tooth Group) |
|---|---|---|---|---|---|
| First upper molars | 128/382 (33%) | 115/128 (90%) | 12/128 (9%) | 1/128 (1%) | 0.0001 |
| Second upper molars | 250/477 (52%) | 206/250 (82%) | 34/250 (14%) | 10/250 (4%) | |
| Third upper molars | 205/263 (78%) | 115/205 (56%) | 67/205 (33%) | 23/205 (11%) | |
| First lower molars | 13/320 (4%) | 12/13 (92%) | 1/13 (8%) | 0/13 (0%) | |
| Second lower molars | 56/483 (12%) | 47/56 (84%) | 7/56 12%) | 2/56 (4%) | |
| Third lower molars | 98/273 (36%) | 84/98 (86%) | 11/98 (11%) | 3/98 (3%) |
| Time Period | Taurodontic Teeth | Hypotaurodontic Teeth | Mesotaurodontic Teeth | Hypertaurodontic Teeth | p Value (Prevalence of Taurodontism across Historical Periods) |
|---|---|---|---|---|---|
| Early Middle Ages | 26/120 (22%) | 21/26 (81%) | 5/26 (19%) | 0/26 (0%) | p = 0.0030 |
| Late Middle Ages | 12/76 (16%) | 9/12 (76%) | 3/12 (25%) | 0/12 (0%) | |
| Modern period (18th–19th centuries) | 138/444 (31%) | 114/138 (83%) | 22/138 (16%) | 2/138 (1%) | |
| Contemporary period (2022) | 750/2198 (34%) | 579/750 (77%) | 132/750 (18%) | 34/750 (5%) | |
| Total | 926/2838 (32%) | 723/926 (78%) | 162/926 (18%) | 36/926 (4%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pach, J.; Regulski, P.A.; Tomczyk, J.; Reymond, J.; Osipowicz, K.; Strużycka, I. Prevalence of Taurodontism in Contemporary and Historical Populations from Radom: A Biometric Analysis of Radiological Data. J. Clin. Med. 2023, 12, 5988. https://doi.org/10.3390/jcm12185988
Pach J, Regulski PA, Tomczyk J, Reymond J, Osipowicz K, Strużycka I. Prevalence of Taurodontism in Contemporary and Historical Populations from Radom: A Biometric Analysis of Radiological Data. Journal of Clinical Medicine. 2023; 12(18):5988. https://doi.org/10.3390/jcm12185988
Chicago/Turabian StylePach, Janusz, Piotr A. Regulski, Jacek Tomczyk, Jerzy Reymond, Katarzyna Osipowicz, and Izabela Strużycka. 2023. "Prevalence of Taurodontism in Contemporary and Historical Populations from Radom: A Biometric Analysis of Radiological Data" Journal of Clinical Medicine 12, no. 18: 5988. https://doi.org/10.3390/jcm12185988
APA StylePach, J., Regulski, P. A., Tomczyk, J., Reymond, J., Osipowicz, K., & Strużycka, I. (2023). Prevalence of Taurodontism in Contemporary and Historical Populations from Radom: A Biometric Analysis of Radiological Data. Journal of Clinical Medicine, 12(18), 5988. https://doi.org/10.3390/jcm12185988







