Transcatheter Aortic Valve Implantation and Cardiac Conduction Abnormalities: Prevalence, Risk Factors and Management
Abstract
:1. Introduction
2. Types of Cardiac Conduction Abnormalities after TAVI and Their Management
3. Factors Associated with Conduction Abnormalities after TAVI
4. Pacing-Induced Cardiomyopathy and Potential Solutions
5. Other Heart Rhythm Disorders after TAVI
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 2021, 43, 561–632. [Google Scholar] [CrossRef]
- Cribier, A.; Eltchaninoff, H.; Bash, A.; Borenstein, N.; Tron, C.; Bauer, F.; Derumeaux, G.; Anselme, F.; Laborde, F.; Leon, M.B. Percutaneous Transcatheter Implantation of an Aortic Valve Prosthesis for Calcific Aortic Stenosis: First Human Case Description. Circ. J. Am. Heart Assoc. 2002, 106, 3006–3008. [Google Scholar] [CrossRef]
- Hudziak, D.; Wojakowski, W.; Malinowski, M.; Gocoł, R.; Żak, A.; Morkisz, Ł.; Ochała, A.; Parma, R.; Smolka, G.; Ciosek, J.; et al. Comparison of the short-term safety and efficacy of transcarotid and transfemoral access routes for transcatheter aortic valve implantation. Kardiol. Pol. 2021, 79, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Bonow, R.O.; Carabello, B.A.; Chatterjee, K.; de Leon, A.C.; Faxon, D.P.; Freed, M.D.; Gaasch, W.H.; Lytle, B.W.; Nishimura, R.A.; O’Gara, P.T.; et al. ACC/AHA 2006 Guidelines for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 1998 Guidelines for the Management of Patients With Valvular Heart Disease): Developed in Collaboration With the Society of Cardiovascular Anesthesiologists: Endorsed by the Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons. Circulation 2006, 114, 84–231. [Google Scholar] [CrossRef]
- Arora, S.; Strassle, P.D.; Kolte, D.; Ramm, C.J.; Falk, K.; Jack, G.; Caranasos, T.G.; Cavender, M.A.; Rossi, J.S.; Vavalle, J.P. Length of Stay and Discharge Disposition After Transcatheter Versus Surgical Aortic Valve Replacement in the United States. Circ. Cardiovasc. Interv. 2018, 11, e006929. [Google Scholar] [CrossRef]
- Adams, D.H.; Popma, J.J.; Reardon, M.J.; Yakubov, S.J.; Coselli, J.S.; Deeb, G.M.; Gleason, T.G.; Buchbinder, M.; Hermiller, J.; Kleiman, N.S.; et al. Transcatheter Aortic-Valve Replacement with a Self-Expanding Prosthesis. N. Engl. J. Med. 2014, 370, 1790–1798. [Google Scholar] [CrossRef]
- Arnold, S.V.; Reynolds, M.R.; Wang, K.; Magnuson, E.A.; Baron, S.J.; Chinnakondepalli, K.M.; Reardon, M.J.; Tadros, P.N.; Zorn, G.L.; Maini, B.; et al. Health Status After Transcatheter or Surgical Aortic Valve Replacement in Patients with Severe Aortic Stenosis at Increased Surgical Risk. JACC Cardiovasc. Interv. 2015, 8, 1207–1217. [Google Scholar] [CrossRef]
- Webb, J.G.; Altwegg, L.; Boone, R.H.; Cheung, A.; Ye, J.; Lichtenstein, S.; Lee, M.; Masson, J.B.; Thompson, C.; Moss, R.; et al. Transcatheter Aortic Valve Implantation: Impact on Clinical and Valve-Related Outcomes. Circulation 2009, 119, 3009–3016. [Google Scholar] [CrossRef]
- Fraccaro, C.; Napodano, M.; Tarantini, G.; Gasparetto, V.; Gerosa, G.; Bianco, R.; Bonato, R.; Pittarello, D.; Isabella, G.; Iliceto, S.; et al. Expanding the Eligibility for Transcatheter Aortic Valve Implantation. JACC Cardiovasc. Interv. 2009, 2, 828–833. [Google Scholar] [CrossRef]
- Scarsini, R.; De Maria, G.L.; Joseph, J.; Fan, L.; Cahill, T.J.; Kotronias, R.A.; Burzotta, F.; Newton, J.D.; Kharbanda, R.; Prendergast, B.; et al. Impact of Complications during Transfemoral Transcatheter Aortic Valve Replacement: How Can They Be Avoided and Managed? J. Am. Hear. Assoc. 2019, 8, e013801. [Google Scholar] [CrossRef]
- Hamdan, A.; Guetta, V.; Klempfner, R.; Konen, E.; Raanani, E.; Glikson, M.; Goitein, O.; Segev, A.; Barbash, I.; Fefer, P.; et al. Inverse Relationship between Membranous Septal Length and the Risk of Atrioventricular Block in Patients Undergoing Transcatheter Aortic Valve Implantation. JACC Cardiovasc. Interv. 2015, 8, 1218–1228. [Google Scholar] [CrossRef] [PubMed]
- Fujita, B.; Kütting, M.; Seiffert, M.; Scholtz, S.; Egron, S.; Prashovikj, E.; Börgermann, J.; Schäfer, T.; Scholtz, W.; Preuss, R.; et al. Calcium distribution patterns of the aortic valve as a risk factor for the need of permanent pacemaker implantation after transcatheter aortic valve implantation. Eur. Heart J. Cardiovasc. Imaging 2016, 17, 1385–1393. [Google Scholar] [CrossRef] [PubMed]
- Lange, R.; Bleiziffer, S.; Mazzitelli, D.; Elhmidi, Y.; Opitz, A.; Krane, M.; Deutsch, M.-A.; Ruge, H.; Brockmann, G.; Voss, B.; et al. Improvements in Transcatheter Aortic Valve Implantation Outcomes in Lower Surgical Risk Patients. J. Am. Coll. Cardiol. 2012, 59, 280–287. [Google Scholar] [CrossRef]
- Schymik, G.; Schröfel, H.; Schymik, J.S.; Wondraschek, R.; Süselbeck, T.; Kiefer, R.; Balthasar, V.; Luik, A.; Posival, H.; Schmitt, C. Acute and Late Outcomes of Transcatheter Aortic Valve Implantation (TAVI) for the Treatment of Severe Symptomatic Aortic Stenosis in Patients at High- and Low-Surgical Risk: Tavi in Patients at High- and Low-Surgical Risk. J. Interv. Cardiol. 2012, 25, 364–374. [Google Scholar] [CrossRef]
- Guyton, R.A. The Placement of Aortic Transcatheter Valve (PARTNER) Trial: The Surgeon’s Perspective: Celebration and Concern. Circulation 2012, 125, 3237–3239. [Google Scholar] [CrossRef] [PubMed]
- Dębiński, M.; Domaradzki, W.; Fil, W.; Milewski, K.; Buszman, P.P.; Kachel, M.; Brączkowski, J.; Gerber, W.; Cisowski, M.; Bochenek, A.; et al. Long-term outcomes of transcatheter self-expanding aortic valve implantations in inoperable and high surgical–risk patients with severe aortic stenosis: A single-center single-valve registry. Kardiol. Pol. 2021, 79, 319–326. [Google Scholar] [CrossRef]
- Voigtländer, L.; Seiffert, M. Expanding TAVI to Low and Intermediate Risk Patients. Front. Cardiovasc. Med. 2018, 5, 92. [Google Scholar] [CrossRef]
- Deeb, G.M.; Reardon, M.J.; Chetcuti, S.; Patel, H.J.; Grossman, P.M.; Yakubov, S.J.; Kleiman, N.S.; Coselli, J.S.; Gleason, T.G.; Lee, J.S.; et al. 3-Year Outcomes in High-Risk Patients Who Underwent Surgical or Transcatheter Aortic Valve Replacement. J. Am. Coll. Cardiol. 2016, 67, 2565–2574. [Google Scholar] [CrossRef]
- Smith, C.R.; Leon, M.B.; Mack, M.J.; Miller, D.C.; Moses, J.W.; Svensson, L.G.; Tuzcu, E.M.; Webb, J.G.; Fontana, G.P.; Makkar, R.R.; et al. Transcatheter versus Surgical Aortic-Valve Replacement in High-Risk Patients. N. Engl. J. Med. 2011, 364, 2187–2198. [Google Scholar] [CrossRef]
- Mack, M.J.; Leon, M.B.; Smith, C.R.; Miller, D.C.; Moses, J.W.; Tuzcu, E.M.; Webb, J.G.; Douglas, P.S.; Anderson, W.N.; Blackstone, E.H.; et al. 5-year outcomes of transcatheter aortic valve replacement or surgical aortic valve replacement for high surgical risk patients with aortic stenosis (PARTNER 1): A randomised controlled trial. Lancet 2015, 385, 2477–2484. [Google Scholar] [CrossRef]
- Thyregod, H.G.H.; Steinbrüchel, D.A.; Ihlemann, N.; Nissen, H.; Kjeldsen, B.J.; Petursson, P.; Chang, Y.; Franzen, O.W.; Engstrøm, T.; Clemmensen, P.; et al. Transcatheter Versus Surgical Aortic Valve Replacement in Patients with Severe Aortic Valve Stenosis. J. Am. Coll. Cardiol. 2015, 65, 2184–2194. [Google Scholar] [CrossRef] [PubMed]
- Leon, M.B.; Smith, C.R.; Mack, M.; Miller, D.C.; Moses, J.W.; Svensson, L.G.; Tuzcu, E.M.; Webb, J.G.; Fontana, G.P.; Makkar, R.R.; et al. Transcatheter Aortic-Valve Implantation for Aortic Stenosis in Patients Who Cannot Undergo Surgery. N. Engl. J. Med. 2010, 363, 1597–1607. [Google Scholar] [CrossRef] [PubMed]
- Thourani, V.H.; Kodali, S.; Makkar, R.R.; Herrmann, H.C.; Williams, M.; Babaliaros, V.; Smalling, R.; Lim, S.; Malaisrie, S.C.; Kapadia, S.; et al. Transcatheter aortic valve replacement versus surgical valve replacement in intermediate-risk patients: A propensity score analysis. Lancet 2016, 387, 2218–2225. [Google Scholar] [CrossRef] [PubMed]
- Reardon, M.J.; Van Mieghem, N.M.; Popma, J.J.; Kleiman, N.S.; Søndergaard, L.; Mumtaz, M.; Adams, D.H.; Deeb, G.M.; Maini, B.; Gada, H.; et al. Surgical or Transcatheter Aortic-Valve Replacement in Intermediate-Risk Patients. N. Engl. J. Med. 2017, 376, 1321–1331. [Google Scholar] [CrossRef] [PubMed]
- Makkar, R.R.; Thourani, V.H.; Mack, M.J.; Kodali, S.K.; Kapadia, S.; Webb, J.G.; Yoon, S.-H.; Trento, A.; Svensson, L.G.; Herrmann, H.C.; et al. Five-Year Outcomes of Transcatheter or Surgical Aortic-Valve Replacement. N. Engl. J. Med. 2020, 382, 799–809. [Google Scholar] [CrossRef]
- Thyregod, H.G.H.; Ihlemann, N.; Jørgensen, T.H.; Nissen, H.; Kjeldsen, B.J.; Petursson, P.; Chang, Y.; Franzen, O.W.; Engstrøm, T.; Clemmensen, P.; et al. Five-Year Clinical and Echocardiographic Outcomes from the NOTION Randomized Clinical Trial in Patients at Lower Surgical Risk. Circulation 2019, 139, 2714–2723. [Google Scholar] [CrossRef]
- Siontis, G.C.M.; Praz, F.; Pilgrim, T.; Mavridis, D.; Verma, S.; Salanti, G.; Søndergaard, L.; Jüni, P.; Windecker, S. Transcatheter aortic valve implantation vs. surgical aortic valve replacement for treatment of severe aortic stenosis: A meta-analysis of randomized trials. Eur. Heart J. 2016, 37, 3503–3512. [Google Scholar] [CrossRef]
- Mack, M.J.; Leon, M.B.; Thourani, V.H.; Makkar, R.; Kodali, S.K.; Russo, M.; Kapadia, S.R.; Malaisrie, S.C.; Cohen, D.J.; Pibarot, P.; et al. Transcatheter Aortic-Valve Replacement with a Balloon-Expandable Valve in Low-Risk Patients. N. Engl. J. Med. 2019, 380, 1695–1705. [Google Scholar] [CrossRef]
- Popma, J.J.; Deeb, G.M.; Yakubov, S.J.; Mumtaz, M.; Gada, H.; O’Hair, D.; Bajwa, T.; Heiser, J.C.; Merhi, W.; Kleiman, N.S.; et al. Transcatheter Aortic-Valve Replacement with a Self-Expanding Valve in Low-Risk Patients. N. Engl. J. Med. 2019, 380, 1706–1715. [Google Scholar] [CrossRef]
- Siontis, G.C.M.; Overtchouk, P.; Cahill, T.J.; Modine, T.; Prendergast, B.; Praz, F.; Pilgrim, T.; Petrinic, T.; Nikolakopoulou, A.; Salanti, G.; et al. Transcatheter aortic valve implantation vs. surgical aortic valve replacement for treatment of symptomatic severe aortic stenosis: An updated meta-analysis. Eur. Heart J. 2019, 40, 3143–3153. [Google Scholar] [CrossRef]
- Leon, M.B.; Mack, M.J.; Hahn, R.T.; Thourani, V.H.; Makkar, R.; Kodali, S.K.; Alu, M.C.; Madhavan, M.V.; Chau, K.H.; Russo, M.; et al. Outcomes 2 Years After Transcatheter Aortic Valve Replacement in Patients at Low Surgical Risk. J. Am. Coll. Cardiol. 2021, 77, 1149–1161. [Google Scholar] [CrossRef]
- Wagner, G.; Steiner, S.; Gartlehner, G.; Arfsten, H.; Wildner, B.; Mayr, H.; Moertl, D. Comparison of transcatheter aortic valve implantation with other approaches to treat aortic valve stenosis: A systematic review and meta-analysis. Syst. Rev. 2019, 8, 44. [Google Scholar] [CrossRef]
- Regueiro, A.; Abdul-Jawad Altisent, O.; Del Trigo, M.; Campelo-Parada, F.; Puri, R.; Urena, M.; Philippon, F.; Rodés-Cabau, J. Impact of New-Onset Left Bundle Branch Block and Periprocedural Permanent Pacemaker Implantation on Clinical Outcomes in Patients Undergoing Transcatheter Aortic Valve Replacement: A Systematic Review and Meta-Analysis. Circ. Cardiovasc. Interv. 2016, 9, e003635. [Google Scholar] [CrossRef]
- Unzué, L.; García, E.; Díaz-Antón, B.; Rodríguez-Rodrigo, F.J.; Rodríguez del Río, M.; Teijeiro, R.; Medina, J.; Parra, F.J. Left Bundle Branch Block after Transcatheter Aortic Valve Implantation with Edwards Sapien 3 Valve: Influence of the Valve Depth Implantation. Cardiovasc. Revascularization Med. 2019, 20, 949–955. [Google Scholar] [CrossRef] [PubMed]
- Auffret, V.; Puri, R.; Urena, M.; Chamandi, C.; Rodriguez-Gabella, T.; Philippon, F.; Rodés-Cabau, J. Conduction Disturbances after Transcatheter Aortic Valve Replacement: Current Status and Future Perspectives. Circulation 2017, 136, 1049–1069. [Google Scholar] [CrossRef] [PubMed]
- Moreno, R.; Dobarro, D.; López de Sá, E.; Prieto, M.; Morales, C.; Calvo Orbe, L.; Moreno-Gomez, I.; Filgueiras, D.; Sanchez-Recalde, A.; Galeote, G.; et al. Cause of Complete Atrioventricular Block after Percutaneous Aortic Valve Implantation: Insights From a Necropsy Study. Circulation 2009, 120, e29–e30. [Google Scholar] [CrossRef]
- Glikson, M.; Nielsen, J.C.; Kronborg, M.B.; Michowitz, Y.; Auricchio, A.; Barbash, I.M.; Barrabés, J.A.; Boriani, G.; Braunschweig, F.; Brignole, M.; et al. 2021 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy. Eur. Heart J. 2021, 42, 3427–3520. [Google Scholar] [CrossRef] [PubMed]
- Kawashima, T.; Sato, F. Visualizing anatomical evidences on atrioventricular conduction system for TAVI. Int. J. Cardiol. 2014, 174, 1–6. [Google Scholar] [CrossRef]
- Siontis, G.C.M.; Jüni, P.; Pilgrim, T.; Stortecky, S.; Büllesfeld, L.; Meier, B.; Wenaweser, P.; Windecker, S. Predictors of Permanent Pacemaker Implantation in Patients with Severe Aortic Stenosis Undergoing TAVR. J. Am. Coll. Cardiol. 2014, 64, 129–140. [Google Scholar] [CrossRef]
- Kooistra, N.H.M.; van Mourik, M.S.; Rodríguez-Olivares, R.; Maass, A.H.; Nijenhuis, V.J.; van der Werf, R.; Ten Berg, J.M.; Kraaijeveld, A.O.; Baan, J., Jr.; Voskuil, M.; et al. Late onset of new conduction disturbances requiring permanent pacemaker implantation following TAVI. Heart 2020, 106, 1244–1251. [Google Scholar] [CrossRef]
- Brunet, M.; Thoman, S.; Gandet, T.; Massin, F.; El Bouazzaoui, R.; Macia, J.C.; Delseny, D.; Granier, M.; Piot, C.; Robert, G.; et al. Indication of permanent cardiac pacing after transcatheter aortic valve implantation (TAVISTIM NCT02337140). Arch. Cardiovasc. Dis. Suppl. 2019, 11, 85–86. [Google Scholar] [CrossRef]
- Nazif, T.M.; Dizon, J.M.; Hahn, R.T.; Xu, K.; Babaliaros, V.; Douglas, P.S.; El-Chami, M.F.; Herrmann, H.C.; Mack, M.; Makkar, R.R.; et al. Predictors and Clinical Outcomes of Permanent Pacemaker Implantation After Transcatheter Aortic Valve Replacement. JACC Cardiovasc. Interv. 2015, 8, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Meduri, C.U.; Kereiakes, D.J.; Rajagopal, V.; Makkar, R.R.; O’Hair, D.; Linke, A.; Waksman, R.; Babliaros, V.; Stoler, R.C.; Mishkel, G.J.; et al. Pacemaker Implantation and Dependency after Transcatheter Aortic Valve Replacement in the REPRISE III Trial. J. Am. Hear. Assoc. 2019, 8, e012594. [Google Scholar] [CrossRef] [PubMed]
- Costa, G.; Zappulla, P.; Barbanti, M.; Cirasa, A.; Todaro, D.; Rapisarda, G.; Picci, A.; Platania, F.; Tosto, A.; Di Grazia, A.; et al. Pacemaker dependency after transcatheter aortic valve implantation: Incidence, predictors and long-term outcomes. EuroIntervention 2019, 15, 875–883. [Google Scholar] [CrossRef]
- Schernthaner, C.; Kraus, J.; Danmayr, F.; Hammerer, M.; Schneider, J.; Hoppe, U.C.; Strohmer, B. Short-term pacemaker dependency after transcatheter aortic valve implantation. Wien. Klin. Wochenschr. 2016, 128, 198–203. [Google Scholar] [CrossRef]
- Dizon, J.M.; Nazif, T.M.; Hess, P.L.; Biviano, A.; Garan, H.; Douglas, P.S.; Kapadia, S.; Babaliaros, V.; Herrmann, H.C.; Szeto, W.Y.; et al. Chronic pacing and adverse outcomes after transcatheter aortic valve implantation. Heart 2015, 101, 1665–1671. [Google Scholar] [CrossRef]
- Jensen, P.N.; Gronroos, N.N.; Chen, L.Y.; Folsom, A.R.; deFilippi, C.; Heckbert, S.R.; Alonso, A. Incidence of and Risk Factors for Sick Sinus Syndrome in the General Population. J. Am. Coll. Cardiol. 2014, 64, 531–538. [Google Scholar] [CrossRef]
- Authors/Task Force Members; Brignole, M.; Auricchio, A.; Baron-Esquivias, G.; Bordachar, P.; Boriani, G.; Breithardt, O.-A.; Cleland, J.; Deharo, J.-C.; Delgado, V.; et al. 2013 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy: The Task Force on cardiac pacing and resynchronization therapy of the European Society of Cardiology (ESC). Developed in collaboration with the European Heart Rhythm Association (EHRA). Europace 2013, 15, 1070–1118. [Google Scholar] [CrossRef]
- Kusumoto, S.; Kawano, H.; Makita, N.; Ichimaru, S.; Kaku, T.; Haruta, D.; Hida, A.; Sera, N.; Imaizumi, M.; Nakashima, E.; et al. Right bundle branch block without overt heart disease predicts higher risk of pacemaker implantation: The study of atomic-bomb survivors. Int. J. Cardiol. 2014, 174, 77–82. [Google Scholar] [CrossRef]
- Rodés-Cabau, J.; Ellenbogen, K.A.; Krahn, A.D.; Latib, A.; Mack, M.; Mittal, S.; Muntané-Carol, G.; Nazif, T.M.; Sondergaard, L.; Urena, M.; et al. Management of Conduction Disturbances Associated with Transcatheter Aortic Valve Replacement. J. Am. Coll. Cardiol. 2019, 74, 1086–1106. [Google Scholar] [CrossRef]
- Rogers, T.; Devraj, M.; Thomaides, A.; Steinvil, A.; Lipinski, M.J.; Buchanan, K.D.; Alraies, M.C.; Koifman, E.; Gai, J.; Torguson, R.; et al. Utility of Invasive Electrophysiology Studies in Patients with Severe Aortic Stenosis Undergoing Transcatheter Aortic Valve Implantation. Am. J. Cardiol. 2018, 121, 1351–1357. [Google Scholar] [CrossRef] [PubMed]
- De Carlo, M.; Giannini, C.; Bedogni, F.; Klugmann, S.; Brambilla, N.; De Marco, F.; Zucchelli, G.; Testa, L.; Oreglia, J.; Petronio, A.S. Safety of a conservative strategy of permanent pacemaker implantation after transcatheter aortic CoreValve implantation. Am. Heart J. 2012, 163, 492–499. [Google Scholar] [CrossRef] [PubMed]
- Kusumoto, F.M.; Schoenfeld, M.H.; Barrett, C.; Edgerton, J.R.; Ellenbogen, K.A.; Gold, M.R.; Goldschlager, N.F.; Hamilton, R.M.; Joglar, J.A.; Kim, R.J.; et al. 2018 ACC/AHA/HRS Guideline on the Evaluation and Management of Patients with Bradycardia and Cardiac Conduction Delay. J. Am. Coll. Cardiol. 2019, 74, e51–e156. [Google Scholar] [CrossRef] [PubMed]
- Fadahunsi, O.O.; Olowoyeye, A.; Ukaigwe, A.; Li, Z.; Vora, A.N.; Vemulapalli, S.; Elgin, E.; Donato, A. Incidence, Predictors, and Outcomes of Permanent Pacemaker Implantation Following Transcatheter Aortic Valve Replacement. JACC Cardiovasc. Interv. 2016, 9, 2189–2199. [Google Scholar] [CrossRef]
- Russo, G.; Tang, G.H.L.; Sangiorgi, G.; Pedicino, D.; Enriquez-Sarano, M.; Maisano, F.; Taramasso, M. Lifetime Management of Aortic Stenosis: Transcatheter Versus Surgical Treatment for Young and Low-Risk Patients. Circ. Cardiovasc. Interv. 2022, 15, 915–927. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- Meguro, K.; Lellouche, N.; Teiger, E. Cardiac resynchronization therapy improved heart failure after left bundle branch block during transcatheter aortic valve implantation. J. Invasive Cardiol. 2012, 24, 132–133. [Google Scholar]
- Osmancik, P.; Stros, P.; Herman, D.; Kocka, V.; Paskova, E. Cardiac resynchronization therapy implantation following transcatheter aortic valve implantation. Europace 2011, 13, 290–291. [Google Scholar] [CrossRef]
- Urena, M.; Webb, J.G.; Tamburino, C.; Muñoz-García, A.J.; Cheema, A.; Dager, A.E.; Serra, V.; Amat-Santos, I.J.; Barbanti, M.; Immè, S.; et al. Permanent Pacemaker Implantation after Transcatheter Aortic Valve Implantation: Impact on Late Clinical Outcomes and Left Ventricular Function. Circulation 2014, 129, 1233–1243. [Google Scholar] [CrossRef]
- Buellesfeld, L.; Stortecky, S.; Heg, D.; Hausen, S.; Mueller, R.; Wenaweser, P.; Pilgrim, T.; Gloekler, S.; Khattab, A.A.; Huber, C.; et al. Impact of Permanent Pacemaker Implantation on Clinical Outcome among Patients Undergoing Transcatheter Aortic Valve Implantation. J. Am. Coll. Cardiol. 2012, 60, 493–501. [Google Scholar] [CrossRef]
- Bagur, R.; Manazzoni, J.M.; Dumont, E.; Doyle, D.; Perron, J.; Dagenais, F.; Mathieu, P.; Baillot, R.; Charbonneau, E.; Metras, J.; et al. Permanent pacemaker implantation following isolated aortic valve replacement in a large cohort of elderly patients with severe aortic stenosis. Heart 2011, 97, 1687–1694. [Google Scholar] [CrossRef]
- Raza, S.S.; Li, J.-M.; John, R.; Chen, L.Y.; Tholakanahalli, V.N.; Mbai, M.; Adabag, A.S. Long-Term Mortality and Pacing Outcomes of Patients with Permanent Pacemaker Implantation after Cardiac Surgery: Pacemakers after Heart Surgery. Pacing Clin. Electrophysiol. 2011, 34, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Mohananey, D.; Jobanputra, Y.; Kumar, A.; Krishnaswamy, A.; Mick, S.; White, J.M.; Kapadia, S.R. Clinical and Echocardiographic Outcomes Following Permanent Pacemaker Implantation after Transcatheter Aortic Valve Replacement: Meta-Analysis and Meta-Regression. Circ. Cardiovasc. Interv. 2017, 10, e005046. [Google Scholar] [CrossRef]
- Zaid, S.; Sengupta, A.; Okoli, K.; Tsoi, M.; Khan, A.; Ahmad, H.; Goldberg, J.B.; Undemir, C.; Rozenshtein, A.; Patel, N.; et al. Novel Anatomic Predictors of New Persistent Left Bundle Branch Block after Evolut Transcatheter Aortic Valve Implantation. Am. J. Cardiol. 2020, 125, 1222–1229. [Google Scholar] [CrossRef] [PubMed]
- Mangieri, A.; Lanzillo, G.; Bertoldi, L.; Jabbour, R.J.; Regazzoli, D.; Ancona, M.B.; Tanaka, A.; Mitomo, S.; Garducci, S.; Montalto, C.; et al. Predictors of Advanced Conduction Disturbances Requiring a Late (≥48 H) Permanent Pacemaker Following Transcatheter Aortic Valve Replacement. JACC Cardiovasc. Interv. 2018, 11, 1519–1526. [Google Scholar] [CrossRef] [PubMed]
- Giustino, G.; Van Der Boon, R.; Molina-Martin De Nicolas, J.; Dumonteil, N.; Chieffo, A.; De Jaegere, P.; Tchetche, D.; Marcheix, B.; Millischer, D.; Cassagneau, R.; et al. Impact of permanent pacemaker on mortality after transcatheter aortic valve implantation: The PRAGMATIC (Pooled Rotterdam-Milan-Toulouse in Collaboration) Pacemaker substudy. EuroIntervention 2016, 12, 1185–1193. [Google Scholar] [CrossRef]
- Abramowitz, Y.; Kazuno, Y.; Chakravarty, T.; Kawamori, H.; Maeno, Y.; Anderson, D.; Allison, Z.; Mangat, G.; Cheng, W.; Gopal, A.; et al. Concomitant mitral annular calcification and severe aortic stenosis: Prevalence, characteristics and outcome following transcatheter aortic valve replacement. Eur. Hear. J. 2016, 38, 1194–1203. [Google Scholar] [CrossRef]
- Mauri, V.; Reimann, A.; Stern, D.; Scherner, M.; Kuhn, E.; Rudolph, V.; Rosenkranz, S.; Eghbalzadeh, K.; Friedrichs, K.; Wahlers, T.; et al. Predictors of Permanent Pacemaker Implantation after Transcatheter Aortic Valve Replacement with the SAPIEN 3. JACC Cardiovasc. Interv. 2016, 9, 2200–2209. [Google Scholar] [CrossRef]
- Maeno, Y.; Abramowitz, Y.; Kawamori, H.; Kazuno, Y.; Kubo, S.; Takahashi, N.; Mangat, G.; Okuyama, K.; Kashif, M.; Chakravarty, T.; et al. A Highly Predictive Risk Model for Pacemaker Implantation after TAVR. JACC Cardiovasc. Imaging 2017, 10, 1139–1147. [Google Scholar] [CrossRef]
- Ledwoch, J.; Franke, J.; Gerckens, U.; Kuck, K.-H.; Linke, A.; Nickenig, G.; Krülls-Münch, J.; Vöhringer, M.; Hambrecht, R.; Erbel, R.; et al. Incidence and predictors of permanent pacemaker implantation following transcatheter aortic valve implantation: Analysis from the german transcatheter aortic valve interventions registry: Predictors of Pacemaker after TAVI. Cathet. Cardiovasc. Intervent. 2013, 82, E569–E577. [Google Scholar] [CrossRef]
- Ruparelia, N.; Prendergast, B.D. Technical aspects of transcatheter aortic valve implantation (TAVI). E-J. Cardiol. Pract. 2016, 14. [Google Scholar]
- Santangelo, G.; Ielasi, A.; Pellicano, M.; Latib, A.; Tespili, M.; Donatelli, F. An Update on New Generation Transcatheter Aortic Valves and Delivery Systems. J. Clin. Med. 2022, 11, 499. [Google Scholar] [CrossRef] [PubMed]
- Moskowitz, G.; Hong, K.N.; Giustino, G.; Gillinov, A.M.; Ailawadi, G.; DeRose, J.J.; Iribarne, A.; Moskowitz, A.J.; Gelijns, A.C.; Egorova, N.N. Incidence and Risk Factors for Permanent Pacemaker Implantation Following Mitral or Aortic Valve Surgery. J. Am. Coll. Cardiol. 2019, 74, 2607–2620. [Google Scholar] [CrossRef] [PubMed]
- Tang, G.H.L.; Zaid, S.; Michev, I.; Ahmad, H.; Kaple, R.; Undemir, C.; Cohen, M.; Lansman, S.L. “Cusp-Overlap” View Simplifies Fluoroscopy-Guided Implantation of Self-Expanding Valve in Transcatheter Aortic Valve Replacement. JACC: Cardiovasc. Interv. 2018, 11, 1663–1665. [Google Scholar] [CrossRef] [PubMed]
- Doldi, P.M.; Stolz, L.; Escher, F.; Steffen, J.; Gmeiner, J.; Roden, D.; Linnemann, M.; Löw, K.; Deseive, S.; Stocker, T.J.; et al. Transcatheter Aortic Valve Replacement with the Self-Expandable Core Valve Evolut Prosthesis Using the Cusp-Overlap vs. Tricusp-View. J. Clin. Med. 2022, 11, 1561. [Google Scholar] [CrossRef] [PubMed]
- Pascual, I.; Hernández-Vaquero, D.; Alperi, A.; Almendarez, M.; Avanzas, P.; Kalavrouziotis, D.; Lorca, R.; Mesnier, J.; Arboine, L.; Mohammadi, S.; et al. Permanent Pacemaker Reduction Using Cusp-Overlapping Projection in TAVR. JACC Cardiovasc. Interv. 2022, 15, 150–161. [Google Scholar] [CrossRef]
- Rodés-Cabau, J.; Urena, M.; Nombela-Franco, L.; Amat-Santos, I.; Kleiman, N.; Munoz-Garcia, A.; Atienza, F.; Serra, V.; Deyell, M.W.; Veiga-Fernandez, G.; et al. Arrhythmic Burden as Determined by Ambulatory Continuous Cardiac Monitoring in Patients with New-Onset Persistent Left Bundle Branch Block Following Transcatheter Aortic Valve Replacement. JACC Cardiovasc. Interv. 2018, 11, 1495–1505. [Google Scholar] [CrossRef]
- Ryś, M.; Hryniewiecki, T.; Witkowski, A.; Michałowska, I.; Zatorska, K.; Stokłosa, P.; Nieznańska, M.; Szymański, P. Association between calcification of mitro-aortic continuity and mitral regurgitation in patients undergoing transcatheter aortic valve replacement. Kardiol. Pol. 2021, 79, 669–675. [Google Scholar] [CrossRef]
- Scisło, P.; Grodecki, K.; Rymuza, B.; Zbroński, K.; Kochman, J.; Wilimski, R.; Huczek, Z. Impact of transcatheter aortic valve implantation on coexistent mitral regurgitation parameters. Kardiol. Pol. 2021, 79, 179–184. [Google Scholar] [CrossRef]
- Kiehl, E.L.; Makki, T.; Kumar, R.; Gumber, D.; Kwon, D.H.; Rickard, J.W.; Kanj, M.; Wazni, O.M.; Saliba, W.I.; Varma, N.; et al. Incidence and predictors of right ventricular pacing-induced cardiomyopathy in patients with complete atrioventricular block and preserved left ventricular systolic function. Heart Rhythm. 2016, 13, 2272–2278. [Google Scholar] [CrossRef]
- Trial, D.D. The DAVID Trial Investigators* Dual-Chamber Pacing or Ventricular Backup Pacing in Patients With an Implantable Defibrillator: The Dual Chamber and VVI Implantable Defibrillator (DAVID) Trial. JAMA 2002, 288, 3115. [Google Scholar] [CrossRef]
- Curtis, A.B.; Worley, S.J.; Adamson, P.B.; Chung, E.S.; Niazi, I.; Sherfesee, L.; Shinn, T.; St. John Sutton, M. Biventricular Pacing for Atrioventricular Block and Systolic Dysfunction. N. Engl. J. Med. 2013, 368, 1585–1593. [Google Scholar] [CrossRef] [PubMed]
- Tops, L.F.; Schalij, M.J.; Bax, J.J. The Effects of Right Ventricular Apical Pacing on Ventricular Function and Dyssynchrony. J. Am. Coll. Cardiol. 2009, 54, 764–776. [Google Scholar] [CrossRef] [PubMed]
- Skalidis, E.I.; Kochiadakis, G.E.; Koukouraki, S.I.; Chrysostomakis, S.I.; Igoumenidis, N.E.; Karkavitsas, N.S.; Vardas, P.E. Myocardial perfusion in patients with permanent ventricular pacing and normal coronary arteries. J. Am. Coll. Cardiol. 2001, 37, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Matusik, P.T. Adverse Clinical Outcomes Related to Right Ventricular Pacing. Eur. Heart J. 2019, 40, 1586–1588. [Google Scholar] [CrossRef]
- Merchant, F.M.; Mittal, S. Pacing induced cardiomyopathy. J. Cardiovasc. Electrophysiol. 2020, 31, 286–292. [Google Scholar] [CrossRef]
- Abdelrahman, M.; Subzposh, F.A.; Beer, D.; Durr, B.; Naperkowski, A.; Sun, H.; Oren, J.W.; Dandamudi, G.; Vijayaraman, P. Clinical Outcomes of His Bundle Pacing Compared to Right Ventricular Pacing. J. Am. Coll. Cardiol. 2018, 71, 2319–2330. [Google Scholar] [CrossRef]
- Zanon, F.; Ellenbogen, K.A.; Dandamudi, G.; Sharma, P.S.; Huang, W.; Lustgarten, D.L.; Tung, R.; Tada, H.; Koneru, J.N.; Bergemann, T.; et al. Permanent His-bundle pacing: A systematic literature review and meta-analysis. EP Eur. 2018, 20, 1819–1826. [Google Scholar] [CrossRef]
- Burri, H.; Jastrzebski, M.; Cano, Ó.; Čurila, K.; de Pooter, J.; Huang, W.; Israel, C.; Joza, J.; Romero, J.; Vernooy, K.; et al. EHRA clinical consensus statement on conduction system pacing implantation: Endorsed by the Asia Pacific Heart Rhythm Society (APHRS), Canadian Heart Rhythm Society (CHRS), and Latin American Heart Rhythm Society (LAHRS). EP Eur. 2023, 25, 1208–1236. [Google Scholar] [CrossRef]
- Ali, N.; Keene, D.; Arnold, A.; Shun-Shin, M.; Whinnett, Z.I.; Sohaib, S.A. His Bundle Pacing: A New Frontier in the Treatment of Heart Failure. Arrhythmia Electrophysiol. Rev. 2018, 7, 103. [Google Scholar] [CrossRef]
- Tokavanich, N.; Prasitlumkum, N.; Mongkonsritragoon, W.; Trongtorsak, A.; Cheungpasitporn, W.; Chokesuwattanaskul, R. QRS area as a predictor of cardiac resynchronization therapy response: A systematic review and meta-analysis. Pacing Clin. Electrophis 2022, 45, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Vijayaraman, P.; Cano, Ó.; Koruth, J.S.; Subzposh, F.A.; Nanda, S.; Pugliese, J.; Ravi, V.; Naperkowski, A.; Sharma, P.S. His-Purkinje Conduction System Pacing Following Transcatheter Aortic Valve Replacement. JACC Clin. Electrophysiol. 2020, 6, 649–657. [Google Scholar] [CrossRef] [PubMed]
- Kron, J.; Padala, S.K. AV Block Post-TAVR. JACC Clin. Electrophysiol. 2020, 6, 658–660. [Google Scholar] [CrossRef] [PubMed]
- De Pooter, J.; Gauthey, A.; Calle, S.; Noel, A.; Kefer, J.; Marchandise, S.; Coeman, M.; Philipsen, T.; Kayaert, P.; Gheeraert, P.; et al. Feasibility of His-bundle pacing in patients with conduction disorders following transcatheter aortic valve replacement. J. Cardiovasc. Electrophysiol. 2020, 31, 813–821. [Google Scholar] [CrossRef] [PubMed]
- Koniari, I.; Tsigkas, G.; Kounis, N.; Velissaris, D.; Chourdakis, E.; Davlouros, P.; Hahalis, G. Incidence, pathophysiology, predictive factors and prognostic implications of new onset atrial fibrillation following transcatheter aortic valve implantation. J. Geriatr. Cardiol. 2018, 15, 50–54. [Google Scholar] [CrossRef]
- Motloch, L.J.; Reda, S.; Rottlaender, D.; Khatib, R.; Müller-Ehmsen, J.; Seck, C.; Strauch, J.; Madershahian, N.; Erdmann, E.; Wahlers, T.; et al. Postprocedural Atrial Fibrillation after Transcatheter Aortic Valve Implantation Versus Surgical Aortic Valve Replacement. Ann. Thorac. Surg. 2012, 93, 124–131. [Google Scholar] [CrossRef]
- Tanawuttiwat, T.; O’Neill, B.P.; Cohen, M.G.; Chinthakanan, O.; Heldman, A.W.; Martinez, C.A.; Alfonso, C.E.; Mitrani, R.D.; Macon, C.J.; Carrillo, R.G.; et al. New-Onset Atrial Fibrillation after Aortic Valve Replacement. J. Am. Coll. Cardiol. 2014, 63, 1510–1519. [Google Scholar] [CrossRef]
- Sannino, A.; Gargiulo, G.; Schiattarella, G.G.; Perrino, C.; Stabile, E.; Losi, M.-A.; Galderisi, M.; Izzo, R.; de Simone, G.; Trimarco, B.; et al. A meta-analysis of the impact of pre-existing and new-onset atrial fibrillation on clinical outcomes in patients undergoing transcatheter aortic valve implantation. EuroIntervention 2016, 12, e1047–e1056. [Google Scholar] [CrossRef]
- Kalra, R.; Patel, N.; Doshi, R.; Arora, G.; Arora, P. Evaluation of the Incidence of New-Onset Atrial Fibrillation after Aortic Valve Replacement. JAMA Intern. Med. 2019, 179, 1122. [Google Scholar] [CrossRef]
- Mentias, A.; Saad, M.; Girotra, S.; Desai, M.; Elbadawi, A.; Briasoulis, A.; Alvarez, P.; Alqasrawi, M.; Giudici, M.; Panaich, S.; et al. Impact of Pre-Existing and New-Onset Atrial Fibrillation on Outcomes after Transcatheter Aortic Valve Replacement. JACC Cardiovasc. Interv. 2019, 12, 2119–2129. [Google Scholar] [CrossRef]
- Biviano, A.B.; Nazif, T.; Dizon, J.; Garan, H.; Fleitman, J.; Hassan, D.; Kapadia, S.; Babaliaros, V.; Xu, K.; Parvataneni, R.; et al. Atrial Fibrillation Is Associated with Increased Mortality in Patients Undergoing Transcatheter Aortic Valve Replacement: Insights from the Placement of Aortic Transcatheter Valve (PARTNER) Trial. Circ. Cardiovasc. Interv. 2016, 9, e002766. [Google Scholar] [CrossRef] [PubMed]
- Tarantini, G.; Mojoli, M.; Urena, M.; Vahanian, A. Atrial fibrillation in patients undergoing transcatheter aortic valve implantation: Epidemiology, timing, predictors, and outcome. Eur. Heart J. 2017, 38, 1285–1293. [Google Scholar] [CrossRef]
- Stähli, B.E.; Grünenfelder, J.; Jacobs, S.; Falk, V.; Landmesser, U.; Wischnewsky, M.B.; Lüscher, T.F.; Corti, R.; Maier, W.; Altwegg, L.A. Assessment of inflammatory response to transfemoral transcatheter aortic valve implantation compared to transapical and surgical procedures: A pilot study. J. Invasive Cardiol. 2012, 24, 407–411. [Google Scholar]
- Sannino, A.; Gargiulo, G.; Schiattarella, G.G.; Brevetti, L.; Perrino, C.; Stabile, E.; Losi, M.A.; Toscano, E.; Giugliano, G.; Scudiero, F.; et al. Increased mortality after transcatheter aortic valve implantation (TAVI) in patients with severe aortic stenosis and low ejection fraction: A meta-analysis of 6898 patients. Int. J. Cardiol. 2014, 176, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Saxena, A.; Dinh, D.T.; Smith, J.A.; Shardey, G.C.; Reid, C.M.; Newcomb, A.E. Usefulness of Postoperative Atrial Fibrillation as an Independent Predictor for Worse Early and Late Outcomes after Isolated Coronary Artery Bypass Grafting (Multicenter Australian Study of 19,497 Patients). Am. J. Cardiol. 2012, 109, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Yankelson, L.; Steinvil, A.; Gershovitz, L.; Leshem-Rubinow, E.; Furer, A.; Viskin, S.; Keren, G.; Banai, S.; Finkelstein, A. Atrial Fibrillation, Stroke, and Mortality Rates after Transcatheter Aortic Valve Implantation. Am. J. Cardiol. 2014, 114, 1861–1866. [Google Scholar] [CrossRef]
- Barbash, I.M.; Minha, S.; Ben-Dor, I.; Dvir, D.; Torguson, R.; Aly, M.; Bond, E.; Satler, L.F.; Pichard, A.D.; Waksman, R. Predictors and clinical implications of atrial fibrillation in patients with severe aortic stenosis undergoing transcatheter aortic valve implantation: AFib in TAVI Patients. Cathet. Cardiovasc. Intervent. 2015, 85, 468–477. [Google Scholar] [CrossRef]
- Stortecky, S.; Buellesfeld, L.; Wenaweser, P.; Heg, D.; Pilgrim, T.; Khattab, A.A.; Gloekler, S.; Huber, C.; Nietlispach, F.; Meier, B.; et al. Atrial Fibrillation and Aortic Stenosis: Impact on Clinical Outcomes Among Patients Undergoing Transcatheter Aortic Valve Implantation. Circ. Cardiovasc. Interv. 2013, 6, 77–84. [Google Scholar] [CrossRef]
- Holmes, D.R.; Brennan, J.M.; Rumsfeld, J.S.; Dai, D.; O’Brien, S.M.; Vemulapalli, S.; Edwards, F.H.; Carroll, J.; Shahian, D.; Grover, F.; et al. Clinical Outcomes at 1 Year Following Transcatheter Aortic Valve Replacement. JAMA 2015, 313, 1019. [Google Scholar] [CrossRef]
- Siontis, G.C.M.; Praz, F.; Lanz, J.; Vollenbroich, R.; Roten, L.; Stortecky, S.; Räber, L.; Windecker, S.; Pilgrim, T. New-onset arrhythmias following transcatheter aortic valve implantation: A systematic review and meta-analysis. Heart 2018, 104, 1208–1215. [Google Scholar] [CrossRef]
- Tempio, D.; Pruiti, G.P.; Conti, S.; Romano, S.A.; Tavano, E.; Capodanno, D.; Liotta, C.; Di Grazia, A.; Tamburino, C.; Calvi, V. Ventricular arrhythmias in aortic valve stenosis before and after transcatheter aortic valve implantation. Europace 2015, 17, 1136–1140. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Lahiri, M.K.; Khan, A.; Schuger, C.D. Bundle branch reentrant ventricular tachycardia after transcatheter aortic valve replacement. Hear. Case Rep. 2017, 3, 177–185. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions, and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions, or products referred to in the content. |

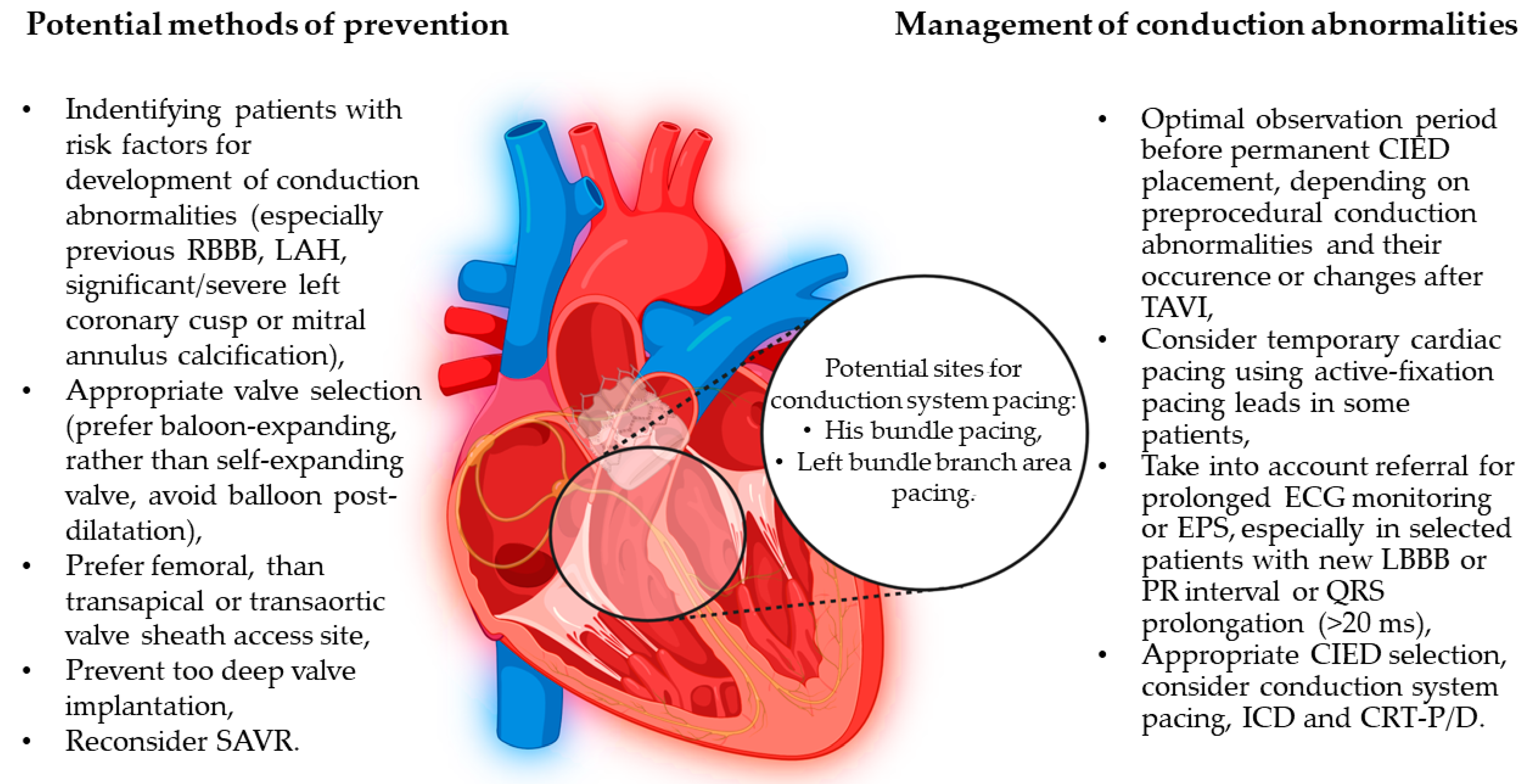

| Management | ESC 2021 Guidelines | ACC/AHA 2018 Guidelines |
|---|---|---|
| PM implantation | PM implantation: high- or third-degree AVB persisting for 24–48 h or new alternating BBB after TAVI procedure (class I recommendation, levels of evidence B or C, respectively), PM implantation within 24 h or immediately after TAVI: previous RBBB with new conduction abnormalities (change in QRS axis, increase in PR interval, transient high-degree AVB) (class IIa recommendation, level of evidence B). | PM implantation before discharge: new AVB with symptoms/hemodynamic instability (class I recommendation, level of evidence B) PM implantation: new persistent LBBB (class IIb recommendation, level of evidence B) |
| Additional examination/observation | Continuous ambulatory ECG monitoring for 7–30 days or EPS ≥ 3 days after TAVI:
| Careful observation: new persistent BBB (class II b recommendation, level of evidence B) |
| Siontis et al. [39] a Meta-Analysis, n = 11,210, 17% Required PM RR (95% CI) | Fadahunsi et al. [54] n = 9785, 6.7% Required PM within 30 Days of TAVI OR (95% CI) * |
|---|---|
| Male sex: 1.23 (1.10–1.38) | Age (per 5-year): 1.07 (1.01–1.15) |
| First-degree AVB: 1.52 (1.15–2.01) | Previous conduction defect: 1.93 (1.63–2.29) |
| LAH: 1.62 (1.17–2.25) | Aortic valve area when ≤0.75 cm2 (per 0.25 cm2): 1.21 (1.00–1.45) |
| Right bundle branch block: 2.89 (2.36–3.54) | Self-expanding MCRS vs. balloon-expanding ESV: 7.56 (5.98–9.56) |
| Intraoperative AVB: 3.49 (2.49–4.89) | Access site (for valve sheath): transapical vs. femoral 1.36 (1.10–1.68) transaortic vs. femoral 1.52 (1.09–2.11) |
| MCRS vs. ESV: 2.54 (2.08–3.12) | Surgical risk (STS PROM): high vs. inoperable/extreme 1.85 (1.54–2.21) intermediate vs. inoperable/extreme 1.78 (1.04–3.04) |
| Home oxygen use: 0.67 (0.49–0.91) | |
| Previous aortic valve procedure: 0.74 (0.57–0.95) | |
| Procedure time (per 30 min): 0.95 (0.92–0.99) |
| PM Implantation Rate after 30 Days or after 1 Year | Name of the Valve and Manufacturer | Type of TAVI System | PM Implantation Rate after 30 Days or after 1 Year |
|---|---|---|---|
| Low risk (<10%) | Acurate (Boston Scientific) | self-expanding | 2.5% |
| J-valve (Jie Cheng Medical Technologies) | 2.3–4.8% ** | ||
| Acurate neo™ (Boston Scientific) | 6.7% | ||
| NEO 2 (Boston Scientific) | 6.0–7.7% | ||
| Myval (Meril Life Sciences) | balloon-expandable | 5.8–8% | |
| Hydra (Vascular Innovations) | self-expanding | 7.5% | |
| Low-moderate risk (5–20%) | Portico (Abbott) | self-expanding | 9.7% or 8.8–15.8% * |
| Sapien 3 (Edwards Lifesciences) | balloon-expandable | 8.2–10.1% | |
| Venus-A (Venus Medtech) | self-expanding | 7.4–18.8% | |
| CoreValve Evolut R (Medtronic) | 8.3–11.7% | ||
| Evolut Pro (Medtronic) | 10.8–11.9% | ||
| JenaValve (JenaValve) | 12.1%/9.1% * | ||
| Moderate risk (10–20%) | Navitor (Abbott) | self-expanding | 15% |
| Direct Flow Medical (Direct Flow Medical) | mechanical | 13–17% | |
| wVR VitaFlow (Microport) | self-expanding | 16.4–19.1% ** | |
| High risk (>20%) | Centera (Edwards Lifesciences) | self-expanding | 27% |
| Engager (Medtronic) | 28.5% | ||
| Lotus™ Valve (Boston Scientific) | mechanical | 23.4–28.6% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szotek, M.; Drużbicki, Ł.; Sabatowski, K.; Amoroso, G.R.; De Schouwer, K.; Matusik, P.T. Transcatheter Aortic Valve Implantation and Cardiac Conduction Abnormalities: Prevalence, Risk Factors and Management. J. Clin. Med. 2023, 12, 6056. https://doi.org/10.3390/jcm12186056
Szotek M, Drużbicki Ł, Sabatowski K, Amoroso GR, De Schouwer K, Matusik PT. Transcatheter Aortic Valve Implantation and Cardiac Conduction Abnormalities: Prevalence, Risk Factors and Management. Journal of Clinical Medicine. 2023; 12(18):6056. https://doi.org/10.3390/jcm12186056
Chicago/Turabian StyleSzotek, Michał, Łukasz Drużbicki, Karol Sabatowski, Gisella R. Amoroso, Koen De Schouwer, and Paweł T. Matusik. 2023. "Transcatheter Aortic Valve Implantation and Cardiac Conduction Abnormalities: Prevalence, Risk Factors and Management" Journal of Clinical Medicine 12, no. 18: 6056. https://doi.org/10.3390/jcm12186056






