Initial and Residual 3D Fracture Displacement Is Predictive for Patient-Reported Functional Outcome at Mid-Term Follow-Up in Surgically Treated Tibial Plateau Fractures
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Patient-Reported Outcome
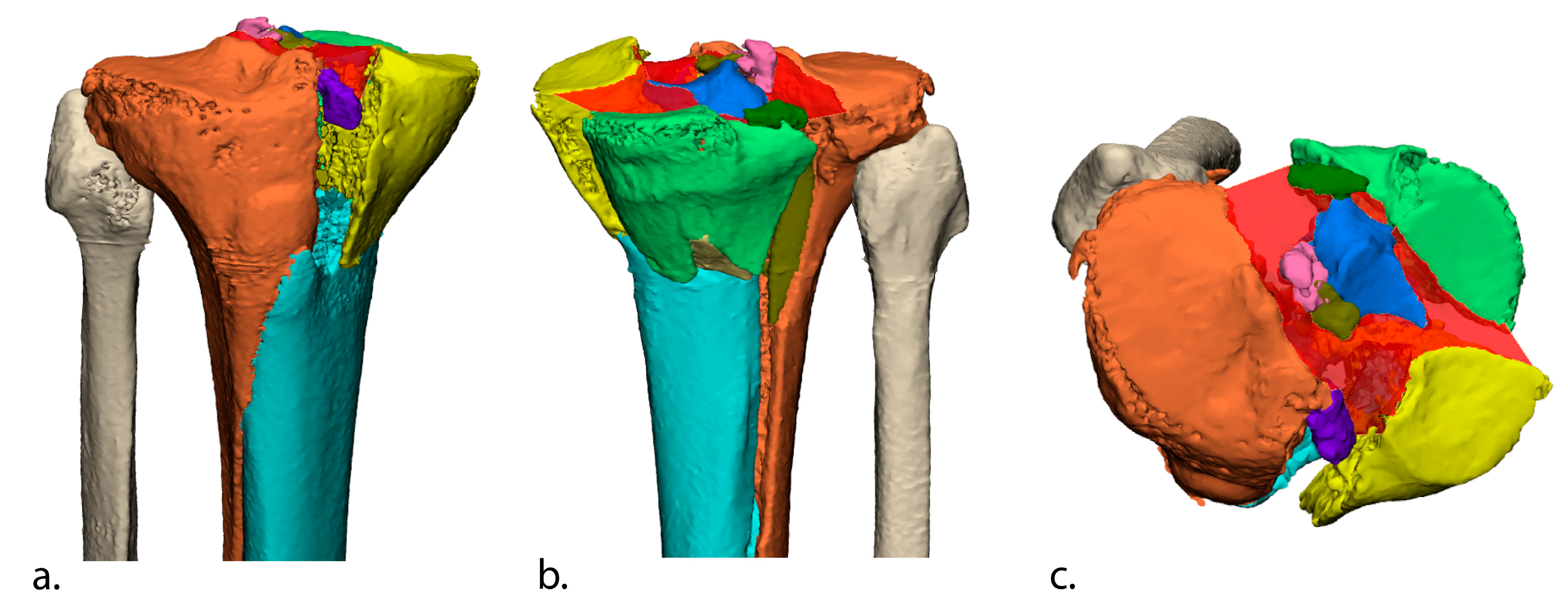
2.4. Three-Dimensional Assessment of Initial (Preoperative) and Residual (Postoperative) Fracture Displacement
2.4.1. Initial Fracture Displacement
2.4.2. Residual Fracture Displacement
2.5. Postoperative Evaluation
2.6. Primary and Secondary Study Goals
2.7. Statistical Analysis
2.8. Analysis of Nonresponders
3. Results
3.1. Association between Initial 3D Displacement and Functional Outcome
3.2. Association between Residual 3D Displacement and Functional Outcome
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A. Full Regression Model for Initial 3D Displacement in Relation to KOOS Functional Outcome
| KOOS-Symptoms (R2 = 0.22, adj R2 = 0.20) | KOOS-Pain (R2 = 0.21, adj R2 = 0.19) | KOOS-ADL (R2 = 0.22, adj R2 = 0.20) | KOOS-Sport (R2 = 0.18, adj R2 = 0.16) | KOOS-QoL (R2 = 0.18, adj R2 = 0.16) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| B (95% CI) | p Value | B (95% CI) | p Value | B (95% CI) | p Value | B (95% CI) | p Value | B (95% CI) | p Value | |
| Preoperative 3D gap area (×100) | −0.9 (−1.3 to −0.5) | <0.001 | −0.9 (−1.4 to −0.5) | <0.001 | −0.8 (−1.2 to −0.4) | 0.002 | −1.4 (−2.1 to −0.7) | <0.001 | −1.1 (−1.7 to −0.5) | <0.001 |
| Age | 0.2 (0.0 to 0.4) | 0.016 | 0.1 (−0.1 to 0.2) | 0.452 | −0.1 (−0.2 to 0.1) | 0.429 | 0.1 (−0.2 to 0.3) | 0.482 | 0.1 (−0.1 to 0.3) | 0.221 |
| Male | 5.2 (0.7 to 9.7) | 0.027 | 6.3 (1.5 to 11.1) | 0.010 | 6.3 (2.0 to 10.7) | 0.004 | 12.1 (4.9 to 19.3) | 0.001 | 4.6 (−1.3 to 10.6) | 0.127 |
| BMI | −0.7 (−1.1 to −0.3) | 0.002 | −0.9 (−1.4 to −0.5) | <0.001 | −1.0 (−1.4 to −0.5 | <0.001 | −1.4 (−2.1 to −0.7) | <0.001 | −0.9 (−1.4 to −0.3) | 0.004 |
| Smoking | −4.0 (−8.8 to 0.8) | 0.104 | −6.5 (−11.7 to −1.3) | 0.014 | −7.1 (−11.8 to −2.4) | 0.003 | −6.3 (−14.1 to 1.5) | 0.115 | −5.6 (−12.0 to 0.9) | 0.090 |
| AO/OTA | −0.8 (−2.4 to 0.9) | 0.374 | −0.3 (−2.1 to 1.5) | 0.714 | −0.7 (−2.3 to 0.9) | 0.410 | −1.6 (−4.2 to 1.1) | 0.256 | −0.8 (−3.0 to 1.4) | 0.494 |
| Postoperative complication | −9.9 (−16.3 to −3.4) | 0.003 | −9.8 (−16.8 to −2.9) | 0.006 | −8.9 (−15.2 to −2.6) | 0.006 | −9.1 (−19.4 to 1.3) | 0.086 | −11.2 (−19.9 to −2.6) | 0.011 |
| Nonanatomical reduction | −5.7 (−10.3 to −1.0) | 0.016 | −5.0 (−10.0 to −0.5) | 0.048 | −2.8 (−7.3 to 1.7) | 0.223 | −4.6 (−12.2 to 2.9) | 0.229 | −7.4 (−13.6 to −1.2) | 0.018 |
| Follow-up time (years) | 0.7 (0.1 to 1.2) | 0.018 | 0.5 (−0.1 to 1.1) | 0.089 | 0.5 (−0.1 to 1.0) | 0.081 | 0.3 (−0.6 to 1.2) | 0.548 | 0.8 (0.0 to 1.5) | 0.045 |
Appendix B. Full Regression Model for Residual 3D Displacement in Relation to KOOS Functional Outcome
| KOOS-Symptoms (R2 = 0.28, adj R2 = 0.19) | KOOS-Pain (R2 = 0.27, adj R2 = 0.17) | KOOS-ADL (R2 = 0.31, adj R2 = 0.21) | KOOS-Sport (R2 = 0.20, adj R2 = 0.09) | KOOS-QoL (R2 = 0.27, adj R2 = 0.17) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| B (95% CI) | p Value | B (95% CI) | p Value | B (95% CI) | p Value | B (95% CI) | p Value | B (95% CI) | p Value | |
| Postoperative 3D gap area (×100) | −2.2 (−3.9 to −0.5) | 0.011 | −2.4 (−4.4 to −0.5) | 0.17 | −2.2 (−3.9 to −0.0) | 0.014 | −2.6 (−5.0 to −0.2) | 0.033 | −2.4 (−4.5 to −0.3) | 0.023 |
| Age | −0.2 (−0.5 to 0.2) | 0.360 | −0.4 (−0.8 to −0.0) | 0.046 | −0.4 (−0.7 to −0.0) | 0.044 | −0.2 (−0.7 to 0.3) | 0.418 | −0.2 (−0.6 to 0.0) | 0.361 |
| Male sex | 6.1 (−4.0 to 16.2) | 0.232 | 2.6 (−9.3 to 14.6) | 0.659 | 2.1 (−8.3 to 12.5) | 0.687 | 2.5 (−11.7 to 16.8) | 0.723 | −4.8 (−17.3 to 7.8) | 0.449 |
| BMI | −0.4 (−1.2 to 0.5) | 0.367 | −0.3 (−1.3 to 0.6) | 0.487 | −0.4 (−1.2 to 0.5) | 0.383 | 0.0 (−1.1 to 1.2) | 0.938 | 0.3 (−0.7 to 1.4) | 0.505 |
| Smoking | −6.3 (−16.6 to 3.8) | 0.216 | −2.5 (−14.6 to 9.5) | 0.675 | −1.9 (−12.4 to 8.6) | 0.723 | −0.8 (−15.2 to 13.6) | 0.912 | −3.5 (−16.1 to 9.2) | 0.588 |
| AO/OTA | −1.1 (−4.3 to 2.0) | 0.479 | −1.2 (−4.9 to 2.6) | 0.532 | −1.3 (−4.6 to 1.9) | 0.424 | −2.1 (−6.6 to 2.2) | 0.339 | −2.9 (−6.9 to 1.0) | 0.143 |
| Postoperative complication | −4.7 (−14.8 to 5.7) | 0.361 | −2.3 (−14.3 to 9.6) | 0.698 | −3.4 (−13.8 to 7.0) | 0.513 | 5.6 (−8.7 to 20.0) | 0.433 | −1.8 (−14.4 to 10.8) | 0.773 |
| Follow-up time (years) | 0.3 (−1.0 to 1.6) | 0.641 | −0.6 (−2.1 to 0.9) | 0.448 | 0.4 (−0.9 to 1.7) | 0.557 | 0.2 (−1.6 to 2.0) | 0.829 | 0.2 (−1.4 to 1.8) | 0.798 |
References
- Weigel, D.P.; Marsh, J.L. High-Energy Fractures of the Tibial Plateau: Knee Function after Longer Follow-Up. JBJS 2002, 84, 1541–1551. [Google Scholar] [CrossRef] [PubMed]
- Meulenkamp, B.; Martin, R.; Desy, N.M.; Duffy, P.; Korley, R.; Puloski, S.; Buckley, R. Incidence, Risk Factors, and Location of Articular Malreductions of the Tibial Plateau. J. Orthop. Trauma 2017, 31, 146–150. [Google Scholar] [CrossRef] [PubMed]
- Tscherne, H.; Lobenhoffer, P. Tibial Plateau Fractures: Management and Expected Results. Clin. Orthop. Related Res. 1993, 292, 87–100. [Google Scholar] [CrossRef]
- Prat-Fabregat, S.; Camacho-Carrasco, P. Treatment Strategy for Tibial Plateau Fractures: An Update. EFORT Open Rev. 2016, 1, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Parkkinen, M.; Madanat, R.; Mustonen, A.; Koskinen, S.K.; Paavola, M.; Lindahl, J. Factors Predicting the Development of Early Osteoarthritis Following Lateral Tibial Plateau Fractures: Mid-Term Clinical and Radiographic Outcomes of 73 Operatively Treated Patients. Scand. J. Surg. 2014, 103, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Giannoudis, P.V.; Tzioupis, C.; Papathanassopoulos, A.; Obakponovwe, O.; Roberts, C. Articular Step-off and Risk of Post-Traumatic Osteoarthritis. Evidence Today. Injury 2010, 41, 986–995. [Google Scholar] [CrossRef] [PubMed]
- Van Dreumel, R.L.M.; Van Wunnik, B.P.W.; Janssen, L.; Simons, P.C.G.; Janzing, H.M.J. Mid-to Long-Term Functional Outcome after Open Reduction and Internal Fixation of Tibial Plateau Fractures. Injury 2015, 46, 1608–1612. [Google Scholar] [CrossRef] [PubMed]
- Assink, N.; Kraeima, J.; Slump, C.H.; ten Duis, K.; de Vries, J.P.P.M.; Meesters, A.M.L.; van Ooijen, P.; Witjes, M.J.H.; IJpma, F.F.A. Quantitative 3D Measurements of Tibial Plateau Fractures. Sci. Rep. 2019, 9, 14395. [Google Scholar] [CrossRef]
- Marsh, J.L.; Buckwalter, J.; Gelberman, R.; Dirschl, D.; Olson, S.; Brown, T.; Llinias, A. Articular Fractures: Does an Anatomic Reduction Really Change the Result? J. Bone Jt. Surg. Ser. A 2002, 84, 1259–1271. [Google Scholar] [CrossRef]
- Watson, N.J.; Asadollahi, S.; Parrish, F.; Ridgway, J.; Tran, P.; Keating, J.L. Reliability of Radiographic Measurements for Acute Distal Radius Fractures. BMC Med. Imaging 2016, 16, 44. [Google Scholar] [CrossRef]
- Assink, N.; Kraeima, J.; Meesters, A.M.L.; El Moumni, M.; Bosma, E.; Nijveldt, R.J.; van Helden, S.H.; de Vries, J.-P.P.M.; Witjes, M.J.H.; IJpma, F.F.A. 3D Assessment of Initial Fracture Displacement of Tibial Plateau Fractures Is Predictive for Risk on Conversion to Total Knee Arthroplasty at Long-Term Follow-Up. Eur. J. Trauma Emerg. Surg. 2022, 49, 867–874. [Google Scholar] [CrossRef] [PubMed]
- Müller, M.E.; Nazarian, S.; Koch, P.; Schatzker, J. The Comprehensive Classification of Fractures of Long Bones; Müller, M.E., Ed.; Springer: Berlin/Heidelberg, Germany, 1990. [Google Scholar]
- De Groot, I.B.; Favejee, M.M.; Reijman, M.; Verhaar, J.A.N.; Terwee, C.B. The Dutch Version of the Knee Injury and Osteoarthritis Outcome Score: A Validation Study. Health Qual. Life Outcomes 2008, 6, 16. [Google Scholar] [CrossRef] [PubMed]
- Heir, S.; Nerhus, T.K.; Røtterud, J.H.; Løken, S.; Ekeland, A.; Engebretsen, L.; Årøen, A. Focal Cartilage Defects in the Knee Impair Quality of Life as Much as Severe Osteoarthritis: A Comparison of Knee Injury and Osteoarthritis Outcome Score in 4 Patient Categories Scheduled for Knee Surgery. Am. J. Sports Med. 2010, 38, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Singleton, N.; Sahakian, V.; Muir, D. Outcome after Tibial Plateau Fracture: How Important Is Restoration of Articular Congruity? J. Orthop. Trauma 2017, 31, 158–163. [Google Scholar] [CrossRef]
- Van Den Berg, J.; Reul, M.; Nunes Cardozo, M.; Starovoyt, A.; Geusens, E.; Nijs, S.; Hoekstra, H. Functional Outcome of Intra-Articular Tibial Plateau Fractures: The Impact of Posterior Column Fractures. Int. Orthop. 2017, 41, 1865–1873. [Google Scholar] [CrossRef] [PubMed]
- Assink, N.; El Moumni, M.; Kraeima, J.; Bosma, E.; Nijveldt, R.J.; van Helden, S.H.; Vaartjes, T.P.; Ten Brinke, J.G.; Witjes, M.J.H.; de Vries, J.-P.P.M. Radiographic Predictors of Conversion to Total Knee Arthroplasty After Tibial Plateau Fracture Surgery: Results in a Large Multicenter Cohort. JBJS J. Bone Jt. Surg. Am. 2023, 105, 1237–1245. [Google Scholar] [CrossRef] [PubMed]
- Barei, D.P.; Nork, S.E.; Mills, W.J.; Bradford Henley, M.; Benirschke, S.K. Complications Associated with Internal Fixation of High-Energy Bicondylar Tibial Plateau Fractures Utilizing a Two-Incision Technique. J. Orthop Trauma 2004, 18, 649–657. [Google Scholar] [CrossRef] [PubMed]
- Barei, D.P.; Nork, S.E.; Mills, W.J.; Coles, C.P.; Henley, M.B.; Benirschke, S.K. Functional Outcomes of Severe Bicondylar Tibial Plateau Fractures Treated with Dual Inci-Sions and Medial and Lateral Plates. Injury 2006, 45, 1980–1984. [Google Scholar]
- Manidakis, N.; Dosani, A.; Dimitriou, R.; Stengel, D.; Matthews, S.; Giannoudis, P. Tibial Plateau Fractures: Functional Outcome and Incidence of Osteoarthritis in 125 Cases. Int. Orthop. 2010, 34, 565–570. [Google Scholar] [CrossRef]
- Parkkinen, M.; Lindahl, J.; Mäkinen, T.J.; Koskinen, S.K.; Mustonen, A.; Madanat, R. Predictors of Osteoarthritis Following Operative Treatment of Medial Tibial Plateau Fractures. Injury 2018, 49, 370–375. [Google Scholar] [CrossRef]
- Jacquet, C.; Pioger, C.; Khakha, R.; Steltzlen, C.; Kley, K.; Pujol, N.; Ollivier, M. Evaluation of the “Minimal Clinically Important Difference”(MCID) of the KOOS, KSS and SF-12 Scores after Open-Wedge High Tibial Osteotomy. Knee Surg. Sports Traumatol. Arthrosc. 2021, 29, 820–826. [Google Scholar] [CrossRef]
- Cole, R.J.; Bindra, R.R.; Evanoff, B.A.; Gilula, L.A.; Yamaguchi, K.; Gelberman, R.H. Radiographic Evaluation of Osseous Displacement Following Intra-Articular Fractures of the Distal Radius: Reliability of Plain Radiography versus Computed Tomography. J. Hand Surg. Am. 1997, 22, 792–800. [Google Scholar] [CrossRef]
- Borrelli, J., Jr.; Goldfarb, C.; Catalano, L.; Evanoff, B.A. Assessment of Articular Fragment Displacement in Acetabular Fractures: A Comparison of Computerized Tomography and Plain Radiographs. J. Orthop. Trauma 2002, 16, 449–456. [Google Scholar] [CrossRef]




| Parameter | Value |
|---|---|
| Age in years | 52 (±14) |
| Women | 250 (69%) |
| BMI in kg/m2 | 26.2 (±4.7) |
| Smoking | 83 (23%) |
| AO/OTA classification | |
| 41-B1 | 19 (5%) |
| 41-B2 | 58 (16%) |
| 41-B3 | 195 (54%) |
| 41-C1 | 22 (6%) |
| 41-C2 | 9 (3%) |
| 41-C3 | 59 (16%) |
| Surgical treatment | |
| Plate osteosynthesis | 294 (81%) |
| Screw osteosynthesis | 68 (19%) |
| Complication | |
| Infection | 21 (6%) |
| Malunion | 11 (3%) |
| Meniscal or ligamental reconstruction | 6 (2%) |
| Nerve damage | 2 (1%) |
| Compartment syndrome | 1 (0%) |
| Follow-up (years) | 7.0 (±3.7) |
| Conversion to total knee arthroplasty | 51 (14%) |
| Initial 3D Gap Area (×100) (n = 362) * | Residual 3D Gap Area (×100) (n = 72) ** | |||
|---|---|---|---|---|
| B (95% CI) | p Value | B (95% CI) | p Value | |
| KOOS-Symptoms | −0.9 (−1.3 to −0.5) | <0.001 | −2.2 (−3.9 to −0.5) | 0.011 |
| KOOS-Pain | −0.9 (−1.4 to −0.5) | <0.001 | −2.4 (−4.4 to 0.2) | 0.17 |
| KOOS-ADL | −0.8 (−1.2 to −0.4) | 0.002 | −2.2 (−3.9 to −0.0) | 0.014 |
| KOOS-Sport | −1.4 (−2.1 to −0.7) | <0.001 | −2.6 (−5.0 to −0.2) | 0.033 |
| KOOS-QoL | −1.1 (−1.7 to −0.5) | <0.001 | −2.4 (−4.5 to −0.3) | 0.023 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Assink, N.; Bosma, E.; Meesters, A.M.L.; van Helden, S.H.; Nijveldt, R.J.; ten Duis, K.; Witjes, M.J.H.; de Vries, J.-P.P.M.; Kraeima, J.; IJpma, F.F.A. Initial and Residual 3D Fracture Displacement Is Predictive for Patient-Reported Functional Outcome at Mid-Term Follow-Up in Surgically Treated Tibial Plateau Fractures. J. Clin. Med. 2023, 12, 6055. https://doi.org/10.3390/jcm12186055
Assink N, Bosma E, Meesters AML, van Helden SH, Nijveldt RJ, ten Duis K, Witjes MJH, de Vries J-PPM, Kraeima J, IJpma FFA. Initial and Residual 3D Fracture Displacement Is Predictive for Patient-Reported Functional Outcome at Mid-Term Follow-Up in Surgically Treated Tibial Plateau Fractures. Journal of Clinical Medicine. 2023; 12(18):6055. https://doi.org/10.3390/jcm12186055
Chicago/Turabian StyleAssink, Nick, Eelke Bosma, Anne M. L. Meesters, Sven H. van Helden, Robert J. Nijveldt, Kaj ten Duis, Max J. H. Witjes, Jean-Paul P. M. de Vries, Joep Kraeima, and Frank F. A. IJpma. 2023. "Initial and Residual 3D Fracture Displacement Is Predictive for Patient-Reported Functional Outcome at Mid-Term Follow-Up in Surgically Treated Tibial Plateau Fractures" Journal of Clinical Medicine 12, no. 18: 6055. https://doi.org/10.3390/jcm12186055









