Endovascular and Open Surgical Treatment of Ruptured Splenic Artery Aneurysms: A Case Report and a Systematic Literature Review
Abstract
1. Introduction
2. Materials and Methods
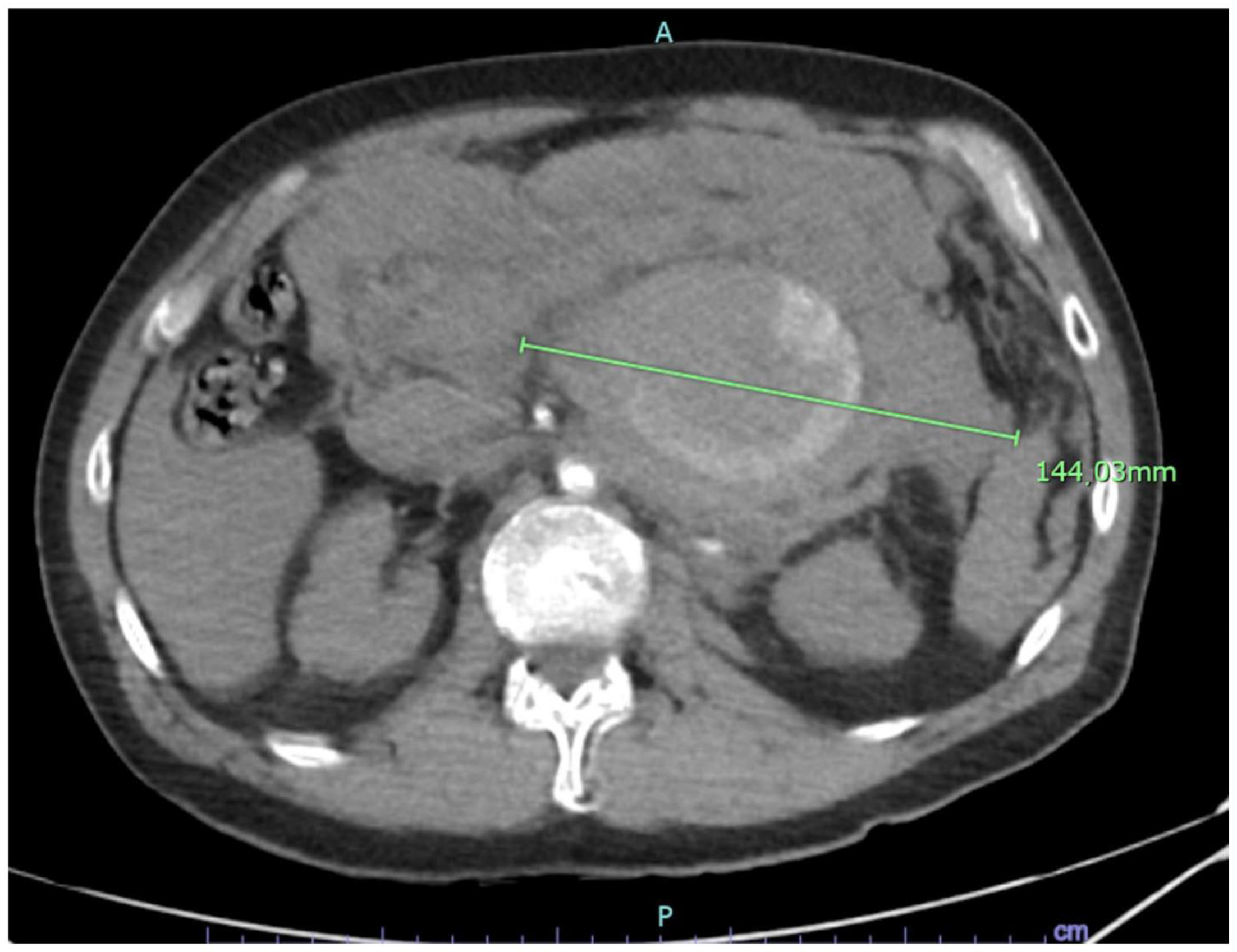
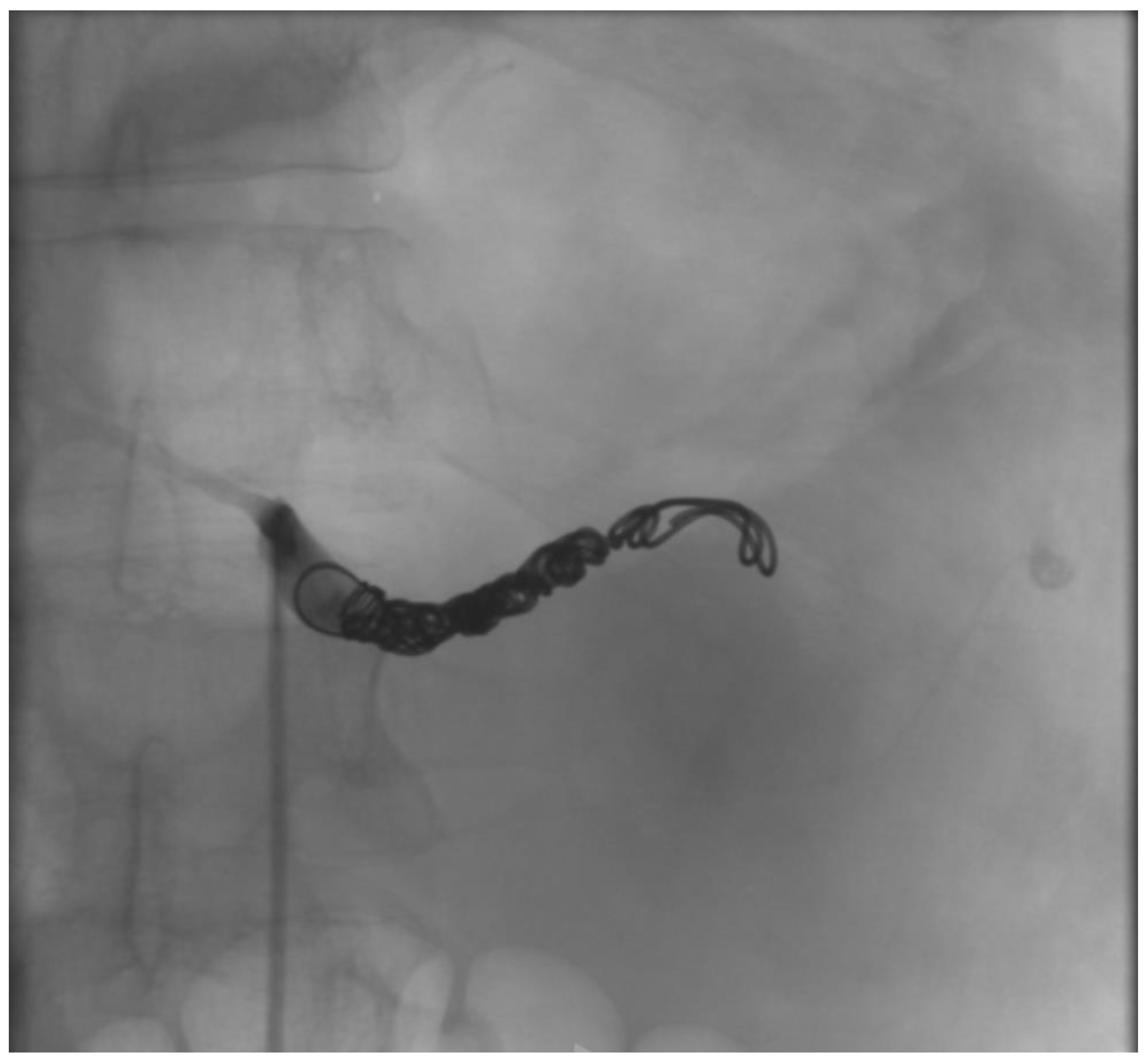
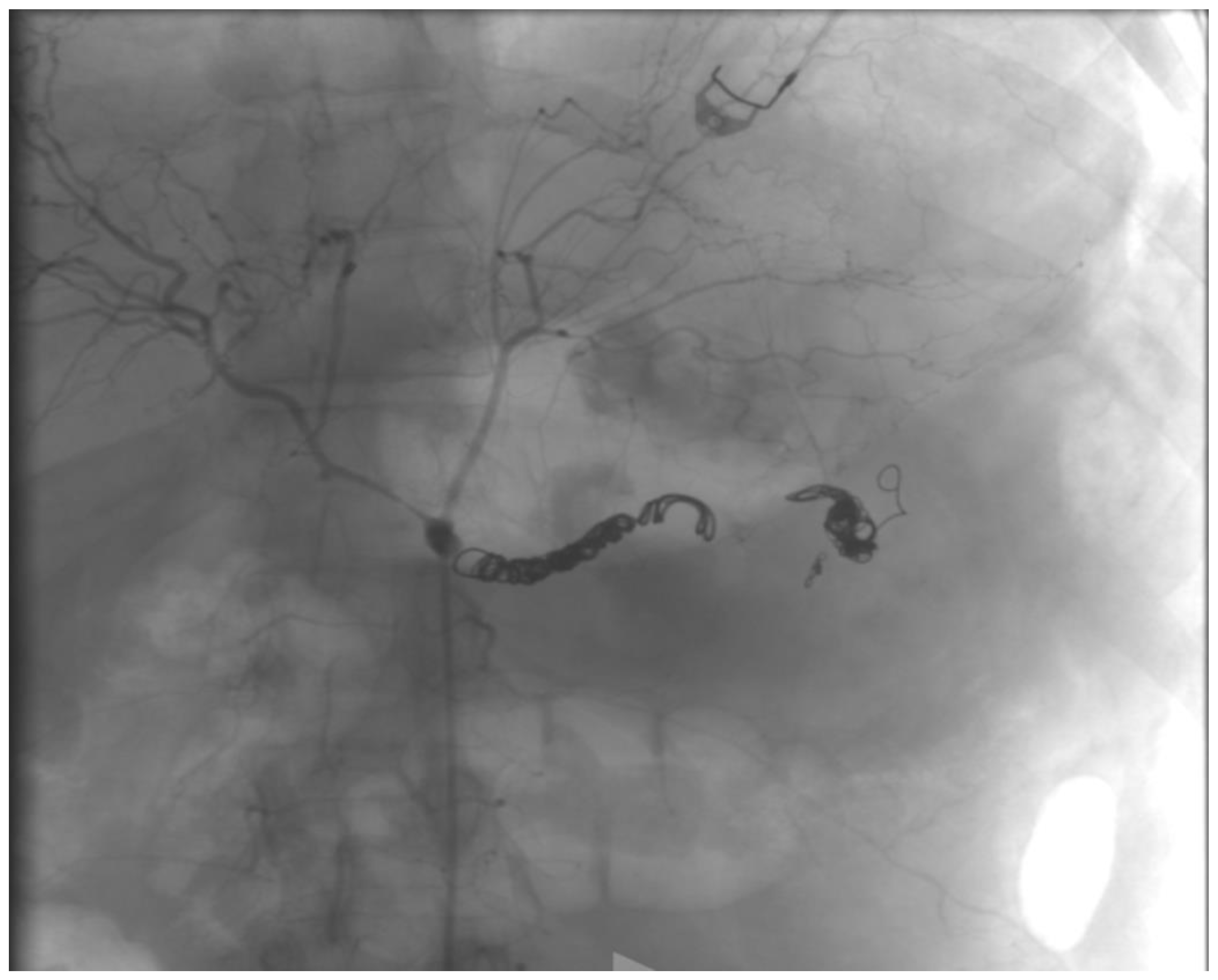
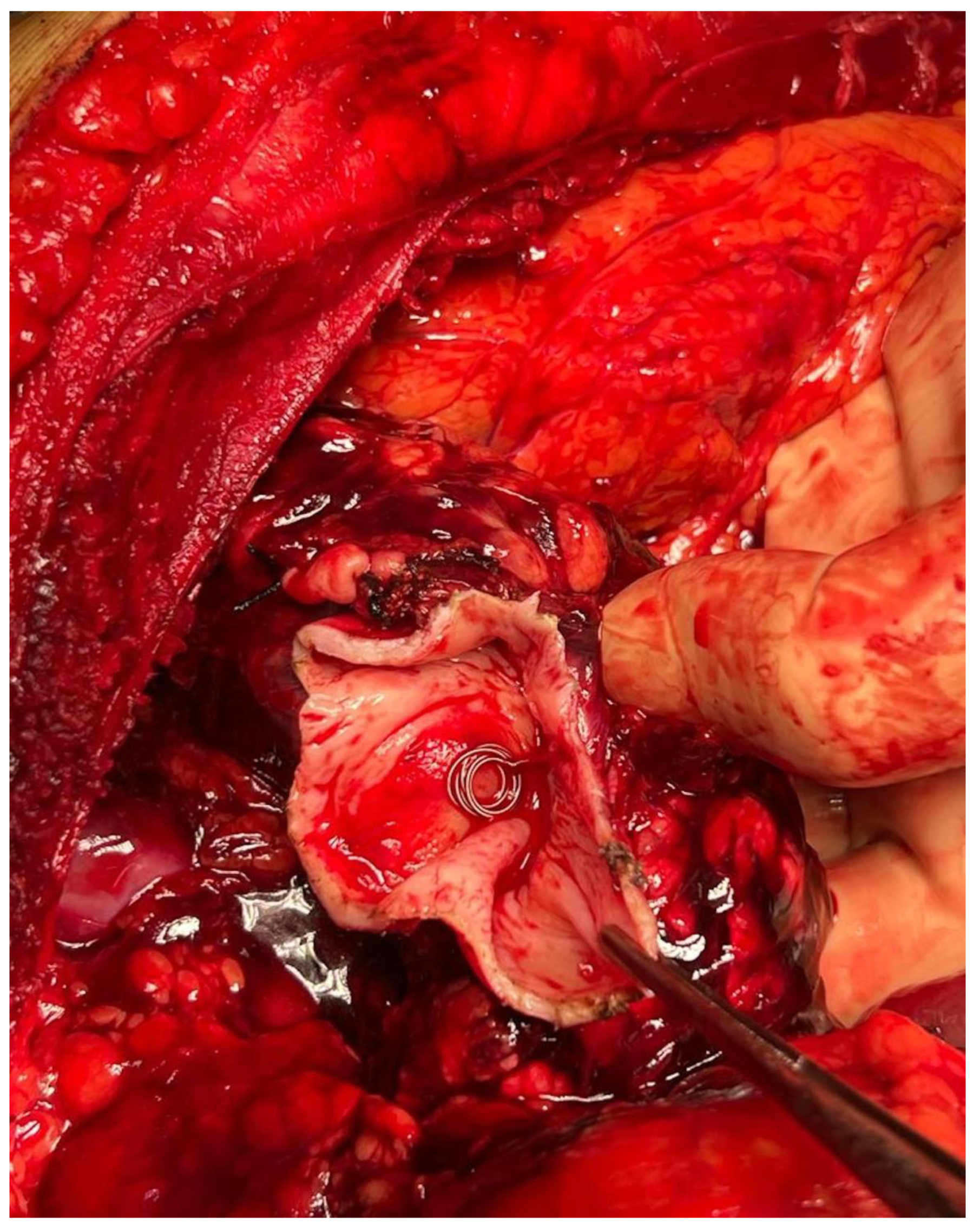
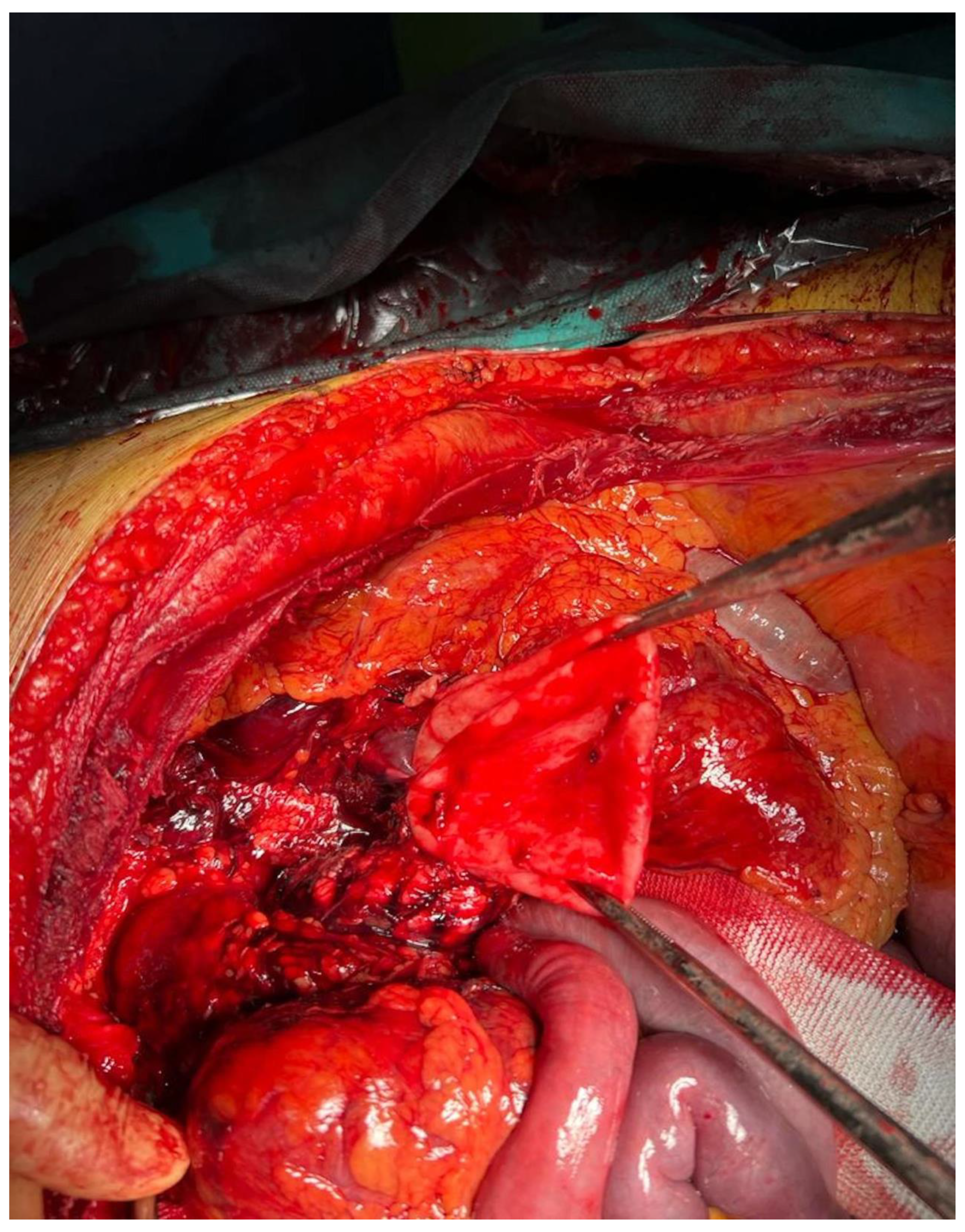
2.1. Case Report
2.2. Literature Review
2.2.1. Search Strategy
2.2.2. Inclusion and Exclusion Criteria
2.2.3. Outcome Measures
2.3. Statistical Analysis
3. Results
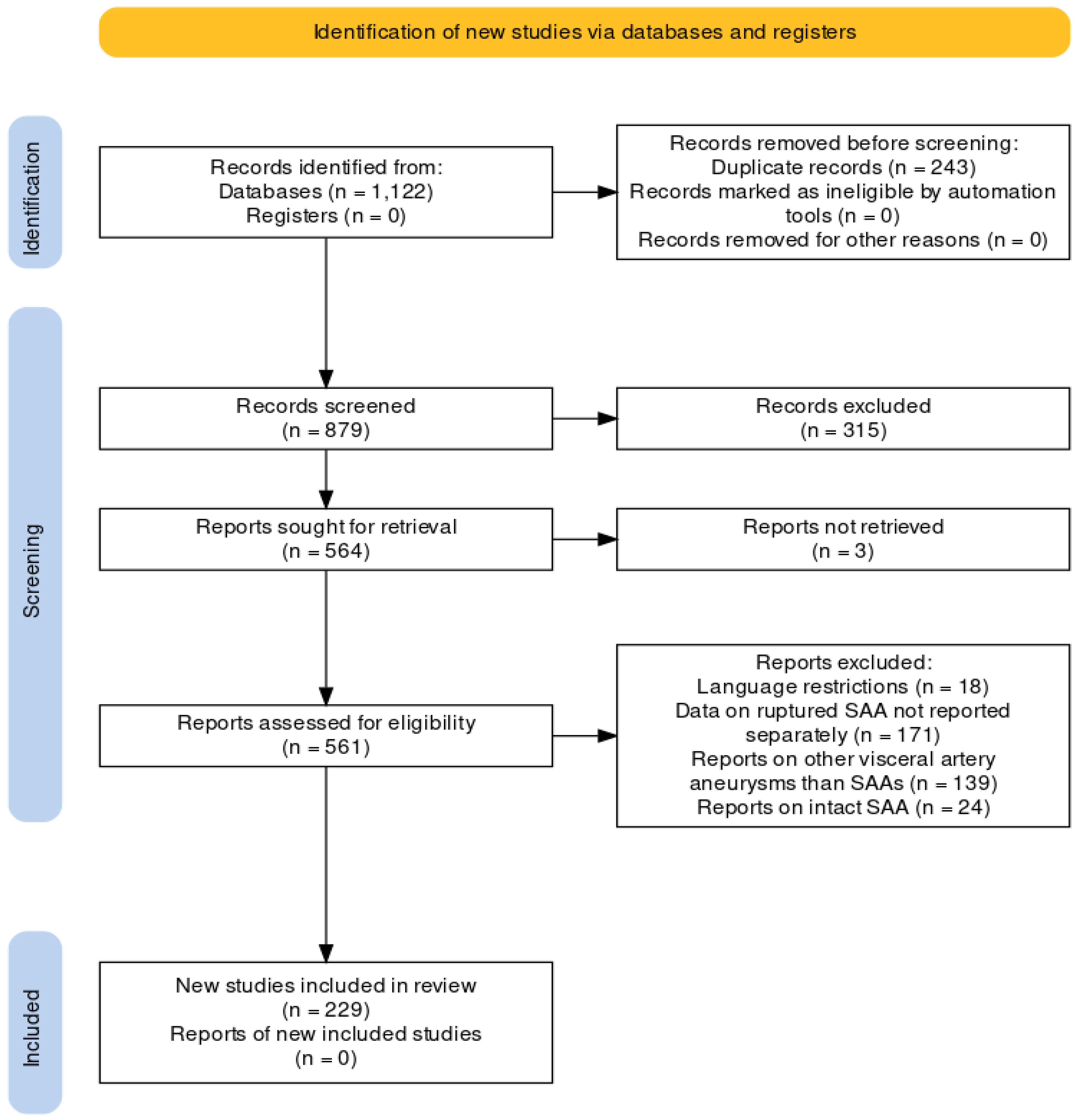
3.1. Study Selection
3.2. Findings
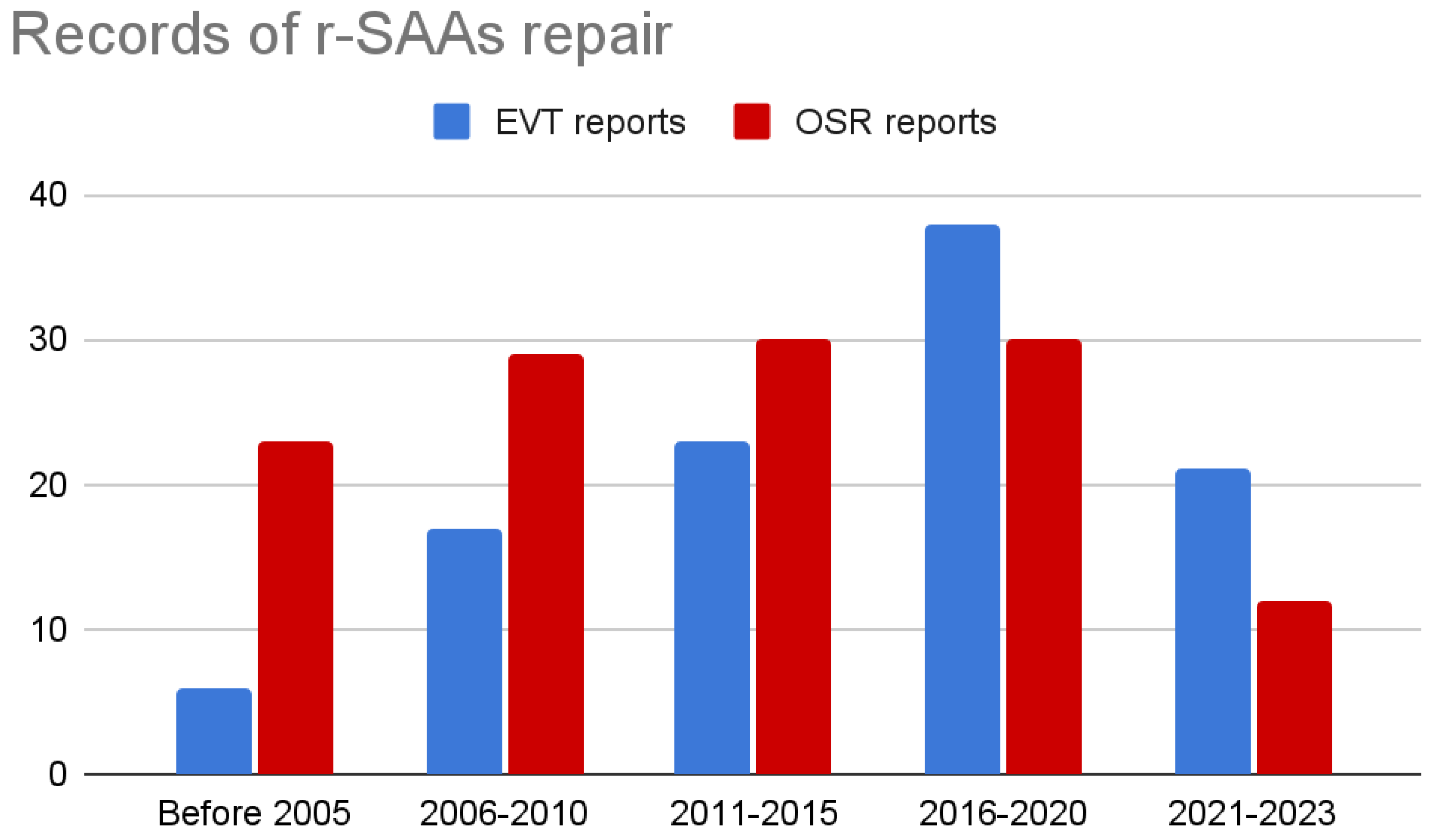
3.2.1. Patients
3.2.2. Outcomes
3.2.3. Regression
4. Discussion
Limitations
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Chaer, R.A.; Abularrage, C.J.; Coleman, D.M.; Eslami, M.H.; Kashyap, V.S.; Rockman, C.; Murad, M.H. The Society for Vascular Surgery clinical practice guidelines on the management of visceral aneurysms. J. Vasc. Surg. 2020, 72, 3S–39S. [Google Scholar] [CrossRef]
- Björck, M.; Koelemay, M.; Acosta, S.; Goncalves, F.B.; Kölbel, T.; Kolkman, J.; Lees, T.; Lefevre, J.; Menyhei, G.; Oderich, G.; et al. Editor’s Choice—Management of the Diseases of Mesenteric Arteries and Veins: Clinical Practice Guidelines of the European Society of Vascular Surgery (ESVS). Eur. J. Vasc. Endovasc. Surg. 2017, 53, 460–510. [Google Scholar] [CrossRef] [PubMed]
- Cordova, A.C.; Sumpio, B.E. Visceral Artery Aneurysms and Pseudoaneurysms—Should They All be Managed by Endovascular Techniques? Ann. Vasc. Dis. 2013, 6, 687–693. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, 71. [Google Scholar] [CrossRef]
- Kramann, B.; Daoyu, H.; Kubale, R.; Schneider, G. Erfahrungen mit der endovaskulären Embolisationstherapie von Aneurysmata der Splanchnikusarterien—Bericht über 13 Fälle. [Experiences with the endovascular embolization therapy of aneurysms of the splanchnic arteries—A report on 13 cases]. In Rofo; Georg Thieme Verlag Stuttgart: Stuttgart, Germany, 1995; Volume 163, pp. 417–423. [Google Scholar] [CrossRef]
- Benz, C.A.; Jakob, P.; Jakobs, R.; Riemann, J.F. Hemosuccus Pancreaticus—A Rare Cause of Gastrointestinal Bleeding: Diagnosis and Interventional Radiological Therapy. Endoscopy 2000, 32, 428–431. [Google Scholar] [CrossRef]
- Kim, Y.S.; Jung, Y.H.; Han, K.W.; Joo, H.S.; Cho, Y.K.; Park, J.W.; Lee, S.H.; Kim, H.C.; Park, S.I.; Jung, I.K.; et al. A case of the ruptured splenic artery aneurysm treated with transcatheter embolization. Korean J. Gastroenterol. 2004, 44, 288–291. (In Korean) [Google Scholar]
- Hasaj, O.; Di Stasi, C.; Perri, V.; Tringali, A.; Costamagna, G. Hemosuccus Pancreaticus Secondary to Intraductal Rupture of a Primary Splenic Artery Aneurysm: Diagnosis by ERCP and Successful Management by Interventional Radiology. Endoscopy 2004, 36, 437–441. [Google Scholar] [CrossRef] [PubMed]
- Kuzuya, A.; Mizuno, K.; Miyaka, H.; Iyomasa, S.; Matsuda, M. Hemosuccus Pancreaticus Caused by Rupture of a True Splenic Artery Aneurysm following a Failure of Coil Embolization. Ann. Vasc. Surg. 2006, 20, 130–133. [Google Scholar] [CrossRef]
- Laganà, D.; Carrafiello, G.; Mangini, M.; Dionigi, G.; Caronno, R.; Castelli, P.; Fugazzola, C. Multimodal approach to endovascular treatment of visceral artery aneurysms and pseudoaneurysms. Eur. J. Radiol. 2006, 59, 104–111. [Google Scholar] [CrossRef]
- Sachdev, U.; Baril, D.T.; Ellozy, S.H.; Lookstein, R.A.; Silverberg, D.; Jacobs, T.S.; Carroccio, A.; Teodorescu, V.J.; Marin, M.L. Management of aneurysms involving branches of the celiac and superior mesenteric arteries: A comparison of surgical and endovascular therapy. J. Vasc. Surg. 2006, 44, 718–724. [Google Scholar] [CrossRef]
- Fernández, E.P.; de la Cruz Burgos, R.; Del Cerro González, J.V.; Polo, M.R. Rotura de bazo espontánea secundaria a aneurisma intraesplénico [Spontaneous rupture of the spleen secondary to intrasplenic aneurysm]. Radiologia 2007, 49, 424–426. (In Spanish) [Google Scholar] [CrossRef] [PubMed]
- Tulsyan, N.; Kashyap, V.S.; Greenberg, R.K.; Sarac, T.P.; Clair, D.G.; Pierce, G.; Ouriel, K. The endovascular management of visceral artery aneurysms and pseudoaneurysms. J. Vasc. Surg. 2007, 45, 276–283, discussion 283. [Google Scholar] [CrossRef] [PubMed]
- Sadat, U.; Noor, N.; Tang, T.; Varty, K. Emergency endovascular repair of ruptured visceral artery aneurysms. World J. Emerg. Surg. 2007, 2, 17. [Google Scholar] [CrossRef]
- Loffroy, R.; Guiu, B.; Cercueil, J.-P.; Lepage, C.; Cheynel, N.; Steinmetz, E.; Ricolfi, F.; Krausé, D. Transcatheter Arterial Embolization of Splenic Artery Aneurysms and Pseudoaneurysms: Short- and Long-Term Results. Ann. Vasc. Surg. 2008, 22, 618–626. [Google Scholar] [CrossRef] [PubMed]
- Pasklinsky, G.; Gasparis, A.P.; Labropoulos, N.; Pagan, J.; Tassiopoulos, A.K.; Ferretti, J.; Ricotta, J.J. Endovascular Covered Stenting for Visceral Artery Pseudoaneurysm Rupture: Report of 2 Cases and a Summary of the Disease Process and Treatment Options. Vasc. Endovasc. Surg. 2008, 42, 601–606. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, S.; Hirota, S.; Maeda, H.; Achiwa, S.; Arai, K.; Kobayashi, K.; Nakao, N. Transcatheter Coil Embolization of Splenic Artery Aneurysm. Cardiovasc. Interv. Radiol. 2008, 31, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Bjerring, O.S.; Pørneki, J.C.; Justesen, P.E. Endovaskulaert behandlet rumperet aneurisme på arteria lienalis [Endovascular treatment of ruptured splenic artery aneurysm]. Ugeskr. Laeger 2008, 170, 158. (In Danish) [Google Scholar]
- Zubaidi, A. Rupture of multiple splenic artery aneurysms: A common presentation of a rare disease with a review of literature. Saudi J. Gastroenterol. 2009, 15, 55–58. [Google Scholar] [CrossRef]
- Manian, U.; Badri, H.; Coyne, P.; Nice, C.; Ashour, H.; Bhattacharya, V. Endovascular Treatment of a Ruptured Splenic Artery Aneurysm using Amplatzer® Vascular Plug. Int. J. Biomed. Sci. 2009, 5, 81–84. [Google Scholar]
- Huang, Y.-C.; Xie, Z.-Y.; Tseng, H.-S.; Yang, C.-F.; Hsiao, L.-T. Splenic artery pseudoaneurysm with rupture by transformed splenic marginal zone B cell lymphoma. Ann. Hematol. 2009, 89, 639–640. [Google Scholar] [CrossRef]
- Iyanaga, M.; Watts, S.; Kasai, T. A Patient with Splenic Artery Aneurysm Rupture and the Importance of Rapid Sonography in the ED. Emerg. Med. Int. 2010, 2010, 893606. [Google Scholar] [CrossRef][Green Version]
- Sadhu, S.; Sarkar, S.; Verma, R.; Dubey, S.; Roy, M. Haemosuccus pancreaticus due to true splenic artery aneurysm: A rare cause of massive upper gastrointestinal bleeding. J. Surg. Case Rep. 2010, 2010, 4. [Google Scholar] [CrossRef]
- Ohyama, Y.; Ishida, H.; Yoshida, C.; Konno, J.; Hoshino, T.; Watanabe, H.; Kudoh, Y.; Furukawa, K.; Watanabe, T. Pseudoaneurysm in a chronic pancreatitis patient: Report of a case, with emphasis on contrast-enhanced sonograms. J. Med. Ultrason. 2010, 37, 75–79. [Google Scholar] [CrossRef]
- Naitoh, I.; Ando, T.; Shimohira, M.; Nakazawa, T.; Hayashi, K.; Okumura, F.; Miyabe, K.; Yoshida, M.; Togawa, H.; Sasaki, S.; et al. Hemosuccus pancreaticus associated with segmental arterial mediolysis successfully treated by transarterial embolization. JOP 2010, 11, 625–629. [Google Scholar]
- Eng, C.W.; Venkatesh, S.K. Clinics in diagnostic imaging (131). Multiple visceral mycotic aneurysms. Singapore Med. J. 2010, 51, 824–829, quiz 830. [Google Scholar]
- Spiliopoulos, S.; Sabharwal, T.; Karnabatidis, D.; Brountzos, E.; Katsanos, K.; Krokidis, M.; Gkoutzios, P.; Siablis, D.; Adam, A. Endovascular Treatment of Visceral Aneurysms and Pseudoaneurysms: Long-term Outcomes from a Multicenter European Study. Cardiovasc. Interv. Radiol. 2012, 35, 1315–1325. [Google Scholar] [CrossRef]
- Cochennec, F.; Riga, C.; Allaire, E.; Cheshire, N.; Hamady, M.; Jenkins, M.; Kobeiter, H.; Wolfe, J.; Becquemin, J.; Gibbs, R. Contemporary Management of Splanchnic and Renal Artery Aneurysms: Results of Endovascular Compared with Open Surgery from Two European Vascular Centers. Eur. J. Vasc. Endovasc. Surg. 2011, 42, 340–346. [Google Scholar] [CrossRef] [PubMed]
- Gehlen, J.M.L.G.; Heeren, P.A.M.; Verhagen, P.F.; Peppelenbosch, A.G. Visceral Artery Aneurysms. Vasc. Endovasc. Surg. 2011, 45, 681–687. [Google Scholar] [CrossRef] [PubMed]
- Fankhauser, G.T.; Stone, W.M.; Naidu, S.G.; Oderich, G.S.; Ricotta, J.J.; Bjarnason, H.; Money, S.R.; Mayo Vascular Research Center Consortium. The minimally invasive management of visceral artery aneurysms and pseudoaneurysms. J. Vasc. Surg. 2011, 53, 966–970. [Google Scholar] [CrossRef]
- Etezadi, V.; Gandhi, R.T.; Benenati, J.F.; Rochon, P.; Gordon, M.; Benenati, M.J.; Alehashemi, S.; Katzen, B.T.; Geisbüsch, P. Endovascular Treatment of Visceral and Renal Artery Aneurysms. J. Vasc. Interv. Radiol. 2011, 22, 1246–1253. [Google Scholar] [CrossRef] [PubMed]
- Ferrero, E.; Ferri, M.; Viazzo, A.; Robaldo, A.; Carbonatto, P.; Pecchio, A.; Chiecchio, A.; Nessi, F. Visceral Artery Aneurysms, an Experience on 32 Cases in a Single Center: Treatment from Surgery to Multilayer Stent. Ann. Vasc. Surg. 2011, 25, 923–935. [Google Scholar] [CrossRef]
- Rosales-Zabal, J.M.; Navarro-Jarabo, J.M.; Rivera-Irigoin, R.; Perez-Aisa, A.; Marcos-Herrero, M.; Sanchez-Cantos, A.M. Rupture of a splenic pseudoaneurysm in the colon as an unusual cause of rectal bleeding. Color. Dis. 2011, 14, e425–e426. [Google Scholar] [CrossRef]
- Wierzbicki, T.; Szmeja, J.; Borejsza-Wysocki, M.; Męczyński, M.; Smuszkiewicz, P.; Katulska, K.; Drews, M. Massive bleeeding from upper gastrointestinal tract as a symptom of rupture of splenic artery aneurysm to stomach. Med. Sci. Monit. 2012, 18, CS8–CS11. [Google Scholar] [CrossRef]
- Taslakian, B.; Khalife, M.; Faraj, W.; Mukherji, D.; Haydar, A. Pancreatitis-associated pseudoaneurysm of the splenic artery presenting as lower gastrointestinal bleeding: Treatment with transcatheter embolisation. BMJ Case Rep. 2012, 2012, bcr2012007403. [Google Scholar] [CrossRef]
- Khurana, J.; Spinello, I.M. Splenic artery aneurysm rupture: A rare but fatal cause for peripartum collapse. J. Intensive Care Med. 2012, 28, 131–133. [Google Scholar] [CrossRef]
- Mazzaccaro, D.; Carmo, M.; Nano, G.; Barbetta, I.; Settembrini, A.M.; Occhiuto, M.T.; Stegher, S.; Dallatana, R.; Malacrida, G.; Settembrini, P.G. Treatment options for visceral artery aneurysms: Ten year experience. J. Cardiovasc. Surg. 2015, 56, 423–432. [Google Scholar]
- Miao, Y.-D.; Ye, B. Intragastric rupture of splenic artery aneurysms: Three case reports and literature review. Pak. J. Med. Sci. 2013, 29, 656–659. [Google Scholar]
- Amico, E.C.; Alves, J.R. Rupture of splenic artery pseudoaneurysm. Pancreatology 2014, 14, 144–145. [Google Scholar] [CrossRef] [PubMed]
- Ünlü, Ç.; Heuvel, D.A.v.D.; Leeuwis, J.W.; de Vries, J.-P.P. Ruptured Aneurysm of the Splenic Artery Associated with Fibromuscular Dysplasia. Ann. Vasc. Surg. 2014, 28, 1799.e15–1799.e18. [Google Scholar] [CrossRef]
- Roberts, K.; McCulloch, N.; Forde, C.; Mahon, B.; Mangat, K.; Olliff, S.; Jones, R. Emergency Treatment of Haemorrhaging Coeliac or Mesenteric Artery Aneurysms and Pseudoaneurysms in the Era of Endovascular Management. Eur. J. Vasc. Endovasc. Surg. 2015, 49, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Akbulut, S.; Otan, E. Management of Giant Splenic Artery Aneurysm: Comprehensive Literature Review. Medicine 2015, 94, e1016. [Google Scholar] [CrossRef] [PubMed]
- Schatz, R.A.; Schabel, S.; Rockey, D.C. Idiopathic Splenic Artery Pseudoaneurysm Rupture as an Uncommon Cause of Hemorrhagic Shock. J. Investig. Med. High Impact Case Rep. 2015, 3, 2324709615577816. [Google Scholar] [CrossRef]
- Pitton, M.B.; Dappa, E.; Jungmann, F.; Kloeckner, R.; Schotten, S.; Wirth, G.M.; Mittler, J.; Lang, H.; Mildenberger, P.; Kreitner, K.-F.; et al. Visceral artery aneurysms: Incidence, management, and outcome analysis in a tertiary care center over one decade. Eur. Radiol. 2015, 25, 2004–2014. [Google Scholar] [CrossRef]
- Reed, N.R.; Oderich, G.S.; Manunga, J.; Duncan, A.; Misra, S.; de Souza, L.R.; Fleming, M.; de Martino, R. Feasibility of endovascular repair of splenic artery aneurysms using stent grafts. J. Vasc. Surg. 2015, 62, 1504–1510. [Google Scholar] [CrossRef] [PubMed]
- Won, Y.; Lee, S.; Kim, Y.; Ku, Y. Clinical efficacy of transcatheter embolization of visceral artery pseudoaneurysms using N-butyl cyanoacrylate (NBCA). Diagn. Interv. Imaging 2015, 96, 563–569. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, S.; Imai, H.; Takaki, H.; Tanemura, A.; Kuriyama, N.; Sakurai, H.; Yamakado, K.; Sakuma, H.; Isaji, S. Spontaneous Rupture of an Intrasplenic Aneurysm After Pancreaticoduodenectomy for Pancreatic Ductal Adenocarcinoma. J. Emerg. Med. 2015, 48, 729–730. [Google Scholar] [CrossRef]
- Matsuda, Y.; Sakamoto, K.; Nishino, E.; Kataoka, N.; Yamaguchi, T.; Tomita, M.; Kazi, A.; Shinozaki, M.; Makimoto, S. Pancreatectomy and splenectomy for a splenic aneurysm associated with segmental arterial mediolysis. World J. Gastrointest. Surg. 2015, 7, 78–81. [Google Scholar] [CrossRef]
- Figueroa-Jiménez, L.A.; González-Márquez, A.L.; Negrón-García, L.; Rosas-Soler, F.; Dones-Rodríguez, A.; De La Paz-López, M.; Santiago-Casiano, M.; Rodríguez-Cruz, E.; Cáceres-Pérkins, W.; Béez-Díaz, L. Uncommon cause of life-threatening retroperitoneal hemorrhage in a healthy young Hispanic patient: Splenic artery aneurysm rupture. Bol. Asoc. Medica Puerto Rico 2015, 107, 45–48. [Google Scholar]
- Guo, B.; Guo, D.; Xu, X.; Chen, B.; Shi, Z.; Luo, J.; Jiang, J.; Fu, W. Early and intermediate results of endovascular treatment of symptomatic and asymptomatic visceral artery aneurysms. J. Vasc. Surg. 2016, 64, 140–148. [Google Scholar] [CrossRef][Green Version]
- Tannoury, J.; Honein, K.; Abboud, B. Splenic artery aneurysm presenting as a submucosal gastric lesion: A case report. World J. Gastrointest. Endosc. 2016, 8, 496–500. [Google Scholar] [CrossRef]
- Jiang, R.; Ding, X.; Jian, W.; Jiang, J.; Hu, S.; Zhang, Z. Combined Endovascular Embolization and Open Surgery for Splenic Artery Aneurysm with Arteriovenous Fistula. Ann. Vasc. Surg. 2016, 30, 311.e1–311.e4. [Google Scholar] [CrossRef]
- Davis, T.; Minardi, J.; Knight, J.; Larrabee, H.; Schaefer, G. Ruptured Splenic Artery Aneurysm: Rare Cause of Shock Diagnosed with Bedside Ultrasound. West J. Emerg. Med. 2015, 16, 762–765. [Google Scholar] [CrossRef] [PubMed]
- Aoki, M.; Hagiwara, S.; Miyazaki, M.; Kaneko, M.; Murata, M.; Nakajima, J.; Ohyama, Y.; Tamura, J.; Tsushima, Y.; Oshima, K. Genuine splenic artery aneurysm rupture treated by N-butyl cyanoacrylate and metallic coils under resuscitative endovascular balloon occlusion of the aorta. Acute Med. Surg. 2016, 3, 286–289. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sul, H.R.; Lee, H.W.; Kim, J.W.; Cha, S.J.; Choi, Y.S.; Kim, G.H.; Kwak, B.K. Endovascular management of hemosuccus pancreaticus, a rare case report of gastrointestinal bleeding. BMC Gastroenterol. 2016, 16, 5. [Google Scholar] [CrossRef]
- Toukouki, A.; Verbeeck, N.; Weber, J.; Lens, V. Intragastric Rupture of a Splenic Artery Aneurysm Associated with a Pancreatic Cancer. J. Belg. Soc. Radiol. 2016, 100, 26. [Google Scholar] [CrossRef]
- Regus, S.; Lang, W. Rupture Risk and Etiology of Visceral Artery Aneurysms and Pseudoaneurysms: A Single-Center Experience. Vasc. Endovasc. Surg. 2016, 50, 10–15. [Google Scholar] [CrossRef]
- Frasnelli, A. Successful resuscitation after splenic artery aneurysm rupture. J. Emerg. Trauma Shock 2016, 9, 38–39. [Google Scholar] [CrossRef] [PubMed]
- Quiles, M.P.; López, V.; Fernández, J.; Cascales, P.; Valero, J.S.; Parrilla, P. Spontaneous rupture of a splenic aneurysm in classic Ehlers-Danlos syndrome. Cir. Esp. 2016, 94, 543–544, (In English and Spanish). [Google Scholar] [CrossRef]
- O’brien, J.; Muscara, F.; Farghal, A.; Shaikh, I. Haematochezia from a Splenic Artery Pseudoaneurysm Communicating with Transverse Colon: A Case Report and Literature Review. Case Rep. Vasc. Med. 2016, 2016, 8461501. [Google Scholar] [CrossRef]
- Kingma, K.D.; van der Linden, A.N.; Roumen, R.M.H. Rebleeding of a Splenic Artery Aneurysm after Coil Embolisation. Case Rep. Surg. 2016, 2016, 1858461. [Google Scholar] [CrossRef]
- Venturini, M.; Marra, P.; Colombo, M.; Panzeri, M.; Gusmini, S.; Sallemi, C.; Salvioni, M.; Lanza, C.; Agostini, G.; Balzano, G.; et al. Endovascular Repair of 40 Visceral Artery Aneurysms and Pseudoaneurysms with the Viabahn Stent-Graft: Technical Aspects, Clinical Outcome and Mid-Term Patency. Cardiovasc. Interv. Radiol. 2018, 41, 385–397. [Google Scholar] [CrossRef] [PubMed]
- Welch, B.T.; Brinjikji, W.; Stockland, A.H.; Lanzino, G. Subarachnoid and intraperitoneal hemorrhage secondary to segmental arterial mediolysis: A case report and review of the literature. Interv. Neuroradiol. 2017, 23, 378–381. [Google Scholar] [CrossRef] [PubMed]
- Blázquez, M.J.P.; Ortiz, A.N. Hemosuccus pancreaticus secondary to pseudoaneurysm of the splenic artery. Rev. Esp. Enferm. Dig. 2017, 109, 727. [Google Scholar] [CrossRef]
- Koutserimpas, C.; Papachristou, E.; Nikitakis, N.; Zannes, N.; Tellos, A.; Velimezis, G. Spontaneous splenic artery aneurysm rupture in a 38-year old female: A case report. G. Chir. 2017, 38, 205–208. [Google Scholar] [CrossRef] [PubMed]
- Meyer, A.; Uder, M.; Lang, W.; Croner, R. Aneurysmen an viszeralen Arterien [Visceral artery aneurysms]. Zentralbl. Chir. 2010, 135, 416–420. (In German) [Google Scholar] [CrossRef]
- Pfister, K.; Kasprzak, P.; Oikonomou, K.; Apfelbeck, H.; Derwich, W.; Uller, W.; Stehr, A.; Schierling, W. Management von Viszeralarterienaneurysmen unter besonderer Berücksichtigung der Organperfusion—Erfahrungen über mehr als 20 Jahre [Management of Visceral Artery Aneurysms with Preservation of Organ Perfusion: More Than Twenty Years Experience]. Zentralbl. Chir. 2018, 143, 516–525. [Google Scholar] [CrossRef]
- Hayashi, S.; Hosoda, K.; Nishimoto, Y.; Nonaka, M.; Higuchi, S.; Miki, T.; Negishi, M. Unexpected intraabdominal hemorrhage due to segmental arterial mediolysis following subarachnoid hemorrhage: A case of ruptured intracranial and intraabdominal aneurysms. Surg. Neurol. Int. 2018, 9, 175. [Google Scholar] [CrossRef] [PubMed]
- Junior, P.R.P.; Fagundes, F.B.; Marchon, L.R.C.; Maciel, R.d.R.T.; Martins, I.M.; Riguetti-Pinto, C.R. Endovascular treatment of acute gastrointestinal bleeding from a large splenic artery pseudoaneurysm: Case report and literature review. J. Vasc. Bras. 2018, 17, 234–242. [Google Scholar] [CrossRef]
- Ouchi, T.; Kato, N.; Nakajima, K.; Higashigawa, T.; Hashimoto, T.; Chino, S.; Sakuma, H. Splenic Artery Aneurysm Treated with Endovascular Stent Grafting: A Case Report and Review of Literature. Vasc. Endovasc. Surg. 2018, 52, 663–668. [Google Scholar] [CrossRef] [PubMed]
- Pratap, A.; Pokala, B.; Vargas, L.M.; Oleynikov, D.; Kothari, V. Laparoscopic endoscopic combined surgery for removal of migrated coil after embolization of ruptured splenic artery aneurysm. J. Surg. Case Rep. 2018, 2018, rjx242. [Google Scholar] [CrossRef]
- Fang, G.; Chen, B.; Fu, W.; Guo, D.; Xu, X.; Jiang, J.; Luo, J.; Dong, Z. Strategies for endovascular treatment of complicated splenic artery aneurysms. J. Vasc. Surg. 2018, 68, 787–794. [Google Scholar] [CrossRef] [PubMed]
- Erben, Y.; Brownstein, A.J.; Rajaee, S.; Li, Y.; Rizzo, J.A.; Mojibian, H.; Ziganshin, B.A.; Elefteriades, J.A. Natural history and management of splanchnic artery aneurysms in a single tertiary referral center. J. Vasc. Surg. 2018, 68, 1079–1087. [Google Scholar] [CrossRef] [PubMed]
- Santos, F.S.; Sousa, K.M.d.S.; de Castro, T.A.C.; Neto, F.C.; de Oliveira, R.G.; de Araujo, W.J.B.; dos Santos, L.C.P.; de Souza, R.C.A. Endovascular treatment of pseudoaneurysms secondary to chronic pancreatitis: Reports of two cases. J. Vasc. Bras. 2018, 17, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Ikeda, A.; Itokawa, Y.; Kusumoto, K.; Nakai, Y.; Azechi, H.; Fujii, S.; Kusaka, T.; Kokuryu, H. [A case of relapsed hemosuccus pancreaticus successfully treated with interventional radiology]. Nihon Shokakibyo Gakkai Zasshi 2018, 115, 825–832. (In Japanese) [Google Scholar]
- Olivieri, J.F.; Jeyakumar, A.; Shivaram, G.M.; Koo, K.S.; Monroe, E.J. Emergent embolization of a ruptured splenic artery aneurysm complicating Menkes disease. Radiol. Case Rep. 2018, 13, 1267–1270. [Google Scholar] [CrossRef]
- Martinelli, O.; Giglio, A.; Irace, L.; Di Girolamo, A.; Gossetti, B.; Gattuso, R. Single-Center Experience in the Treatment of Visceral Artery Aneurysms. Ann. Vasc. Surg. 2019, 60, 447–454. [Google Scholar] [CrossRef]
- Najafi, A.; Sheikh, G.T.; Binkert, C. Extensive Embolization of Splanchnic Artery Aneurysms due to Segmental Arterial Mediolysis. In Rofo; Georg Thieme Verlag Stuttgart: Stuttgart, Germany, 2019; Volume 191, pp. 1010–1014, (In English and German). [Google Scholar] [CrossRef]
- Patel, R.; Girgis, M. Splenic artery pseudoaneurysm with hemosuccus pancreaticus requiring multimodal treatment. J. Vasc. Surg. 2019, 69, 592–595. [Google Scholar] [CrossRef]
- Suri, K.; Tran, K.; Whang, G. Gastric remnant perforation in a gastric bypass patient secondary to splenic artery pseudoaneurysm: Radiologic-surgical correlation. Clin. Imaging 2019, 54, 159–162. [Google Scholar] [CrossRef]
- Wang, W.; Chang, H.; Liu, B.; Wang, W.; Yu, Z.; Chen, C.; Li, Y.; Wang, Z.; Wang, Y. Long-term outcomes of elective transcatheter dense coil embolization for splenic artery aneurysms: A two-center experience. J. Int. Med. Res. 2020, 48, 300060519873256. [Google Scholar] [CrossRef]
- Nagao, K.; Kuroda, K.; Fujii, M.; Shirasaka, D.; Era, Y.; Tsuda, K.; Tanaka, S.; Miyazaki, H.; Hiemori, A.; Asada, Y. Hematemesis due to rupture of splenic artery pseudoaneurysm in association with segmental arterial mediolysis: A case report. Nihon Shokakibyo Gakkai Zasshi 2020, 117, 165–170. (In Japanese) [Google Scholar] [CrossRef]
- Effraemidou, E.; Souftas, V.; Kofina, K.; Karanikas, M.; Lyratzopoulos, N. Spontaneous rupture of a splenic artery aneurysm treated with a spleen-preserving procedure: A case report. J. Surg. Case Rep. 2020, 2020, rjz412. [Google Scholar] [CrossRef] [PubMed]
- Hori, E.; Shibata, T.; Okamoto, S.; Kubo, M.; Horie, Y.; Kuroda, S. An Intraabdominal Hemorrhage Affected by Catecholamine Surges Caused by Subarachnoid Hemorrhage:A Case Report. No Shinkei Geka 2020, 48, 835–840. [Google Scholar] [CrossRef]
- Chipaila, J.; Kato, H.; Iizawa, Y.; Motonori, N.; Noguchi, D.; Gyoten, K.; Hayasaki, A.; Fujii, T.; Tanemura, A.; Murata, Y.; et al. Prolonged operating time is a significant perioperative risk factor for arterial pseudoaneurysm formation and patient death following hemorrhage after pancreaticoduodenectomy. Pancreatology 2020, 20, 1540–1549. [Google Scholar] [CrossRef]
- Kumari, M.; Parwez, M.; Jain, A.; Pandya, B. Management of a delayed, post-traumatic rupture of splenic artery pseudoaneurysm in a patient with life threatening co-morbidities: A treatment challenge. Int. J. Surg. Case Rep. 2020, 75, 479–482. [Google Scholar] [CrossRef] [PubMed]
- Tipaldi, M.A.; Krokidis, M.; Orgera, G.; Pignatelli, M.; Ronconi, E.; Laurino, F.; Laghi, A.; Rossi, M. Endovascular management of giant visceral artery aneurysms. Sci. Rep. 2021, 11, 700. [Google Scholar] [CrossRef]
- Carr, S.C.; Mahvi, D.M.; Hoch, J.R.; Archer, C.W.; Turnipseed, W.D. Visceral artery aneurysm rupture. J. Vasc. Surg. 2001, 33, 806–811. [Google Scholar] [CrossRef] [PubMed]
- Vanetta, C.; González Salazar, E.; Goransky, J.; Arbues, G.; Palavecino, M. Tratamiento endovascular del aneurisma esplénico incidental y en la urgencia [Endovascular treatment of incidental and emergency splenic aneurysm]. Medicina 2021, 81, 96–98. [Google Scholar] [PubMed]
- Sonanis, S.; Layton, B.; Nicholson, O.; Subar, D. Splenic artery pseudoaneurysm and resultant haematosuccus pancreaticus. BMJ Case Rep. 2021, 14, e239485. [Google Scholar] [CrossRef]
- Law, N.L.; Villada, A.F.; Kruse, M.J. Rupture of splenic artery aneurysm in a man with polycythemia vera and acquired von Willebrand syndrome. BMJ Case Rep. 2021, 14, e243316. [Google Scholar] [CrossRef]
- Borzelli, A.; Amodio, F.; Pane, F.; Coppola, M.; Silvestre, M.; Di Serafino, M.; Corvino, F.; Giurazza, F.; Niola, R. Successful endovascular embolization of a giant splenic artery pseudoaneurysm secondary to a huge pancreatic pseudocyst with concomitant spleen invasion. Pol. J. Radiol. 2021, 86, e489–e495. [Google Scholar] [CrossRef]
- Xu, Y.; Wu, Z. A case of a pregnant woman with a special splenic artery aneurysm. Malawi Med. J. 2022, 34, 220–222. [Google Scholar] [CrossRef] [PubMed]
- Mulpuri, V.B.; Gurijala, P.; Yerolla, B.R.; Krishna, R.; Pandey, A.; Ramachandran, G. Cross clamping of the supraceliac aorta is effective for bleeding control in ruptured giant splenic artery pseudoaneurysm when proximal and distal control of the splenic artery is not possible: A case report. J. Vasc. Bras. 2022, 21, e20210210. [Google Scholar] [CrossRef] [PubMed]
- Tesolin, D.; Alaref, A.; Ibrahim, M.F.K. A case of splenic artery aneurysm and rupture in a patient on a vascular endothelial growth factor inhibitor for renal cell carcinoma. Cancer Rep. 2021, 5, e1567. [Google Scholar] [CrossRef] [PubMed]
- Luan, N.D.; Duc, N.M.; Son, N.H.; Hien, T.M.; Huy, L.A.; Tai, N.T.; Kinh, B.T.; Loi, H.M. A rare case report of acute upper gastrointestinal hemorrhage due to splenic artery pseudoaneurysm. SAGE Open Med. Case Rep. 2021, 9, 2050313X211061910. [Google Scholar] [CrossRef]
- Vaughan, E.; Carlsson, T.; Brooks, M.; Elhodaiby, M. Splenic artery aneurysm rupture in pregnancy: Challenges in diagnosis and the importance of multidisciplinary management. BMJ Case Rep. 2022, 15, e249227. [Google Scholar] [CrossRef]
- Patel, D. Acute Abdomen from Spontaneous Splenic Artery Rupture with Coincidental Metastatic Disease: A Case Report. Am. J. Case Rep. 2022, 23, e936987. [Google Scholar] [CrossRef]
- Lee, S.H.; Yang, S.; Park, I.; Im, Y.C.; Kim, G.Y. Ruptured splenic artery aneurysms in pregnancy and usefulness of endovascular treatment in selective patients: A case report and review of literature. World J. Clin. Cases 2022, 10, 9057–9063. [Google Scholar] [CrossRef] [PubMed]
- Okumura, T.; Kimura, T.; Nakajima, D.; Kondo, S.; Kako, S.; Takahashi, Y.; Yamaka, K.; Ichinohe, F.; Tsukahara, Y.; Nagaya, T.; et al. Splenic artery pseudoaneurysm resulting from gastric ulcer presenting acute upper gastrointestinal bleeding. Radiol. Case Rep. 2022, 18, 97–99. [Google Scholar] [CrossRef] [PubMed]
- Schellenberg, M.; Owattanapanich, N.; Emigh, B.; Nichols, C.; Dilday, J.; Ugarte, C.; Onogawa, A.; Matsushima, K.; Martin, M.J.; Inaba, K. Pseudoaneurysms after high-grade blunt solid organ injury and the utility of delayed computed tomography angiography. Eur. J. Trauma Emerg. Surg. 2023, 49, 1315–1320. [Google Scholar] [CrossRef]
- Pennetta, F.F.; Ferrer, C.; Tonidandel, L.; Coscarella, C.; Vagnarelli, S.; Giudice, R. Disappearing multiple visceral aneurysms in Vascular Ehlers-Danlos syndrome. Vascular 2023, 17085381231162126. [Google Scholar] [CrossRef]
- Liu, X.; Chen, S.; Yang, G.; Hong, J.; Lin, Y.; Lin, Z.; Zhang, Y.; Chiang, T.-Y. A super-selective coil impregnation therapy for pancreatic duct haemorrhage caused by pseudoaneurysm rupture. Technol. Health Care 2023, 31, 441–447. [Google Scholar] [CrossRef]
- Alexander, E.; Santos, E. Endovascular management of incidentally discovered splenic arteriovenous fistula resulting from ruptured splenic aneurysm: Case report and review of the literature. Radiol. Case Rep. 2023, 18, 2465–2469. [Google Scholar] [CrossRef] [PubMed]
- Aoki, R.; Kobayashi, Y.; Nawata, S.; Kamide, H.; Sekikawa, Z.; Utsunomiya, D. Gastrointestinal Bleeding Due to the Rupture of Splenic Artery Caused by Pancreatic Carcinoma: A Case Requiring Repeated Transcatheter Arterial Embolization in a Short Period of Time. Interv. Radiol. 2023, 8, 88–91. [Google Scholar] [CrossRef]
- Palughi, M.; Sirignano, P.; Stella, N.; Rossi, M.; Fiorani, L.; Taurino, M. Rupture of Splenic Artery Aneurysm in Patient with ACTN2 Mutation. J. Clin. Med. 2023, 12, 4729. [Google Scholar] [CrossRef] [PubMed]
- LoCurto, P.; Farulla, M.A.; Di Lorenzo, G.; Amico, M.; Ciaccio, G. Acute massive bleeding from splenic artery aneurysm rupture: A case report. G. Chir. 2019, 40, 530–534. [Google Scholar]
- Panzera, F.; Inchingolo, R.; Rizzi, M.; Biscaglia, A.; Schievenin, M.G.; Tallarico, E.; Pacifico, G.; Di Venere, B. Giant splenic artery aneurysm presenting with massive upper gastrointestinal bleeding: A case report and review of literature. World J. Gastroenterol. 2020, 26, 3110–3117. [Google Scholar] [CrossRef] [PubMed]
- Quandalle, P.; Gambiez, L.; Brami, F.; Ghisbain, H.; André, J.; Zahredine, A.; Saudemont, A. Gastrointestinal hemorrhage caused by rupture of an aneurysm of visceral arteries. Presentation of 4 cases. Chirurgie 1998, 123, 139–147. [Google Scholar] [CrossRef]
- De Silva, W.S.L.; Gamlaksha, D.S.; Jayasekara, D.P.; Rajamanthri, S.D. A splenic artery aneurysm presenting with multiple episodes of upper gastrointestinal bleeding: A case report. J. Med. Case Rep. 2017, 11, 123. [Google Scholar] [CrossRef]
- Pinarbaşi, B.; Poturoğlu, S.; Yanar, H.; Güven, K.; Akyüz, F.; Dizdaroğlu, F.; Güllüoğlu, M.; Taviloğlu, K.; Kaymakoğlu, S.; Mungan, Z. A rare cause of hemosuccus pancreaticus: Primary splenic artery aneurysm ruptured into pancreatic serous cystadenoma. Turk. J. Gastroenterol. 2008, 19, 57–63. [Google Scholar]
- Igari, K.; Ochiai, T.; Aihara, A.; Kumagai, Y.; Iida, M.; Yamazaki, S. Hemosuccus pancreaticus caused by a primary splenic artery aneurysm as a rare cause of gastrointestinal bleeding: Report of a case. Int. Surg. 2010, 95, 325–328. [Google Scholar]
- Herrera-Fernández, F.A.; Palomeque-Jiménez, A.; Serrano-Puche, F.; Calzado-Baeza, S.F.; Reyes-Moreno, M. Rupture of splenic artery pseudoaneurysm: An unusual cause of upper gastrointetinal bleeding. Cir. Cir. 2014, 82, 551–555. [Google Scholar]
- Carmeci, C.; McClenathan, J. Visceral artery aneurysms as seen in a community hospital. Am. J. Surg. 2000, 179, 486–489. [Google Scholar] [CrossRef] [PubMed]
- Popov, P.; Boskovic, S.; Sagic, D.; Radevic, B.; Ilijevski, N.; Nenezic, D.; Tasic, N.; Davidovic, L.; Radak, D. Treatment of visceral artery aneurysms: Retrospective study of 35 cases. Vasa 2007, 36, 191–198. [Google Scholar] [CrossRef]
- Muscari, F.; Barret, A.; Chaufour, X.; Bossavy, J.; Bloom, E.; Pradère, B.; Gouzi, J. Management of visceral artery aneurysms. Retrospective study of 23 cases. Ann. Chir. 2002, 127, 281–288. (In French) [Google Scholar] [CrossRef] [PubMed]
- Rao, S.; Sivina, M.; Willis, I.; Sher, T.; Habibnejad, S. Massive Lower Gastrointestinal Tract Bleeding Due to Splenic Artery Aneurysm: A Case Report. Ann. Vasc. Surg. 2007, 21, 388–391. [Google Scholar] [CrossRef]
- Colombo, P.L.; Tinozzi, F.P.; Abelli, M.; Pini, G.; Benedetti, M.; Morone, G.; Moglia, P.; Albertario, S.; Laera, M.R.; Valenti, L.; et al. Aneurysms of the visceral arteries: Report of 5 cases. Ann. Ital. Chir. 2002, 73, 219–229. [Google Scholar] [PubMed]
- Massani, M.; Bridda, A.; Caratozzolo, E.; Bonariol, L.; Antoniutti, M.; Bassi, N. Hemosuccus pancreaticus due to primary splenic artery aneurysm: A diagnostic and therapeutic challenge. JOP 2009, 10, 48–52. [Google Scholar] [PubMed]
- Hordiychuk, A.; Mehanna, D. Spontaneous rupture of splenic artery pseudoaneurysm. J. Surg. Case Rep. 2022, 2022, rjac604. [Google Scholar] [CrossRef]
- Dave, S.P.; Reis, E.D.; Hossain, A.; Taub, P.J.; Kerstein, M.D.; Hollier, L.H. Splenic Artery Aneurysm in the 1990s. Ann. Vasc. Surg. 2000, 14, 223–229. [Google Scholar] [CrossRef]
- Windham, T.C.; Risin, S.A.; Tamm, E.P. Spontaneous Rupture of a Nontraumatic Intrasplenic Aneurysm. N. Engl. J. Med. 2000, 342, 1999–2000. [Google Scholar] [CrossRef]
- Gaglio, P.; Regenstein, F.; Slakey, D.; Cheng, S.; Takiff, H.; Rinker, R.; Dick, D.; Thung, S. alpha-1 antitrypsin deficiency and splenic artery aneurysm rupture: An association? Am. J. Gastroenterol. 2000, 95, 1531–1534. [Google Scholar] [CrossRef] [PubMed]
- Asokan, S.; Chew, E.K.; Ng, K.Y.; Thanaletchimy, N.; Asmiati, A.; Kong, N.M. Post partum splenic artery aneurysm rupture. J. Obstet. Gynaecol. Res. 2000, 26, 199–201. [Google Scholar] [CrossRef]
- Arrieta, F.M.; Muguerza, J.; Sancho, L.G.; Ayuso, M.; Rustarazu, M.; Valenzuela, P. Rupture of splenic artery aneurysm during pregnancy and posterior evolution of gestation. Zentralbl Gynakol. 2000, 122, 579–580. [Google Scholar] [CrossRef]
- Shahabi, S.; Jani, J.; Masters, L.; Cobin, L.; Greindl, J. Spontaneous Rupture of a Splenic Artery Aneurysm in Pregnancy: Report of Two Cases. Acta Chir. Belg. 2000, 100, 231–233. [Google Scholar] [CrossRef]
- Sam, C.E.; Rabl, M.; Joura, A.E. Aneurysm of the splenic artery: Rupture in pregnancy. Wien. Klin. Wochenschr. 2000, 112, 896–898. (In German) [Google Scholar] [PubMed]
- Fotopoulos, N.; Kyriakidis, A.; Dimopoulos, K.; Karkaletsis, A.; Faros, E.; Dalamarinis, K.; Raitsiou, B.; Antsaklis, G. Splenic Artery Aneurysm Rupture. Dig. Surg. 2001, 18, 325–326. [Google Scholar] [CrossRef] [PubMed]
- Balsarkar, D.J.; Joshi, M.A. Rupture of splenic artery pseudoaneurysm presenting with massive upper gastrointestinal bleed. Am. J. Surg. 2002, 183, 197–198. [Google Scholar] [CrossRef]
- Brocas, E.; Tenaillon, A. Rupture spontanée de la rate au second trimestre de grossesse [Spontaneous splenic rupture in the second quarter of pregnancy]. Ann. Fr. Anesth. Reanim. 2002, 21, 231–234. (In French) [Google Scholar] [CrossRef]
- Heestand, G.; Sher, L.; Lightfoote, J.; Palmer, S.; Mateo, R.; Singh, G.; Moser, J.; Selby, R.; Genyk, Y.; Jabbour, N. Characteristics and Management of Splenic Artery Aneurysm in Liver Transplant Candidates and Recipients. Am. Surg. 2003, 69, 933–940. [Google Scholar] [CrossRef]
- Selo-Ojeme, D.; Robarts, P. Spontaneous rupture of splenic artery aneurysm in pregnancy. J. Obstet. Gynaecol. 2004, 24, 460–461. [Google Scholar] [CrossRef]
- Woo, E.Y.; Fairman, R.M. Treatment of multiple visceral aneurysms in a 20-year-old patient. J. Vasc. Surg. 2004, 40, 167–169. [Google Scholar] [CrossRef][Green Version]
- Khan, H.R.; Low, S.; Selinger, M.; Nelson, N. Splenic artery aneurysm rupture in pregnancy. J. Coll. Physicians Surg. Pak. 2004, 14, 298–299, Erratum in J. Coll. Physicians Surg. Pak. 2004, 14, 452. [Google Scholar] [PubMed]
- Popham, P.; Buettner, A. Arterial aneurysms of the lienorenal axis during pregnancy. Int. J. Obstet. Anesth. 2003, 12, 117–119. [Google Scholar] [CrossRef]
- Al Asfar, F.; Saber, M.; Dhar, P.M.; Al Awadhi, N. Rupture of Splenic Artery Aneurysm during Labor: A Case Report of Maternal and Fetal Survival. Med. Princ. Pract. 2004, 14, 53–54. [Google Scholar] [CrossRef]
- Dolar, E.; Uslusoy, H.; Kiyici, M.; Gurel, S.; Nak, S.G.; Gulten, M.; Zorluoglu, A.; Saricaoglu, H.; Memik, F. Rupture of the splenic arterial aneurysm due to Behçet’s disease. Rheumatology 2005, 44, 1327–1328. [Google Scholar] [CrossRef]
- Saltzberg, S.S.; Maldonado, T.S.; Lamparello, P.J.; Cayne, N.S.; Nalbandian, M.M.; Rosen, R.J.; Jacobowitz, G.R.; Adelman, M.A.; Gagne, P.J.; Riles, T.S.; et al. Is Endovascular Therapy the Preferred Treatment for All Visceral Artery Aneurysms? Ann. Vasc. Surg. 2005, 19, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Brook, O.R.; Ghersin, E.; Guralnik, L.; Israelit, S.H.; Engel, A. Abdominal apoplexy due to spontaneous rupture of an aberrant visceral artery pseudoaneurysm. Emerg. Radiol. 2005, 11, 228–230. [Google Scholar] [CrossRef] [PubMed]
- Richardson, A.J.; Bahlool, S.; Knight, J. Ruptured splenic artery aneurysm in pregnancy presenting in a manner similar to pulmonary embolus. Anaesthesia 2006, 61, 187–189. [Google Scholar] [CrossRef]
- Chaichian, S.; Mehdizadeh, A.; Akbarian, A.; Groohi, B.; Khanahmadi, N.; Alaghehbandan, R. Rupture of Splenic Artery Aneurysm with Portal Hypertension During Pregnancy: A Case Report. J. Obstet. Gynaecol. Can. 2006, 28, 303–304. [Google Scholar] [CrossRef] [PubMed]
- El-Shawarby, S.A.; Franklin, O.; South, M.; Goodman, J. Caesarean splenectomy for spontaneous rupture of splenic artery aneurysm at 34 weeks gestation with survival of the mother and the preterm fetus. J. Obstet. Gynaecol. 2006, 26, 468–469. [Google Scholar] [CrossRef]
- Cioppa, T.; De Stefano, A.; Marrelli, D.; Neri, A.; Rossi, S.; De Marco, G.; Pinto, E.; Roviello, F. Pseudoaneurysm of the splenic artery fistulized in the stomach and associated to a pancreatic pseudocyst: Case report. Minerva Chir. 2006, 61, 261–264. (In English) [Google Scholar] [PubMed]
- Kalko, Y.; Ugurlucan, M.; Basaran, M.; Kafali, E.; Aydin, U.; Kafa, U.; Kosker, T.; Ozcaliskan, O.; Yilmaz, E.; Alpagut, U.; et al. Visceral Artery Aneurysms. Heart Surg. Forum 2007, 10, E24–E29. [Google Scholar] [CrossRef]
- Tarifa-Castila, A.; Salvoch-Arnedo, F.J.; Arín-Palacios, B.; Lera-Tricas, J.M. Rupture of a splenic artery aneurysm. Cir. Esp. 2007, 81, 159. (In Spanish) [Google Scholar] [CrossRef] [PubMed]
- Mattick, A.; Gawthrope, I. Splenic artery aneurysm rupture: Case report of this uncommon presentation. Emerg. Med. J. 2007, 24, 863. [Google Scholar] [CrossRef] [PubMed]
- Toyoki, Y.; Hakamada, K.; Narumi, S.; Nara, M.; Ishido, K.; Sasaki, M. Hemosuccus pancreaticus: Problems and pitfalls in diagnosis and treatment. World J. Gastroenterol. 2008, 14, 2776–2779. [Google Scholar] [CrossRef]
- Upadhyaya, P.K.; Chava, S.; Bin-Sangheer, S.; Sudan, R.; Mittal, S.K.; Cemaj, S. Delayed Rupture of a Splenic Artery Pseudoaneurysm After Biliopancreatic Diversion. Obes. Surg. 2008, 18, 890–892. [Google Scholar] [CrossRef]
- Fernández, E.L.-T.; Delgado-Plasencia, L.; Arteaga-Gonzalez, I.; Carrillo-Pallares, A.; Diaz-Romero, F. Posttraumatic Intrasplenic Pseudoaneurysm with High-Flow Arteriovenous Fistula: New Lessons to Learn. Eur. J. Trauma Emerg. Surg. 2008, 34, 305–308. [Google Scholar] [CrossRef] [PubMed]
- Nordanstig, J.; Gerdes, H.; Kocys, E. Spontaneous Isolated Dissection of the Celiac Trunk with Rupture of the Proximal Splenic Artery: A Case Report. Eur. J. Vasc. Endovasc. Surg. 2009, 37, 194–197. [Google Scholar] [CrossRef]
- Sinha, A.; Meldrum, D.; Sinha, B.; Thakor, A. Postpartum rupture of a splenic artery aneurysm presenting as disseminated intravascular coagulation. Int. J. Obstet. Anesth. 2009, 18, 95–96. [Google Scholar] [CrossRef]
- Patrelli, T.S.; Anfuso, S.; Verrotti, C.; Fadda, G.M.; Gramellini, D.; Nardelli, G.B. Intrapancreatic rupture of a splenic artery aneurysm during pregnancy—A rare case report with fetal and maternal survival. J. Matern. Neonatal Med. 2009, 22, 362–364. [Google Scholar] [CrossRef]
- Kourabi, M.; Reibel, N.; Perez, M.; Grosdidier, G. A serious late complication of non-operative management of splenic trauma: Rupture of splenic artery aneurysm. J. Chir. 2008, 145, 605–607. (In French) [Google Scholar] [CrossRef]
- Mordant, P.; Trésallet, C.; Royer, B.; Brouquet, A.; Turrin, N.; Ménégaux, F. Post-coital rupture of a splenic artery aneurysm. J. Chir. 2008, 145, 623–624. (In French) [Google Scholar] [CrossRef]
- Mattick, A.; Gawthrope, I. Splenic artery aneurysm rupture: Case report of this uncommon presentation. BMJ Case Rep. 2009, 2009, bcr10.2008.1148. [Google Scholar] [CrossRef] [PubMed]
- Sbihi, L.; Dafiri, R. Unusual cause of hematemesis in a child: Rupture of a splenic artery aneurysm. J. Radiol. 2009, 90, 315–317. (In French) [Google Scholar] [CrossRef]
- Varela, C.A.; Gómez, J.M.; Reina, M.A.; López, A.; Galindo, S.; Arruga, A.M. Reversal of acenocoumarol anticoagulation with activated factor VII in massive hemorrhage following rupture of a splenic artery pseudoaneurysm. Rev. Esp. Anestesiol. Reanim. 2009, 56, 245–248. (In Spanish) [Google Scholar] [CrossRef] [PubMed]
- Chookun, J.; Bounes, V.; Ducassé, J.L.; Fourcade, O. Rupture of splenic artery aneurysm during early pregnancy: A rare and catastrophic event. Am. J. Emerg. Med. 2009, 27, 898.e5–898.e6. [Google Scholar] [CrossRef]
- Charokopos, N.A.; Foroulis, C.N.; Rouska, E.G.; Papakonstantinou, C. Fatal rupture of splenic artery mycotic aneurysm after mitral valve replacement for infective endocarditis. Eur. J. Cardio-Thoracic Surg. 2009, 36, 783–784; author reply 784–785. [Google Scholar] [CrossRef] [PubMed]
- Sarikaya, S.; Ekci, B.; Aktas, C.; Cetin, A.; Ay, D.; Demirag, A. A rare clinic presentation of abdominal pain: Rupture of splenic artery aneurysm: A case report. Cases J. 2009, 2, 148. [Google Scholar] [CrossRef]
- Thomson, M.J.; Seshadri, S.; Swami, S.; Strandvik, G.F.; Neales, K. The pitfalls of protocols—A case of postpartum splenic artery aneurysm rupture. BMJ Case Rep. 2010, 2010, bcr0220102743. [Google Scholar] [CrossRef]
- Ousadden, A.; Ibnmajdoub, K.H.; Elbouhaddouti, H.; Mazaz, K.; AitTaleb, K. Intragastric rupture of a splenic artery aneurysm—A case report. Cases J. 2009, 2, 202. [Google Scholar] [CrossRef]
- Betal, D.; Khangura, J.S.; Swan, P.J.; Mehmet, V. Spontaneous ruptured splenic artery aneurysm: A case report. Cases J. 2009, 2, 7150. [Google Scholar] [CrossRef]
- Groussolles, M., Jr.; Merveille, M.; Alacoque, X.; Vayssiere, C.; Reme, J.M.; Parant, O. Rupture of a Splenic Artery Aneurysm in the First Trimester of Pregnancy. J. Emerg. Med. 2010, 41, e13–e16. [Google Scholar] [CrossRef]
- Tsankova, M.; Jankova, J.; Dimitrova, V.; Grigorov, G.; Dzherov, L. Rupture of splenic artery aneurysm--life-threatening condition for women during pregnancy and after birth (with report of one case). Akush Ginekol 2010, 49, 59–62. (In Bulgarian) [Google Scholar]
- Lakin, R.O.; Bena, J.F.; Sarac, T.P.; Shah, S.; Krajewski, L.P.; Srivastava, S.D.; Clair, D.G.; Kashyap, V.S. The contemporary management of splenic artery aneurysms. J. Vasc. Surg. 2011, 53, 958–964; discussion 965. [Google Scholar] [CrossRef]
- Rahmoune, F.C.; Aya, G.; Biard, M.; Belkhayat, G.; Hamza, J.; Leperc, J.; Ouchtati, M. Splenic artery aneurysm rupture in late pregnancy: A case report and review of the literature. Ann. Fr. Anesth. Reanim. 2011, 30, 156–158. [Google Scholar] [CrossRef] [PubMed]
- Dhinakar, M.; Al Mashini, S.; Golash, V. Rupture of Splenic Artery Aneurysm during Pregnancy: A Report of two Cases. Oman Med. J. 2011, 26, e025. [Google Scholar] [CrossRef] [PubMed]
- Michel, P.; Jarry, J.; Pagliano, G. Faux anévrisme iatrogène de l’artère splénique après duodénopancréatectomie céphalique [Iatrogenic false aneurysm of the splenic artery after cephalic duodenopancreatectomy]. J. Mal. Vasc. 2012, 37, 22–25. [Google Scholar] [CrossRef] [PubMed]
- Kalavský, M.; Smetka, J. Krvácanie do hrubého creva spôsobené ruptúrou pseudoaneuryzmy arterie lienalis komplikujúcej pankreatickú pseudocystu [Large intestine bleeding caused by the lienal artery pseudoaneurysm rupture complicating a pancreatic pseudocyst]. Rozhl. Chir. 2011, 90, 590–593. (In Slovak) [Google Scholar]
- Perino, A.; Proto, E.; Calagna, G.; Granese, R.; Agrusa, A.; Guarneri, F.; Cucinella, G. Spontaneous rupture of splenic artery aneurysm in pregnancy: Is splenectomy always necessary? Acta Obstet. Gynecol. Scand. 2012, 91, 1349–1350. [Google Scholar] [CrossRef]
- Pavlis, T.; Seretis, C.; Gourgiotis, S.; Aravosita, P.; Mystakelli, C.; Aloizos, S. Spontaneous Rupture of Splenic Artery Aneurysm during the First Trimester of Pregnancy: Report of an Extremely Rare Case and Review of the Literature. Case Rep. Obstet. Gynecol. 2012, 2012, 528051. [Google Scholar] [CrossRef] [PubMed]
- Boumans, D.; Weerink, L.B.; Leyssius, A.T.R.; Swartbol, P.; Veneman, T.F. Splenic Artery Rupture During Pregnancy Concealed by a Pancreatic Lymphangioma: A Rare Co-Occurrence. Ann. Vasc. Surg. 2013, 27, 112.e1–112.e4. [Google Scholar] [CrossRef]
- Green, A.; Bowman-Burns, C.; Cumberbatch, G. Abdominal pain and collapse in the emergency department. BMJ Case Rep. 2013, 2013, bcr2013009925. [Google Scholar] [CrossRef]
- Boufettal, H.; Moussaïd, I.; Salmi, S.; Mahdaoui, S.; Hermas, S.; Samouh, N. Spontaneous rupture of a splenic artery aneurysm in peri-partum. Ann. Fr. Anesth. Reanim. 2013, 32, 722. [Google Scholar] [CrossRef] [PubMed]
- Benali, M.; Charrada, H.; Bouassida, M.; Bahloul, A.; Jmal, K.; Dhouib, F.; Saied, M.R.; Khaddar, M.K. Splenic artery aneurysm rupture in late pregnancy: A case report. Ann. Fr. Anesth. Reanim. 2013, 32, 721–722. [Google Scholar] [CrossRef]
- Oakley, E.; Ho, J.D.; Johnson, V.; VanCamp, J.; Melson, T.; Hick, J.L. Splenic Artery Aneurysm: An Important Cause of Hemoperitoneum and Shock. J. Emerg. Med. 2014, 46, e65–e67. [Google Scholar] [CrossRef] [PubMed]
- Khoshnevis, J.; Lotfollahzadeh, S.; Sobhiyeh, M.R.; Sepas, H.N.; Nejad, M.A.; Rahbari, A.; Behnaz, N.; Mahdi, Z. Ruptured Aneurysm of the Splenic Artery: A Rare Cause of Abdominal Pain after Blunt Trauma. Trauma Mon. 2013, 18, 46–49. [Google Scholar] [CrossRef] [PubMed]
- Papadomichelakis, A.; Anyfantakis, D.; Kastanakis, M.; Karona, P.; Bobolakis, E. Rupture of a splenic artery aneurysm in a previously healthy 53-year-old male. J. Med. Life 2014, 7, 69–70. [Google Scholar]
- Phillips, C.; Bulmer, J. Splenic Artery Aneurysm Rupture During Pregnancy. Nurs. Women’s Health 2013, 17, 508–517; quiz 518. [Google Scholar] [CrossRef]
- Goshayeshi, L.; Vosoghinia, H.; Rajabzadeh, F.; Ahadi, M.; Sakhmaresi, T.A.; Farzanehfar, M.R. Splenic Artery Aneurysm as an Unusual Cause of New Onset Ascites: A Case Report. Middle East J. Dig. Dis. 2014, 6, 37–41. [Google Scholar]
- Jackson, H.T.; Diaconu, S.C.; Maluso, P.J.; Abell, B.; Lee, J. Ruptured Splenic Artery Aneurysms and the Use of an Adapted Fast Protocol in Reproductive Age Women with Hemodynamic Collapse: Case Series. Case Rep. Emerg. Med. 2014, 2014, 454923. [Google Scholar] [CrossRef][Green Version]
- Kazaryan, A.M.; Wiborg, J.; Hauss, K.; Anundsen, T.K.; Flemmen, O.J.; Holm, T.E.; Lauzikas, G. Spontaneous non-traumatic massive intraabdominal spleen bleeding in young females: Importance of ATLS principles and trauma alarm. Am. J. Case Rep. 2014, 15, 189–193. [Google Scholar] [CrossRef][Green Version]
- Zeren, S.; Bayhan, Z.; Sönmez, Y.; Mestan, M.; Korkmaz, M.; Kadıoglu, E.; Ucar, B.I.; Devir, C.; Ekici, F.M.; Sanal, B. Spontaneous splenic artery aneurysm rupture: Mimicking acute myocardial infarct. Am. J. Emerg. Med. 2014, 32, 1563.e1–1563.e3. [Google Scholar] [CrossRef]
- Abdulrahman, A.; Shabkah, A.; Hassanain, M.; Aljiffry, M. Ruptured spontaneous splenic artery aneurysm: A case report and review of the literature. Int. J. Surg. Case Rep. 2014, 5, 754–757. [Google Scholar] [CrossRef] [PubMed]
- Corey, E.K.; Harvey, S.A.; Sauvage, L.M.; Bohrer, J.C. A Case of Ruptured Splenic Artery Aneurysm in Pregnancy. Case Rep. Obstet. Gynecol. 2014, 2014, 793735. [Google Scholar] [CrossRef] [PubMed]
- Heitkamp, A.C.; Dickhoff, C.; Nederhoed, J.H.; Franschman, G.; de Vries, J.I. Saved from a fatal flight: A ruptured splenic artery aneurysm in a pregnant woman. Int. J. Surg. Case Rep. 2015, 8C, 32–34. [Google Scholar] [CrossRef][Green Version]
- Kataoka, J.; Nitta, T.; Fujii, K.; Yamaguchi, T.; Hirata, Y.; Kawakami, K.; Kawasaki, H.; Higashino, T.; Ishibashi, T. A case of ruptured giant splenic artery aneurysm. Nihon Shokakibyo Gakkai Zasshi 2015, 112, 101–107. (In Japanese) [Google Scholar] [CrossRef] [PubMed]
- Le Tinier, B.; Jungo-Nançoz, C.J.-N.; McCarey, C.; Jastrow, N. Rupture of maternal splenic artery aneurysm and fetal demise. Clin. Exp. Obstet. Gynecol. 2015, 42, 381–383. [Google Scholar] [CrossRef]
- Pejkic, S.; Tomic, I.; Opacic, D.; Pejinovic, L.; Grubor, N.; Cinara, I.; Davidovic, L. Splenic artery aneurysms: Two cases of varied etiology, clinical presentation and treatment outcome. Srp. Arh. Celok. Lek. 2015, 143, 326–331. [Google Scholar] [CrossRef]
- Sawicki, M.; Marlicz, W.; Czapla, N.; Łokaj, M.; Skoczylas, M.M.; Donotek, M.; Kołaczyk, K. Massive Upper Gastrointestinal Bleeding from a Splenic Artery Pseudoaneurysm Caused by a Penetrating Gastric Ulcer: Case Report and Review of Literature. Pol. J. Radiol. 2015, 80, 384–387. [Google Scholar] [CrossRef]
- Barišić, T.; Šutalo, N.; Letica, L.; Kordić, A.V. Rupture of splenic artery aneurysm in primipara five days after cesarean section: Case report and review of the literature. Wien. Klin. Wochenschr. 2015, 127, 896–898. [Google Scholar] [CrossRef]
- Chia, C.; Pandya, G.J.; Kamalesh, A.; Shelat, V.G. Splenic Artery Pseudoaneurysm Masquerading as a Pancreatic Cyst—A Diagnostic Challenge. Int. Surg. 2015, 100, 1069–1071. [Google Scholar] [CrossRef]
- Hiltrop, N.; Vanhauwaert, A.; Palmers, P.-J.L.H.; Cool, M.; DeBoever, G.; Lambrecht, G. Hemosuccus pancreaticus caused by rupture of a splenic artery pseudoaneurysm complicating chronic alcoholic pancreatitis: An uncommon cause of gastrointestinal bleeding. Acta Gastro Enterol. Belg. 2015, 78, 427–430. [Google Scholar]
- Lee, J.W.; Kim, T.N.; Kim, S.B.; Kim, K.H. Splenic rupture following transcatheter arterial embolization of splenic artery pseudoaneurysm caused by acute pancreatitis. Korean J. Intern. Med. 2016, 31, 620–621. [Google Scholar] [CrossRef] [PubMed]
- Aydın, M.T.; Fersahoğlu, M.M.; Tezer, S.; Okuducu, M.; Ağca, B.; Memişoğlu, K. Spontaneous rupture of the splenic artery aneurysm: A rare clinical presentation of acute abdomen. Ulus. Travma. Acil. Cerrahi. Derg. 2016, 22, 106–108. [Google Scholar] [CrossRef][Green Version]
- Robaldo, A.; Gramondo, F.; Beccaria, F.; Colotto, P. Giant splenic artery aneurysm rupture. Clin. Case Rep. 2016, 4, 620–622. [Google Scholar] [CrossRef]
- Monti, J.D. A rare cause of abdominal pain and hypotension in pregnancy. J. Am. Acad. Physician Assist. 2016, 29, 31–34. [Google Scholar] [CrossRef]
- Hostinská, E.; Huml, K.; Pilka, R. Acute pancreatitis in pregnancy, complicated by rupture of aneurysm of artery lienalis. Ceska Gynekol. 2016, 81, 208–211. (In Czech) [Google Scholar]
- Jacobson, J.; Gorbatkin, C.; Good, S.; Sullivan, S. Splenic artery aneurysm rupture in pregnancy. Am. J. Emerg. Med. 2017, 35, 935.e5–935.e8. [Google Scholar] [CrossRef]
- Szpakowicz, J.; Szpakowicz, P.; Urbanik, A.; Markuszewski, L. Splenic Artery Pseudoaneurysm Rupture into a Pancreatic Pseudocyst with its Subsequent Perforation as the Cause of a Massive Intra-Abdominal Bleeding—Case Report. Pol. Przegl. Chir. 2016, 88, 350–355. [Google Scholar] [CrossRef]
- Kim, J.H.; Chung, H.S.; Kim, J.H.; Park, S.Y.; Lee, S.B.; Do, B.S. Splenic artery aneurysm with the double-rupture phenomenon. Clin. Exp. Emerg. Med. 2017, 4, 113–116. [Google Scholar] [CrossRef] [PubMed]
- Ologun, G.; Sharpton, K.; Granet, P. Successful use of resuscitative endovascular balloon occlusion of the aorta in the treatment of ruptured 8.5-cm splenic artery aneurysm. J. Vasc. Surg. 2017, 66, 1873–1875. [Google Scholar] [CrossRef][Green Version]
- Bacalja, I.D.; Koprek, D.; Pavic, P.; Cvjetko, I.; Krpina, K.; Diklic, D. Spontaneous rupture of a splenic artery aneurysm in a male patient. Neth. J. Med. 2017, 75, 357. [Google Scholar]
- Sakuraba, S.; Orita, H.; Ueda, S.; Tokuda, S.; Ito, T.; Kushida, T.; Sakurada, M.; Maekawa, H.; Wada, R.; Sato, K. A Case of Segmental Arterial Mediolysis Presenting as Mucosal Gastric Hematoma. Case Rep. Gastrointest. Med. 2017, 2017, 3634967. [Google Scholar] [CrossRef] [PubMed]
- Maharaj, R.; Raghunanan, B.; Mohammed, W.; Rambally, R.; Sookdeo, V.D.; Harnanan, D.; Warner, A.W. A rare case of massive lower gastrointestinal bleeding from a ruptured splenic artery aneurysm. J. Surg. Case Rep. 2018, 2018, rjy003. [Google Scholar] [CrossRef]
- Martin, D.; Farinha, H.T.; Dattner, N.; Rotman, S.; Demartines, N.; Sauvain, O.M. Spontaneous non-traumatic splenic artery aneurysm rupture: A case report and review of the literature. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 3147–3150. [Google Scholar] [CrossRef]
- Ktenidis, K.; Manaki, V.; Kapoulas, K.; Kourtellari, E.; Gionis, M. Giant Splenic Aneurysm with Arteriovenous (A-V) Shunt, Portal Hypertension, and Ascites. Am. J. Case Rep. 2018, 19, 1410–1415. [Google Scholar] [CrossRef]
- Chen, G.; Yang, J.; Qian, G.; Jiang, K.; Lv, Y.; Shi, N.; Zhu, T. Spontaneous rupture of a splenic artery aneurysm with splenic epithelioid hemangioendothelioma: A case report. J. Int. Med. Res. 2019, 47, 1059–1063. [Google Scholar] [CrossRef] [PubMed]
- Abdul, R.; Teelucksingh, S.; Omar, M.; Chow, A.C.; Boppana, L.K.T.; Goli, S.; Naraynsingh, V.; Teelucksingh, S. Splenic artery pseudoaneurysm presenting with massive rectal bleeding. Radiol. Case Rep. 2019, 14, 791–794. [Google Scholar] [CrossRef]
- Abhari, P.; Abhari, S.; Jackson, A.; Moustafa, A.S.Z.; Mercer, L.; Ashraf, M. Splenic Artery Aneurysm Case Report. Case Rep. Obstet. Gynecol. 2019, 2019, 8347983. [Google Scholar] [CrossRef]
- Wiener, Y.; Tomashev, R.; Neeman, O.; Itzhakov, Z.; Heldenberg, E.; Melcer, Y.; Maymon, R. Splenic artery aneurysms during pregnancy: An obstetric nightmare. Eur. J. Obstet. Gynecol. Reprod. Biol. 2019, 237, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Ballout, A.R.; Ghanem, R.; Nassar, A.; Hallal, A.H.; Ghulmiyyah, L.M. Splenic Artery Aneurysm (SAA) Rupture in Pregnancy: A Case Report of a Rare but Life-Threatening Obstetrical Complication. J. Women’s Health Dev. 2019, 2, 19–27. [Google Scholar] [CrossRef]
- Tlili, A.; Trigui, A.; Dkhil, O.; Feki, W.; Rejab, H.; Ben Ameur, H.; Boujelbene, S.; Mnif, Z. Rupture d’un anévrisme de l’artère splénique en fin de grossesse: À propos d’un cas [Splenic artery aneurysm rupture at the end of pregnancy: A case study]. Pan Afr. Med. J. 2019, 34, 63. [Google Scholar] [CrossRef]
- Pararas, N.; Rajendiran, S.; Taha, I.; Powar, R.R.; Holguera, C.; Tadros, E. Spontaneous Rupture of a Huge Splenic Artery Aneurysm: A Case Report. Am. J. Case Rep. 2020, 21, e919956. [Google Scholar] [CrossRef]
- Montrief, T.; Parris, M.A.; Auerbach, J.S.; Scott, J.M.; Cabrera, J. Spontaneous Splenic Artery Pseudoaneurysm Rupture Causing Hemorrhagic Shock. Cureus 2020, 12, e8286. [Google Scholar] [CrossRef]
- Huff, J.; Valle, O. Rupture of splenic artery aneurysm in pregnancy with double-rupture phenomenon: A case report. Case Rep. Women’s Health 2020, 27, e00230. [Google Scholar] [CrossRef] [PubMed]
- Fujii, M.; Yamashita, S.; Fudono, A.; Yanai, S.; Tashiro, J.; Takenaka, Y.; Yamasaki, K.; Ito, E.; Masaki, Y. Splenic artery aneurysm rupture during pregnancy: A case report of maternal and fetal survival. Int. J. Surg. Case Rep. 2020, 76, 94–97. [Google Scholar] [CrossRef] [PubMed]
- Holt, J.N.; Schwalb, E.H. A case of splenic artery pseudoaneurysm rupture presenting as rectal bleeding in a regional hospital. J. Surg. Case Rep. 2020, 2020, rjaa504. [Google Scholar] [CrossRef]
- Vieujean, S.; Dauby, M.; Remacle, G.; Kridelka, F.; Dewandre, P.Y.; Capelle, X. Spontaneous rupture of a splenic artery aneurysm during the third trimester of pregnancy. Rev. Med. Liege. 2021, 76, 18–22. (In French) [Google Scholar] [PubMed]
- Tan, M.Y.Q.; Wong, A.J.T.-Y.; Aung, L.; Ng, W.M.; Lee, W.F.; Lim, B.L. Circulatory collapse from rupture of splenic artery aneurysm: A case study. Ann. Acad. Med. Singap. 2021, 50, 86–87. [Google Scholar] [CrossRef]
- El Aidaoui, K.; Bensaad, A.; Habi, J.; El Yamani, K.; El Kettani, C. Hemorrhagic Shock Revealing Rupture of Splenic Artery Pseudoaneurysm Three Years After Post-Traumatic Pancreatitis. Cureus 2021, 13, e15678. [Google Scholar] [CrossRef]
- Wang, A.; Gao, J. Spontaneous rupture of a splenic artery aneurysm during pregnancy. Asian J. Surg. 2021, 45, 739–741. [Google Scholar] [CrossRef]
- Ornaghi, S.; Crippa, I.; Di Nicola, S.; Giardini, V.; La Milia, L.; Locatelli, L.; Corso, R.; Roncaglia, N.; Vergani, P. Splenic artery aneurysm in obstetrical patients: A series of four cases with different clinical presentation and outcome. Int. J. Gynaecol. Obstet. 2022, 159, 474–479. [Google Scholar] [CrossRef] [PubMed]
- Betancourth Alvarenga, J.E.; Santiago Martínez, S.; Jiménez Gómez, S.J.; San Vicente Vela, M.B.; Gaspar Pérez, M.; Álvarez García, N.; Güizzo, J.R.; Jiménez Arribas, P.; Esteva Miró, C.; Núñez García, B. Management of splenic and/or hepatic pseudoaneurysm following abdominal trauma in pediatric patients. Cir. Pediatr. 2022, 35, 80–84, (In English and Spanish). [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, M.M.; Quiyum, M.A.; Mohammed, S.; Karim, R. Hemosuccus Pancreaticus: A Rare Cause of Gastrointestinal Bleeding. Mymensingh. Med. J. 2022, 31, 872–875. [Google Scholar]
- Yoshikawa, C.; Yamato, I.; Nakata, Y.; Nakagawa, T.; Inoue, T.; Nakatani, M.; Nezu, D.; Doi, S.; Kuroda, Y.; Fujii, K.; et al. Giant splenic artery aneurysm rupture into the stomach that was successfully managed with emergency distal pancreatectomy. Surg. Case Rep. 2022, 8, 148. [Google Scholar] [CrossRef] [PubMed]
- Hussein, M.M.; Al-Mollah, M.; Kanaan, T. Splenic artery aneurysm rupture post-anterior cervical discectomy and fusion: Case report & literature review. Int. J. Surg. Case Rep. 2022, 99, 107704. [Google Scholar] [CrossRef]
- Hosseinzadeh, A.; Shahriarirad, R.; Majdazar, V.A.; Farsani, M.M.; Tadayon, S.M.K. Spontaneous rupture of a large splenic artery aneurysm in a 59-year-old male patient with pemphigus vulgaris: A case report. J. Med. Case Rep. 2022, 16, 382. [Google Scholar] [CrossRef]
- Hamilton, E.J.; Ngugi, S.; Kotakadeniya, R. Surgical Management of Atraumatic Rupture of Splenic Artery Aneurysm with Spleen Preservation in a Regional Australian Hospital. Case Rep. Surg. 2023, 2023, 5738806. [Google Scholar] [CrossRef]
- Shalhub, S.; Nkansah, R.; El-Ghazali, A.; Hillenbrand, C.J.; Vaidya, S.S.; Schwarze, U.; Byers, P.H. Splenic artery pathology presentation, operative interventions, and outcomes in 88 patients with vascular Ehlers-Danlos syndrome. J. Vasc. Surg. 2023, 78, 394–404. [Google Scholar] [CrossRef] [PubMed]
- Barrionuevo, P.; Malas, M.B.; Nejim, B.; Haddad, A.; Morrow, A.; Ponce, O.; Hasan, B.; Seisa, M.; Chaer, R.; Murad, M.H. A systematic review and meta-analysis of the management of visceral artery aneurysms. J. Vasc. Surg. 2019, 70, 1694–1699. [Google Scholar] [CrossRef]
- Chiaradia, M.; Novelli, L.; Deux, J.-F.; Tacher, V.; Mayer, J.; You, K.; Djabbari, M.; Luciani, A.; Rahmouni, A.; Kobeiter, H. Ruptured visceral artery aneurysms. Diagn. Interv. Imaging 2015, 96, 797–806. [Google Scholar] [CrossRef]
- Marone, E.M.; Mascia, D.; Kahlberg, A.; Brioschi, C.; Tshomba, Y.; Chiesa, R. Is Open Repair Still the Gold Standard in Visceral Artery Aneurysm Management? Ann. Vasc. Surg. 2011, 25, 936–946. [Google Scholar] [CrossRef] [PubMed]
- Shera, F.A.; Shera, T.A.; Choh, N.A.; Bhat, M.H.; Shah, O.A.; Shaheen, F.A.; Robbani, I.; Gojwari, T. Clinical Profile, Management, and Outcome of Visceral Artery Pseudoaneurysms: 5-Year Experience in a Tertiary Care Hospital. Int. J. Angiol. 2023, 32, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Gong, C.; Sun, M.-S.; Leng, R.; Ren, H.-L.; Zheng, K.; Wang, S.-X.; Zhu, R.-M.; Li, C.-M. Endovascular embolization of visceral artery aneurysm: A retrospective study. Sci. Rep. 2023, 13, 6936. [Google Scholar] [CrossRef] [PubMed]
- Fargion, A.T.; Falso, R.; Speziali, S.; Biancofiore, B.; Esposito, D.; Giacomelli, E.; Dorigo, W.; Pulli, R. Results of current endovascular treatments for visceral artery aneurysms. J. Vasc. Surg. 2023, 78, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Marone, E.M.; Rinaldi, L.F. Current Debates in the Management of Visceral Artery Aneurysms: Where the Guidelines Collide. J. Clin. Med. 2023, 12, 3267. [Google Scholar] [CrossRef]



| OSR (%) | EVT (%) | p-Value | |
|---|---|---|---|
| Male sex | 63/185 (34%) | 57/165 (34.5%) | 1 |
| Female sex | 68/185 (36.75%) | 27/165 (16.5%) | 0.01 |
| Sex non reported | 54/185 (29.25%) | 81/165 (49%) | - |
| Mean age | 41.5 ± 15.3 | 53.5 ± 16.9 | 0.02 |
| SAPAs | 23/185 (12.4%) | 69/165 (41.8%) | <0.01 |
| Hemodynamic stability | 19 (10.6%) | 81 (49.1%) | <0.01 |
| Hemodynamic instability | 142 (76.5%) | 81 (49.1%) | <0.01 |
| Hemodynamic status non reported | 24 (12.9%) | 3 (1.8%) | - |
| Rupture during pregnancy | 79 (42.7%) | 7 (4.2%) | 0.01 |
| OSR | EVT | p-Value | |
|---|---|---|---|
| Overall mortality | 24/185 (12.9%) | 13/165 (7.8%) | 0.84 |
| Mortality in HD stable patients | 0/19 (0%) | 7/81 (8.6%) | Logistic regression analysis on HD instability as predictor of mortality: p-value (OSR): 0.02 p-value (EVT): 0.18 |
| Mortality in HD unstable patients | 13/142 * (9.1%) | 3/81 * (3.7%) | |
| Early reintervention conversion to OSR | 5/185 (2.7%) - | 37/165 (22.4%) 31/165 (18.8%) | <0.01 - |
| Reinterventions/conversions in HD stable patients | 2/19 (10.5%) | 9/81 (11.1%) | Logistic regression analysis on HD instability as predictor of reintervention: p-value (OSR): 0.30 p-value (EVT): 0.01 |
| Reinterventions/conversions in HD stable patients | 1/142 * (7%) | 21/81 * (25.9%) |
| OSR | EVT | Total | p-Value | |
|---|---|---|---|---|
| Systemic complications | 4/185 (2 sepsis, 1 stroke, 1 ileus) | 4/165 (2 MI, 1 pneumonia, 1 ileus) | 8/350 | 1 |
| Splenic abscess | 1/185 | 3/165 | 4/350 | 0.8 |
| Splenic infarction | 0/185 | 4/165 | 4/350 | 0.3 |
| Pancreatitis | 2/185 | 0/165 | 2/350 | 0.7 |
| Total | 7/185 (3.8%) | 11/165 (6.7%) | 18/350 (5.1%) | 0.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rinaldi, L.F.; Brioschi, C.; Marone, E.M. Endovascular and Open Surgical Treatment of Ruptured Splenic Artery Aneurysms: A Case Report and a Systematic Literature Review. J. Clin. Med. 2023, 12, 6085. https://doi.org/10.3390/jcm12186085
Rinaldi LF, Brioschi C, Marone EM. Endovascular and Open Surgical Treatment of Ruptured Splenic Artery Aneurysms: A Case Report and a Systematic Literature Review. Journal of Clinical Medicine. 2023; 12(18):6085. https://doi.org/10.3390/jcm12186085
Chicago/Turabian StyleRinaldi, Luigi Federico, Chiara Brioschi, and Enrico Maria Marone. 2023. "Endovascular and Open Surgical Treatment of Ruptured Splenic Artery Aneurysms: A Case Report and a Systematic Literature Review" Journal of Clinical Medicine 12, no. 18: 6085. https://doi.org/10.3390/jcm12186085
APA StyleRinaldi, L. F., Brioschi, C., & Marone, E. M. (2023). Endovascular and Open Surgical Treatment of Ruptured Splenic Artery Aneurysms: A Case Report and a Systematic Literature Review. Journal of Clinical Medicine, 12(18), 6085. https://doi.org/10.3390/jcm12186085






