Promising Novel Therapies in the Treatment of Aortic and Visceral Aneurysms
Abstract
:1. Introduction
2. Definition and Epidemiology of Aneurysms
3. Etiology of Aortic and Visceral Aneurysms: Genetic Syndromes vs. Sporadic Disease
4. Symptomatology of Aortic and Visceral Aneurysms
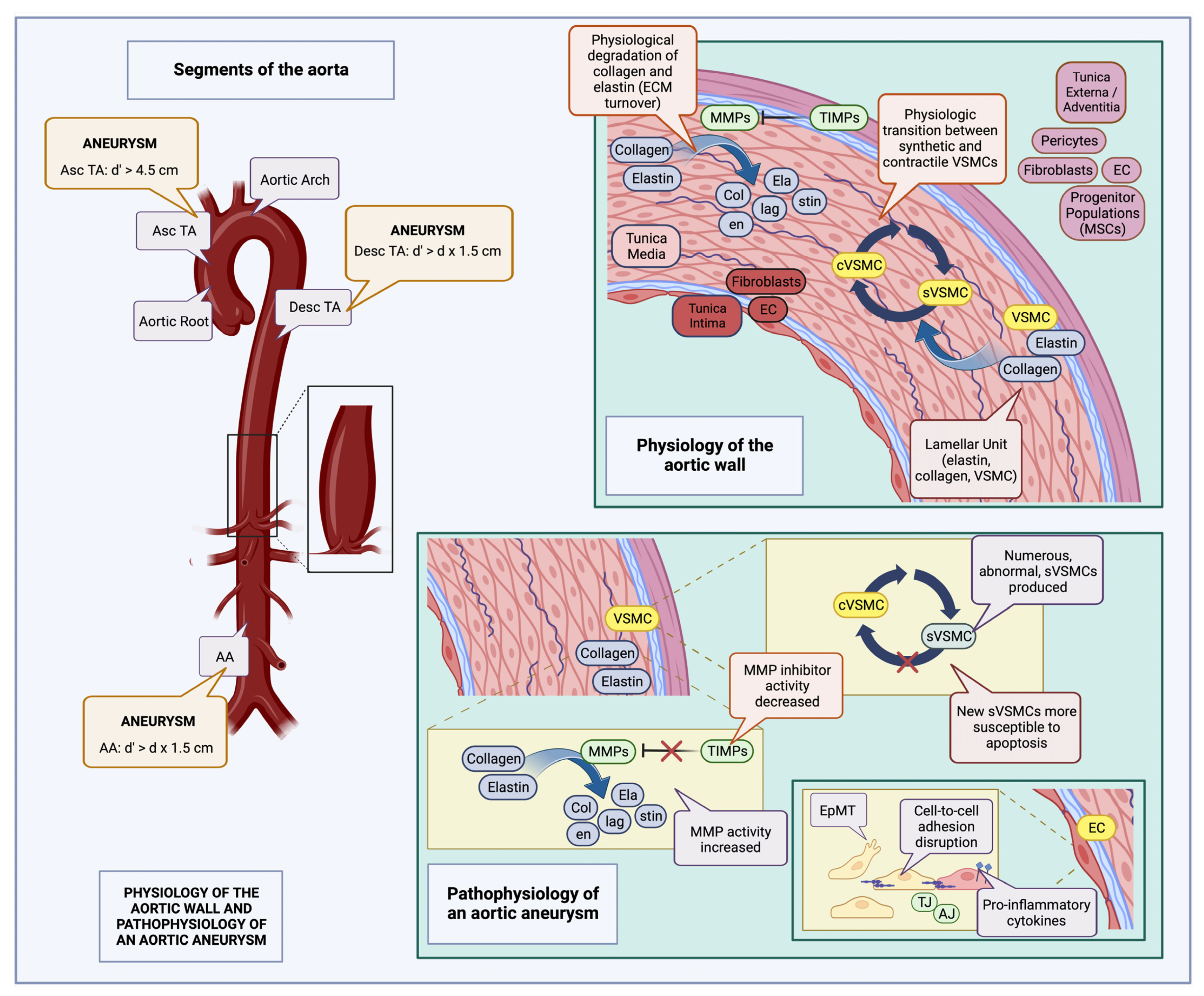
5. Molecular Pathophysiology of Aortic and Visceral Aneurysms
6. Animal Models of Aneurysm
7. Current Treatment Strategies
7.1. Conservative Management
7.2. Criteria and Forms of Surgical Intervention
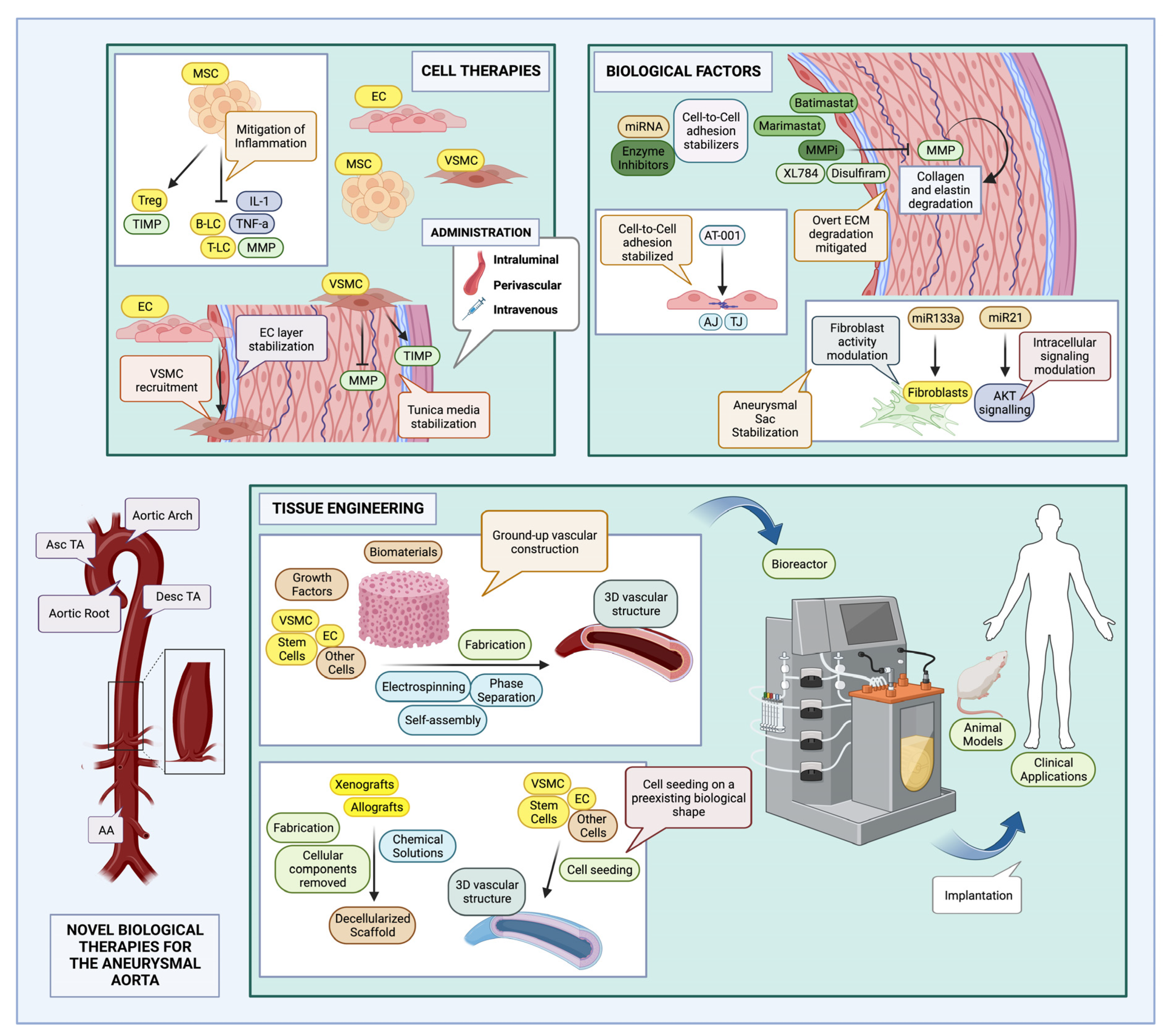
8. Promising New Updates
8.1. A Discovery of Stem Cells: Stem Cell Therapies for Aneurysms
8.1.1. Stem and Progenitor Cells: Definitions, Classification, and Derivation
8.1.2. Cell Therapies for Aneurysms
8.2. Tissue Engineering (TE)
Tissue Engineered Vascular Grafts (TEVGs) and Large Vessel Replacement
8.3. Biological Factors
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kwon, S.G.; Kwon, Y.W.; Lee, T.W.; Park, G.T.; Kim, J.H. Recent Advances in Stem Cell Therapeutics and Tissue Engineering Strategies. Biomater. Res. 2018, 22, 36. [Google Scholar] [CrossRef]
- Alrefai, M.T.; Murali, D.; Paul, A.; Ridwan, K.M.; Connell, J.M.; Shum-Tim, D. Cardiac Tissue Engineering and Regeneration Using Cell-Based Therapy. Stem Cells Cloning 2015, 8, 81–101. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, I.C.P.; Kaasi, A.; Maciel Filho, R.; Jardini, A.L.; Gabriel, L.P. Cardiac Tissue Engineering: Current State-of-the-Art Materials, Cells and Tissue Formation. Einstein 2018, 16, eRB4538. [Google Scholar] [CrossRef] [PubMed]
- Nugent, H.M.; Edelman, E.R. Tissue Engineering Therapy for Cardiovascular Disease. Circ. Res. 2003, 92, 1068–1078. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Li, L.K.; Dai, T.; Wang, A.; Li, S. Adult Stem Cells in Vascular Remodeling. Theranostics 2018, 8, 815–829. [Google Scholar] [CrossRef]
- Yu, B.; Chen, Q.; Le Bras, A.; Zhang, L.; Xu, Q. Vascular Stem/Progenitor Cell Migration and Differentiation in Atherosclerosis. Antioxid. Redox Signal. 2018, 29, 219–235. [Google Scholar] [CrossRef]
- Tulsyan, N.; Kashyap, V.S.; Greenberg, R.K.; Sarac, T.P.; Clair, D.G.; Pierce, G.; Ouriel, K. The Endovascular Management of Visceral Artery Aneurysms and Pseudoaneurysms. J. Vasc. Surg. 2007, 45, 276–283. [Google Scholar] [CrossRef]
- Writing Committee Members; Isselbacher, E.M.; Preventza, O.; Hamilton, B.I.J.; Augoustides, J.G.; Beck, A.W.; Bolen, M.A.; Braverman, A.C.; Bray, B.E.; Brown-Zimmerman Maya, M.; et al. 2022 ACC/AHA Guideline for the Diagnosis and Management of Aortic Disease. J. Am. Coll. Cardiol. 2022, 80, e223–e393. [Google Scholar] [CrossRef]
- Upchurch, G.R.; Perry, R.J. Thoracic and Thoracoabdominal Aortic Aneurysms: Etiology, Epidemiology, Natural History, Medical Management, and Decision Making. In Rutherford’s Vascular Surgery and Endovascular Therapy; Elsevier: Philadelphia, PA, USA, 2019; ISBN 978-0-323-42791-3. [Google Scholar]
- Safi, H.J.; Anthony, L. Estrera Direct Surgical Repair of Aneurysms of the Thoracic and Thoracoabdominal Aorta. In Atlas of Vascular and Endovascular Therapy—Anatomy and Technique; Elsevier Saunders: Philadelphia, PA, USA, 2015; ISBN 978-1-4160-6841-9. [Google Scholar]
- Juntermanns, B.; Bernheim, J.; Karaindros, K.; Walensi, M.; Hoffmann, J.N. Visceral Artery Aneurysms. Gefasschirurgie 2018, 23, 19–22. [Google Scholar] [CrossRef]
- Shen, Y.H.; LeMaire, S.A. Molecular Pathogenesis of Genetic and Sporadic Aortic Aneurysms and Dissections. Curr. Probl. Surg. 2017, 54, 95–155. [Google Scholar] [CrossRef]
- Isselbacher, E.M. Thoracic and Abdominal Aortic Aneurysms. Circulation 2005, 111, 816–828. [Google Scholar] [CrossRef] [PubMed]
- Meyer, A.; Uder, M.; Lang, W.; Croner, R. Visceral artery aneurysms. Zent. Chir. 2010, 135, 416–420. [Google Scholar] [CrossRef] [PubMed]
- Germain, D.P. Ehlers-Danlos Syndrome Type IV. Orphanet J. Rare Dis. 2007, 2, 32. [Google Scholar] [CrossRef]
- Saeyeldin, A.; Zafar, M.A.; Velasquez, C.A.; Ip, K.; Gryaznov, A.; Brownstein, A.J.; Li, Y.; Rizzo, J.A.; Erben, Y.; Ziganshin, B.A.; et al. Natural History of Aortic Root Aneurysms in Marfan Syndrome. Ann. Cardiothorac. Surg. 2017, 6, 625–632. [Google Scholar] [CrossRef]
- Cury, M.; Zeidan, F.; Lobato, A.C. Aortic Disease in the Young: Genetic Aneurysm Syndromes, Connective Tissue Disorders, and Familial Aortic Aneurysms and Dissections. Int. J. Vasc. Med. 2013, 2013, 267215. [Google Scholar] [CrossRef] [PubMed]
- Corbitt, H.; Gutierrez, J.; Silberbach, M.; Maslen, C.L. The Genetic Basis of Turner Syndrome Aortopathy. Am. J. Med. Genet. C Semin. Med. Genet. 2019, 181, 117–125. [Google Scholar] [CrossRef]
- Pomianowski, P.; Elefteriades, J.A. The Genetics and Genomics of Thoracic Aortic Disease. Ann. Cardiothorac. Surg. 2013, 2, 27179–27279. [Google Scholar] [CrossRef]
- Diletta, L.; Enrico, R.; Germano, M. Thoracoabdominal Aortic Aneurysm in Connective Tissue Disorder Patients. Indian. J. Thorac. Cardiovasc. Surg. 2022, 38, 146–156. [Google Scholar] [CrossRef]
- Goyal, A.; Keramati, A.R.; Czarny, M.J.; Resar, J.R.; Mani, A. The Genetics of Aortopathies in Clinical Cardiology. Clin. Med. Insights Cardiol. 2017, 11, 1179546817709787. [Google Scholar] [CrossRef]
- Shalhub, S.; Black, J.H.; Cecchi, A.C.; Xu, Z.; Griswold, B.F.; Safi, H.J.; Milewicz, D.M.; McDonnell, N.B. Molecular Diagnosis in Vascular Ehlers-Danlos Syndrome Predicts Pattern of Arterial Involvement and Outcomes. J. Vasc. Surg. 2014, 60, 160–169. [Google Scholar] [CrossRef]
- Panneton, J.M.; Hollier, L.H. Nondissecting Thoracoabdominal Aortic Aneurysms: Part I. Ann. Vasc. Surg. 1995, 9, 503–514. [Google Scholar] [CrossRef]
- Badea, R. Splanchnic Artery Aneurysms: The Diagnostic Contribution of Ultrasonography in Correlation with Other Imaging Methods. J. Gastrointestin Liver Dis. 2008, 17, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Miranda, V.; Sousa, J.; Mansilha, A. Spinal Cord Injury in Endovascular Thoracoabdominal Aortic Aneurysm Repair: Prevalence, Risk Factors and Preventive Strategies. Int. Angiol. 2018, 37, 112–126. [Google Scholar] [CrossRef]
- DeRoo, E.; Stranz, A.; Yang, H.; Hsieh, M.; Se, C.; Zhou, T. Endothelial Dysfunction in the Pathogenesis of Abdominal Aortic Aneurysm. Biomolecules 2022, 12, 509. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.; Hu, M.; Fedak, P.W.M.; Oudit, G.Y.; Kassiri, Z. Cell-Specific Functions of ADAM17 Regulate the Progression of Thoracic Aortic Aneurysm. Circ. Res. 2018, 123, 372–388. [Google Scholar] [CrossRef]
- Graham, L.M.; Stanley, J.C.; Whitehouse, W.M.; Zelenock, G.B.; Wakefield, T.W.; Cronenwett, J.L.; Lindenauer, S.M. Celiac Artery Aneurysms: Historic (1745–1949) versus Contemporary (1950–1984) Differences in Etiology and Clinical Importance. J. Vasc. Surg. 1985, 2, 757–764. [Google Scholar] [CrossRef]
- Popov, P.; Radak, Đ.; Popov, P.; Radak, Đ. Visceral Artery Aneurysms. In Aortic Aneurysm—Recent Advances; IntechOpen: Rijeka, Croatia, 2013; ISBN 978-953-51-1081-1. [Google Scholar]
- Dejana, E.; Hirschi, K.K.; Simons, M. The Molecular Basis of Endothelial Cell Plasticity. Nat. Commun. 2017, 8, 14361. [Google Scholar] [CrossRef]
- Jauhiainen, S.; Kiema, M.; Hedman, M.; Laakkonen, J.P. Large Vessel Cell Heterogeneity and Plasticity: Focus in Aortic Aneurysms. Arterioscler. Thromb. Vasc. Biol. 2022, 42, 811–818. [Google Scholar] [CrossRef]
- Van Doren, S.R. Matrix Metalloproteinase Interactions with Collagen and Elastin. Matrix Biol. 2015, 44–46, 224–231. [Google Scholar] [CrossRef]
- Gao, J.; Cao, H.; Hu, G.; Wu, Y.; Xu, Y.; Cui, H.; Lu, H.S.; Zheng, L. The Mechanism and Therapy of Aortic Aneurysms. Signal Transduct. Target. 2023, 8, 1–20. [Google Scholar] [CrossRef]
- Ikonomidis, J.S.; Barbour, J.R.; Amani, Z.; Stroud, R.E.; Herron, A.R.; McClister, D.M.; Camens, S.E.; Lindsey, M.L.; Mukherjee, R.; Spinale, F.G. Effects of Deletion of the Matrix Metalloproteinase 9 Gene on Development of Murine Thoracic Aortic Aneurysms. Circulation 2005, 112, I-242. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.A.; Ruddy, J.M.; Bouges, S.; Zavadzkas, J.A.; Brinsa, T.A.; Stroud, R.E.; Mukherjee, R.; Spinale, F.G.; Ikonomidis, J.S. Alterations in Membrane Type-1 Matrix Metalloproteinase Abundance after the Induction of Thoracic Aortic Aneurysm in a Murine Model. Am. J. Physiol. Heart Circ. Physiol. 2010, 299, H114–H124. [Google Scholar] [CrossRef] [PubMed]
- Rombouts, K.B.; van Merrienboer, T.A.R.; Ket, J.C.F.; Bogunovic, N.; van der Velden, J.; Yeung, K.K. The Role of Vascular Smooth Muscle Cells in the Development of Aortic Aneurysms and Dissections. Eur. J. Clin. Investig. 2022, 52, e13697. [Google Scholar] [CrossRef] [PubMed]
- Kiema, M.; Sarin, J.K.; Kauhanen, S.P.; Torniainen, J.; Matikka, H.; Luoto, E.-S.; Jaakkola, P.; Saari, P.; Liimatainen, T.; Vanninen, R.; et al. Wall Shear Stress Predicts Media Degeneration and Biomechanical Changes in Thoracic Aorta. Front. Physiol. 2022, 13, 934941. [Google Scholar] [CrossRef]
- Piasek, E.; Sojka, M.; Kuczyńska, M.; Światłowski, Ł.; Drelich-Zbroja, A.; Furmaga, O.; Jargiełło, T. Visceral Artery Aneurysms –Classification, Diagnosis and Treatment. J. Ultrason. 2018, 18, 148–151. [Google Scholar] [CrossRef]
- Ghosh, A.; DiMusto, P.D.; Ehrlichman, L.K.; Sadiq, O.; McEvoy, B.; Futchko, J.S.; Henke, P.K.; Eliason, J.L.; Upchurch, G.R. The Role of Extracellular Signal-Related Kinase During Abdominal Aortic Aneurysm Formation. J. Am. Coll. Surg. 2012, 215, 668–680.e1. [Google Scholar] [CrossRef]
- Shen, Y.H.; Zhang, L.; Ren, P.; Nguyen, M.T.; Zou, S.; Wu, D.; Wang, X.L.; Coselli, J.S.; LeMaire, S.A. AKT2 Confers Protection Against Aortic Aneurysms and Dissections. Circ. Res. 2013, 112, 618–632. [Google Scholar] [CrossRef]
- Chung, A.W.Y.; Au Yeung, K.; Cortes, S.F.; Sandor, G.G.S.; Judge, D.P.; Dietz, H.C.; van Breemen, C. Endothelial Dysfunction and Compromised ENOS/Akt Signaling in the Thoracic Aorta during the Progression of Marfan Syndrome. Br. J. Pharmacol. 2007, 150, 1075–1083. [Google Scholar] [CrossRef]
- Chen, P.-Y.; Qin, L.; Li, G.; Wang, Z.; Dahlman, J.E.; Malagon-Lopez, J.; Gujja, S.; Cilfone, N.A.; Kauffman, K.J.; Sun, L.; et al. Endothelial TGF-β Signalling Drives Vascular Inflammation and Atherosclerosis. Nat. Metab. 2019, 1, 912–926. [Google Scholar] [CrossRef]
- Tingting, T.; Wenjing, F.; Qian, Z.; Hengquan, W.; Simin, Z.; Zhisheng, J.; Shunlin, Q. The TGF-β Pathway Plays a Key Role in Aortic Aneurysms. Clin. Chim. Acta 2020, 501, 222–228. [Google Scholar] [CrossRef]
- Iddawela, S.; Ravendren, A.; Harky, A. Bio-Chemo-Mechanics of the Thoracic Aorta. Vasc. Biol. 2021, 3, R25–R33. [Google Scholar] [CrossRef] [PubMed]
- Jana, S.; Hu, M.; Shen, M.; Kassiri, Z. Extracellular Matrix, Regional Heterogeneity of the Aorta, and Aortic Aneurysm. Exp. Mol. Med. 2019, 51, 1–15. [Google Scholar] [CrossRef]
- Canfield, A.E.; Doherty, M.J.; Wood, A.C.; Farrington, C.; Ashton, B.; Begum, N.; Harvey, B.; Poole, A.; Grant, M.E.; Boot-Handford, R.P. Role of Pericytes in Vascular Calcification: A Review. Z. Kardiol. 2000, 89, S020–S027. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Xu, Q. Adventitial Biology. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 1523–1529. [Google Scholar] [CrossRef] [PubMed]
- Berman, A.G.; Romary, D.J.; Kerr, K.E.; Gorazd, N.E.; Wigand, M.M.; Patnaik, S.S.; Finol, E.A.; Cox, A.D.; Goergen, C.J. Experimental Aortic Aneurysm Severity and Growth Depend on Topical Elastase Concentration and Lysyl Oxidase Inhibition. Sci. Rep. 2022, 12, 99. [Google Scholar] [CrossRef]
- Halabi, C.M.; Mecham, R.P. Chapter 12–Elastin Purification and Solubilization. In Methods in Cell Biology; Mecham, R.P., Ed.; Methods in Extracellular Matrix Biology; Academic Press: New York, NY, USA, 2018; Volume 143, pp. 207–222. [Google Scholar]
- Deng, J.; Li, D.; Zhang, X.; Lu, W.; Rong, D.; Wang, X.; Sun, G.; Jia, S.; Zhang, H.; Jia, X.; et al. Murine Model of Elastase-Induced Proximal Thoracic Aortic Aneurysm through a Midline Incision in the Anterior Neck. Front. Cardiovasc. Med. 2023, 10, 953514. [Google Scholar] [CrossRef]
- Xue, C.; Zhao, G.; Zhao, Y.; Chen, Y.E.; Zhang, J. Mouse Abdominal Aortic Aneurysm Model Induced by Perivascular Application of Elastase. J. Vis. Exp. 2022, 180. e63608. [Google Scholar] [CrossRef]
- Chiou, A.C.; Chiu, B.; Pearce, W.H. Murine Aortic Aneurysm Produced by Periarterial Application of Calcium Chloride. J. Surg. Res. 2001, 99, 371–376. [Google Scholar] [CrossRef]
- Cao, J.; Wu, Q.; Geng, L.; Chen, X.; Shen, W.; Wu, F.; Chen, Y. Rapamycin Inhibits CaCl2-Induced Thoracic Aortic Aneurysm Formation in Rats through MTOR-Mediated Suppression of Proinflammatory Mediators. Mol. Med. Rep. 2017, 16, 1911–1919. [Google Scholar] [CrossRef]
- Coscas, R.; Dupont, S.; Mussot, S.; Louedec, L.; Etienne, H.; Morvan, M.; Chiocchia, G.; Massy, Z.; Jacob, M.-P.; Michel, J.-B. Exploring Antibody-Dependent Adaptive Immunity against Aortic Extracellular Matrix Components in Experimental Aortic Aneurysms. J. Vasc. Surg. 2018, 68, 60S–71S.e3. [Google Scholar] [CrossRef]
- Authors/Task Force Members; Erbel, R.; Aboyans, V.; Boileau, C.; Bossone, E.; Bartolomeo, R.D.; Eggebrecht, H.; Evangelista, A.; Falk, V.; Frank, H.; et al. 2014 ESC Guidelines on the Diagnosis and Treatment of Aortic Diseases: Document Covering Acute and Chronic Aortic Diseases of the Thoracic and Abdominal Aorta of the AdultThe Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society of Cardiology (ESC). Eur. Heart J. 2014, 35, 2873–2926. [Google Scholar] [CrossRef] [PubMed]
- Sode, B.F.; Nordestgaard, B.G.; Grønbæk, M.; Dahl, M. Tobacco Smoking and Aortic Aneurysm: Two Population-Based Studies. Int. J. Cardiol. 2013, 167, 2271–2277. [Google Scholar] [CrossRef]
- Sweeting, M.J.; Thompson, S.G.; Brown, L.C.; Powell, J.T. RESCAN collaborators Meta-Analysis of Individual Patient Data to Examine Factors Affecting Growth and Rupture of Small Abdominal Aortic Aneurysms. Br. J. Surg. 2012, 99, 655–665. [Google Scholar] [CrossRef]
- Wan, X.; Wang, W.; Liu, J.; Tong, T. Estimating the Sample Mean and Standard Deviation from the Sample Size, Median, Range and/or Interquartile Range. BMC Med. Res. Methodol. 2014, 14, 135. [Google Scholar] [CrossRef]
- Li, S.; Zhang, L.; Zhu, G.; Feng, R.; Zhou, J.; Jing, Z. Diabetes Mellitus Lowers the Risk of Aortic Dissection: A Systematic Review and Meta-Analysis. Ann. Vasc. Surg. 2021, 74, 209–219. [Google Scholar] [CrossRef]
- Yiu, R.S.; Cheng, S.W.K. Natural History and Risk Factors for Rupture of Thoracic Aortic Arch Aneurysms. J. Vasc. Surg. 2016, 63, 1189–1194. [Google Scholar] [CrossRef] [PubMed]
- Grundy, S.M.; Stone, N.J.; Bailey, A.L.; Beam, C.; Birtcher, K.K.; Blumenthal, R.S.; Braun, L.T.; de Ferranti, S.; Faiella-Tommasino, J.; Forman, D.E.; et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019, 139, e1082–e1143. [Google Scholar] [CrossRef] [PubMed]
- Lindholt, J.S.; Björck, M.; Michel, J.B. Anti-Platelet Treatment of Middle-Sized Abdominal Aortic Aneurysms. Curr. Vasc. Pharm. 2013, 11, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.C.; Allen, J.; Blair, S.N.; Bonow, R.O.; Brass, L.M.; Fonarow, G.C.; Grundy, S.M.; Hiratzka, L.; Jones, D.; Krumholz, H.M.; et al. AHA/ACC Guidelines for Secondary Prevention for Patients with Coronary and Other Atherosclerotic Vascular Disease: 2006 Update. Circulation 2006, 113, 2363–2372. [Google Scholar] [CrossRef] [PubMed]
- Chaer, R.A.; Abularrage, C.J.; Coleman, D.M.; Eslami, M.H.; Kashyap, V.S.; Rockman, C.; Murad, M.H. The Society for Vascular Surgery Clinical Practice Guidelines on the Management of Visceral Aneurysms. J. Vasc. Surg. 2020, 72, 3S–39S. [Google Scholar] [CrossRef]
- Davies, R.R.; Gallo, A.; Coady, M.A.; Tellides, G.; Botta, D.M.; Burke, B.; Coe, M.P.; Kopf, G.S.; Elefteriades, J.A. Novel Measurement of Relative Aortic Size Predicts Rupture of Thoracic Aortic Aneurysms. Ann. Thorac. Surg. 2006, 81, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Gloviczki, P. Surgical Repair of Thoracoabdominal Aneurysms: Patient Selection, Techniques and Results. Cardiovasc. Surg. 2002, 10, 434–441. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, A.; Smith, H.N.; Safi, H.J.; Estrera, A.L. Open Treatments for Thoracoabdominal Aortic Aneurysm Repair. Methodist. Debakey Cardiovasc. J. 2023, 19, 49–58. [Google Scholar] [CrossRef]
- Tian, D.H.; De Silva, R.P.; Wang, T.; Yan, T.D. Open Surgical Repair for Chronic Type B Aortic Dissection: A Systematic Review. Ann. Cardiothorac. Surg. 2014, 3, 340–350. [Google Scholar] [CrossRef]
- Ouzounian, M.; LeMaire, S.A.; Weldon, S.; Coselli, J.S. Open Repair of Thoracoabdominal Aortic Aneurysm: Step-by-Step. Oper. Tech. Thorac. Cardiovasc. Surg. 2018, 23, 2–20. [Google Scholar] [CrossRef]
- Ouzounian, M.; Tadros, R.O.; Svensson, L.G.; Lyden, S.P.; Oderich, G.S.; Coselli, J.S. Thoracoabdominal Aortic Disease and Repair: JACC Focus Seminar, Part 3. J. Am. Coll. Cardiol. 2022, 80, 845–856. [Google Scholar] [CrossRef] [PubMed]
- Tenorio, E.R.; Dias-Neto, M.F.; Lima, G.B.B.; Estrera, A.L.; Oderich, G.S. Endovascular Repair for Thoracoabdominal Aortic Aneurysms: Current Status and Future Challenges. Ann. Cardiothorac. Surg. 2021, 10, 744–767. [Google Scholar] [CrossRef]
- Oderich, G.S.; Tenorio, E.R.; Mendes, B.C.; Lima, G.B.B.; Marcondes, G.B.; Saqib, N.; Hofer, J.; Wong, J.; Macedo, T.A. Midterm Outcomes of a Prospective, Nonrandomized Study to Evaluate Endovascular Repair of Complex Aortic Aneurysms Using Fenestrated-Branched Endografts. Ann. Surg. 2021, 274, 491–499. [Google Scholar] [CrossRef]
- Daye, D.; Walker, T.G. Complications of Endovascular Aneurysm Repair of the Thoracic and Abdominal Aorta: Evaluation and Management. Cardiovasc. Diagn. 2018, 8, S138–S156. [Google Scholar] [CrossRef]
- Can, A. A Concise Review on the Classification and Nomenclature of Stem Cells. Turk. J. Haematol. 2008, 25, 57–59. [Google Scholar]
- Baker, C.L.; Pera, M.F. Capturing Totipotent Stem Cells. Cell. Stem Cell. 2018, 22, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Murry, C.E.; Keller, G. Differentiation of Embryonic Stem Cells to Clinically Relevant Populations: Lessons from Embryonic Development. Cell. 2008, 132, 661–680. [Google Scholar] [CrossRef]
- Pera, M.F.; Reubinoff, B.; Trounson, A. Human Embryonic Stem Cells. J. Cell. Sci. 2000, 113, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Thomson, J.A.; Itskovitz-Eldor, J.J.; Shapiro, S.S.; Waknitz, M.A.; Swiergiel, J.J.; Marshall, V.S.; Jones, J.M. Embryonic Stem Cell Lines Derived from Human Blastocysts. Science 1998, 282, 1145–1147. [Google Scholar] [CrossRef]
- Yu, J.; Vodyanik, M.A.; Smuga-Otto, K.; Antosiewicz-Bourget, J.; Frane, J.L.; Tian, S.; Nie, J.; Jonsdottir, G.A.; Ruotti, V.; Stewart, R.; et al. Induced Pluripotent Stem Cell Lines Derived from Human Somatic Cells. Science 2007, 318, 1917. [Google Scholar] [CrossRef]
- Takahashi, K.; Yamanaka, S. Induction of Pluripotent Stem Cells from Mouse Embryonic and Adult Fibroblast Cultures by Defined Factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of Pluripotent Stem Cells from Adult Human Fibroblasts by Defined Factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.B.; Sebastiano, V.; Wu, G.; Araúzo-Bravo, M.J.; Sasse, P.; Gentile, L.; Ko, K.; Ruau, D.; Ehrich, M.; van den Boom, D.; et al. Oct4-Induced Pluripotency in Adult Neural Stem Cells. Cell 2009, 136, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Mitalipov, S.; Wolf, D. Totipotency, Pluripotency and Nuclear Reprogramming. Adv. Biochem. Eng. Biotechnol. 2009, 114, 185–199. [Google Scholar] [CrossRef]
- Kim, Y.; Rim, Y.A.; Yi, H.; Park, N.; Park, S.-H.; Ju, J.H. The Generation of Human Induced Pluripotent Stem Cells from Blood Cells: An Efficient Protocol Using Serial Plating of Reprogrammed Cells by Centrifugation. Stem Cells Int. 2016, 2016, 1329459. [Google Scholar] [CrossRef]
- Zhou, T.; Benda, C.; Duzinger, S.; Huang, Y.; Li, X.; Li, Y.; Guo, X.; Cao, G.; Chen, S.; Hao, L.; et al. Generation of Induced Pluripotent Stem Cells from Urine. J. Am. Soc. Nephrol. 2011, 22, 1221–1228. [Google Scholar] [CrossRef]
- Charbord, P. Bone Marrow Mesenchymal Stem Cells: Historical Overview and Concepts. Hum. Gene 2010, 21, 1045–1056. [Google Scholar] [CrossRef]
- Li, X.; Wen, H.; Lv, J.; Luan, B.; Meng, J.; Gong, S.; Wen, J.; Xin, S. Therapeutic Efficacy of Mesenchymal Stem Cells for Abdominal Aortic Aneurysm: A Meta-Analysis of Preclinical Studies. Stem Cell. Res. Ther. 2022, 13, 81. [Google Scholar] [CrossRef]
- Allaire, E.; Muscatelli-Groux, B.; Guinault, A.-M.; Pages, C.; Goussard, A.; Mandet, C.; Bruneval, P.; Méllière, D.; Becquemin, J.-P. Vascular Smooth Muscle Cell Endovascular Therapy Stabilizes Already Developed Aneurysms in a Model of Aortic Injury Elicited by Inflammation and Proteolysis. Ann. Surg. 2004, 239, 417–427. [Google Scholar] [CrossRef]
- Wen, H.; Wang, M.; Gong, S.; Li, X.; Meng, J.; Wen, J.; Wang, Y.; Zhang, S.; Xin, S. Human Umbilical Cord Mesenchymal Stem Cells Attenuate Abdominal Aortic Aneurysm Progression in Sprague-Dawley Rats: Implication of Vascular Smooth Muscle Cell Phenotypic Modulation. Stem Cells Dev. 2020, 29, 981–993. [Google Scholar] [CrossRef] [PubMed]
- Parvizi, M.; Petersen, A.H.; van Spreuwel-Goossens, C.a.F.M.; Kluijtmans, S.G.J.M.; Harmsen, M.C. Perivascular Scaffolds Loaded with Adipose Tissue-Derived Stromal Cells Attenuate Development and Progression of Abdominal Aortic Aneurysm in Rats. J. Biomed. Mater. Res. Part A 2018, 106, 2494–2506. [Google Scholar] [CrossRef]
- Blose, K.J.; Ennis, T.L.; Arif, B.; Weinbaum, J.S.; Curci, J.A.; Vorp, D.A. Periadventitial Adipose-Derived Stem Cell Treatment Halts Elastase-Induced Abdominal Aortic Aneurysm Progression. Regen. Med. 2014, 9, 733–741. [Google Scholar] [CrossRef] [PubMed]
- Zidi, M.; Allaire, E. Mechanical Behavior of Abdominal Aorta Aneurysm in Rat Model Treated by Cell Therapy Using Mesenchymal Stem Cells. Biomech. Model. Mechanobiol. 2015, 14, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Schneider, F.; Saucy, F.; de Blic, R.; Dai, J.; Mohand, F.; Rouard, H.; Ricco, J.-B.; Becquemin, J.-P.; Gervais, M.; Allaire, E. Bone Marrow Mesenchymal Stem Cells Stabilize Already-Formed Aortic Aneurysms More Efficiently than Vascular Smooth Muscle Cells in a Rat Model. Eur. J. Vasc. Endovasc. Surg. 2013, 45, 666–672. [Google Scholar] [CrossRef]
- Gopalarethinam, J.; Nair, A.P.; Iyer, M.; Vellingiri, B.; Subramaniam, M.D. Advantages of Mesenchymal Stem Cell over the Other Stem Cells. Acta Histochem. 2023, 125, 152041. [Google Scholar] [CrossRef]
- Franck, G.; Dai, J.; Fifre, A.; Ngo, S.; Justine, C.; Michineau, S.; Allaire, E.; Gervais, M. Reestablishment of the Endothelial Lining by Endothelial Cell Therapy Stabilizes Experimental Abdominal Aortic Aneurysms. Circulation 2013, 127, 1877–1887. [Google Scholar] [CrossRef] [PubMed]
- Allaire, E.; Muscatelli-Groux, B.; Mandet, C.; Guinault, A.-M.; Bruneval, P.; Desgranges, P.; Clowes, A.; Méllière, D.; Becquemin, J.-P. Paracrine Effect of Vascular Smooth Muscle Cells in the Prevention of Aortic Aneurysm Formation. J. Vasc. Surg. 2002, 36, 1018–1026. [Google Scholar] [CrossRef] [PubMed]
- Gonfiotti, A.; Jaus, M.O.; Barale, D.; Baiguera, S.; Comin, C.; Lavorini, F.; Fontana, G.; Sibila, O.; Rombolà, G.; Jungebluth, P.; et al. The First Tissue-Engineered Airway Transplantation: 5-Year Follow-up Results. Lancet 2014, 383, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Macchiarini, P.; Jungebluth, P.; Go, T.; Asnaghi, M.A.; Rees, L.E.; Cogan, T.A.; Dodson, A.; Martorell, J.; Bellini, S.; Parnigotto, P.P.; et al. Clinical Transplantation of a Tissue-Engineered Airway. Lancet 2008, 372, 2023–2030. [Google Scholar] [CrossRef]
- Mastrullo, V.; Cathery, W.; Velliou, E.; Madeddu, P.; Campagnolo, P. Angiogenesis in Tissue Engineering: As Nature Intended? Front. Bioeng. Biotechnol. 2020, 8, 188. [Google Scholar] [CrossRef]
- Thottappillil, N.; Nair, P.D. Scaffolds in Vascular Regeneration: Current Status. Vasc. Health Risk Manag. 2015, 11, 79–91. [Google Scholar] [CrossRef]
- Eldeeb, A.E.; Salah, S.; Elkasabgy, N.A. Biomaterials for Tissue Engineering Applications and Current Updates in the Field: A Comprehensive Review. AAPS PharmSciTech 2022, 23, 267. [Google Scholar] [CrossRef]
- Lin, C.-H.; Hsia, K.; Ma, H.; Lee, H.; Lu, J.-H. In Vivo Performance of Decellularized Vascular Grafts: A Review Article. Int. J. Mol. Sci. 2018, 19, 2101. [Google Scholar] [CrossRef]
- Han, E.X. Arterial Grafts for Transplantation. In Reference Module in Biomedical Sciences; Elsevier: Amsterdam, The Netherlands, 2019; p. B9780128012383656000. ISBN 978-0-12-801238-3. [Google Scholar]
- Sarker, M.D.; Naghieh, S.; Sharma, N.K.; Ning, L.; Chen, X. Bioprinting of Vascularized Tissue Scaffolds: Influence of Biopolymer, Cells, Growth Factors, and Gene Delivery. J. Healthc. Eng. 2019, 2019, 9156921. [Google Scholar] [CrossRef]
- Ren, X.; Feng, Y.; Guo, J.; Wang, H.; Li, Q.; Yang, J.; Hao, X.; Lv, J.; Ma, N.; Li, W. Surface Modification and Endothelialization of Biomaterials as Potential Scaffolds for Vascular Tissue Engineering Applications. Chem. Soc. Rev. 2015, 44, 5680–5742. [Google Scholar] [CrossRef]
- Sagnella, S.; Anderson, E.; Sanabria, N.; Marchant, R.E.; Kottke-Marchant, K. Human Endothelial Cell Interaction with Biomimetic Surfactant Polymers Containing Peptide Ligands from the Heparin Binding Domain of Fibronectin. Tissue Eng. 2005, 11, 226–236. [Google Scholar] [CrossRef]
- Konig, G.; McAllister, T.N.; Dusserre, N.; Garrido, S.A.; Iyican, C.; Marini, A.; Fiorillo, A.; Avila, H.; Wystrychowski, W.; Zagalski, K.; et al. Mechanical Properties of Completely Autologous Human Tissue Engineered Blood Vessels Compared to Human Saphenous Vein and Mammary Artery. Biomaterials 2009, 30, 1542–1550. [Google Scholar] [CrossRef]
- Devillard, C.D.; Marquette, C.A. Vascular Tissue Engineering: Challenges and Requirements for an Ideal Large Scale Blood Vessel. Front. Bioeng. Biotechnol. 2021, 9. [Google Scholar] [CrossRef] [PubMed]
- Serbo, J.V.; Gerecht, S. Vascular Tissue Engineering: Biodegradable Scaffold Platforms to Promote Angiogenesis. Stem Cell. Res. Ther. 2013, 4, 8. [Google Scholar] [CrossRef]
- Luo, J.; Qin, L.; Zhao, L.; Gui, L.; Ellis, M.W.; Huang, Y.; Kural, M.H.; Clark, J.A.; Ono, S.; Wang, J.; et al. Tissue-Engineered Vascular Grafts with Advanced Mechanical Strength from Human IPSCs. Cell. Stem Cell. 2020, 26, 251–261.e8. [Google Scholar] [CrossRef] [PubMed]
- Kannan, R.Y.; Salacinski, H.J.; Butler, P.E.; Hamilton, G.; Seifalian, A.M. Current Status of Prosthetic Bypass Grafts: A Review. J. Biomed. Mater. Res. B. Appl. Biomater. 2005, 74, 570–581. [Google Scholar] [CrossRef]
- Bhattacharya, V.; McSweeney, P.A.; Shi, Q.; Bruno, B.; Ishida, A.; Nash, R.; Storb, R.F.; Sauvage, L.R.; Hammond, W.P.; Wu, M.H.-D. Enhanced Endothelialization and Microvessel Formation in Polyester Grafts Seeded with CD34+ Bone Marrow Cells. Blood 2000, 95, 581–585. [Google Scholar] [CrossRef]
- Hoerstrup, S.P.; Cummings Mrcs, I.; Lachat, M.; Schoen, F.J.; Jenni, R.; Leschka, S.; Neuenschwander, S.; Schmidt, D.; Mol, A.; Günter, C.; et al. Functional Growth in Tissue-Engineered Living, Vascular Grafts: Follow-Up at 100 Weeks in a Large Animal Model. Circulation 2006, 114, I-159–I-166. [Google Scholar] [CrossRef] [PubMed]
- Miyachi, H.; Shoji, T.; Miyamoto, S.; Shinoka, T. Chapter 58–Engineering of Large Diameter Vessels. In Principles of Regenerative Medicine, 3rd ed.; Atala, A., Lanza, R., Mikos, A.G., Nerem, R., Eds.; Academic Press: Boston, IL, USA, 2019; pp. 1029–1040. ISBN 978-0-12-809880-6. [Google Scholar]
- Shinoka, T.; Shum-Tim, D.; Ma, P.X.; Tanel, R.E.; Isogai, N.; Langer, R.; Vacanti, J.P.; Mayer, J.E. Creation Of Viable Pulmonary Artery Autografts Through Tissue Engineering. J. Thorac. Cardiovasc. Surg. 1998, 115, 536–546. [Google Scholar] [CrossRef]
- L’Heureux, N.; Pâquet, S.; Labbé, R.; Germain, L.; Auger, F.A. A Completely Biological Tissue-Engineered Human Blood Vessel. FASEB J. 1998, 12, 47–56. [Google Scholar] [CrossRef] [PubMed]
- L’Heureux, N.; Dusserre, N.; Garrido, S.A.; Manglano, X.; Marini, A.; De La Fuente, L.; McAllister, T. Sheet–Based Tissue Engineering: From Bench Top to the First Clinical Use of a Completely Biological Tissue Engineered Blood Vessel. FASEB J. 2006, 20, 5. [Google Scholar] [CrossRef]
- Gao, G.; Kim, H.; Kim, B.S.; Kong, J.S.; Lee, J.Y.; Park, B.W.; Chae, S.; Kim, J.; Ban, K.; Jang, J.; et al. Tissue-Engineering of Vascular Grafts Containing Endothelium and Smooth-Muscle Using Triple-Coaxial Cell Printing. Appl. Phys. Rev. 2019, 6, 041402. [Google Scholar] [CrossRef]
- GnanaDev, R.; Malkoc, A.; Jeney, A.B.; Mikael, A.; Andacheh, I. A Multicenter Analysis of Revision of Aneurysmal Dialysis Access Using Bovine Carotid Artery Conduit. Ann. Vasc. Surg. 2023, S0890-5096(23)00288-1. [Google Scholar] [CrossRef]
- Bader, A.; Steinhoff, G.; Strobl, K.; Schilling, T.; Brandes, G.; Mertsching, H.; Tsikas, D.; Froelich, J.; Haverich, A. Engineering of Human Vascular Aortic Tissue Based on A Xenogeneic Starter Matrix. Transplantation 2000, 70, 7. [Google Scholar] [PubMed]
- Aldridge, A.; Desai, A.; Owston, H.; Jennings, L.M.; Fisher, J.; Rooney, P.; Kearney, J.N.; Ingham, E.; Wilshaw, S.P. Development and Characterisation of a Large Diameter Decellularised Vascular Allograft. Cell. Tissue Bank. 2018, 19, 287–300. [Google Scholar] [CrossRef]
- Wang, J.; Liu, L.; Wu, Y.; Maitz, M.F.; Wang, Z.; Koo, Y.; Zhao, A.; Sankar, J.; Kong, D.; Huang, N.; et al. Ex Vivo Blood Vessel Bioreactor for Analysis of the Biodegradation of Magnesium Stent Models with and without Vessel Wall Integration. Acta Biomater. 2017, 50, 546–555. [Google Scholar] [CrossRef]
- Pennings, I.; Van Haaften, E.E.; Jungst, T.; Bulsink, J.A.; Rosenberg, A.J.W.P.; Groll, J.; Bouten, C.V.C.; Kurniawan, N.A.; Smits, A.I.P.M.; Gawlitta, D. Layer-Specific Cell Differentiation in Bi-Layered Vascular Grafts under Flow Perfusion. Biofabrication 2019, 12, 015009. [Google Scholar] [CrossRef]
- Wang, C.; Cen, L.; Yin, S.; Liu, Q.; Liu, W.; Cao, Y.; Cui, L. A Small Diameter Elastic Blood Vessel Wall Prepared under Pulsatile Conditions from Polyglycolic Acid Mesh and Smooth Muscle Cells Differentiated from Adipose-Derived Stem Cells. Biomaterials 2010, 31, 621–630. [Google Scholar] [CrossRef]
- Yang, X.; Xu, C.; Yao, F.; Ding, Q.; Liu, H.; Luo, C.; Wang, D.; Huang, J.; Li, Z.; Shen, Y.; et al. Targeting Endothelial Tight Junctions to Predict and Protect Thoracic Aortic Aneurysm and Dissection. Eur. Heart J. 2023, 44, 1248–1261. [Google Scholar] [CrossRef]
- Li, Y.; Wang, W.; Li, L.; Khalil, R.A. MMPs and ADAMs/ADAMTS Inhibition Therapy of Abdominal Aortic Aneurysm. Life Sci. 2020, 253, 117659. [Google Scholar] [CrossRef]
- Ennis, T.; Jin, J.; Bartlett, S.; Arif, B.; Grapperhaus, K.; Curci, J.A. Effect of Novel Limited-Spectrum MMP Inhibitor XL784 in Abdominal Aortic Aneurysms. J. Cardiovasc. Pharm. 2012, 17, 417–426. [Google Scholar] [CrossRef]
- Song, Q.; Yu, H.; Han, J.; Lv, J.; Lv, Q.; Yang, H. Exosomes in Urological Diseases—Biological Functions and Clinical Applications. Cancer Lett. 2022, 544, 215809. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, J.; Hayder, H.; Zayed, Y.; Peng, C. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front. Endocrinol. 2018, 9, 402. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, P.S.; Parkin, R.K.; Kroh, E.M.; Fritz, B.R.; Wyman, S.K.; Pogosova-Agadjanyan, E.L.; Peterson, A.; Noteboom, J.; O’Briant, K.C.; Allen, A.; et al. Circulating MicroRNAs as Stable Blood-Based Markers for Cancer Detection. Proc. Natl. Acad. Sci. USA 2008, 105, 10513–10518. [Google Scholar] [CrossRef]
- Maegdefessel, L.; Azuma, J.; Toh, R.; Deng, A.; Merk, D.R.; Raiesdana, A.; Leeper, N.J.; Raaz, U.; Schoelmerich, A.M.; McConnell, M.V.; et al. MicroRNA-21 Blocks Abdominal Aortic Aneurysm Development and Nicotine-Augmented Expansion. Sci. Transl. Med. 2012, 4, 122ra22. [Google Scholar] [CrossRef] [PubMed]
- Akerman, A.W.; Collins, E.N.; Peterson, A.R.; Collins, L.B.; Harrison, J.K.; DeVaughn, A.; Townsend, J.M.; Vanbuskirk, R.L.; Riopedre-Maqueira, J.; Reyes, A.; et al. MiR-133a Replacement Attenuates Thoracic Aortic Aneurysm in Mice. J. Am. Heart Assoc. 2021, 10, e019862. [Google Scholar] [CrossRef]
| Intervention | Target/Action | Application | Additional Information |
|---|---|---|---|
| MSC | MCP-1, TNF-a, IL-6, MMP/TIMP | Small animal models (AAA) | Meta-analysis of 18 studies in animal models, by Li et al. [87], showed significant effect on aortic diameter, inflammatory mediators, and elastin content; there were also effects on enzyme activity, with decreased MMP and increased TIMP expression levels. |
| EC | Tunica intima stabilization, VSMC recruitment | Small animal models (AAA) | Administration in a rat model by Franck et al. [95] prevented new AAA formation and expansion of preexisting AAAs. |
| Observed effects by Franck et al. [95]: Indirect stabilization of the endothelium through paracrine effects, and augmentation of aortic wall components through ECM secretion via VSMC recruitment. | |||
| VSMC | Tunica media stabilization, MMP/TIMP | Small animal models (AAA) | Intraluminal use in a rat model by Allaire et al. [88,96]; decrease in inflammatory cell infiltrate, decreased MMP, and increased TIMP activity. |
| TEVG | Large vessel replacement | Canine model | Large artery synthetic graft (Dacron®) infused with CD34+ BMCs by Bhattacharya et al. [112]; successful endothelialization and microvessel formation; incomplete integration by host. |
| Large vessel replacement | Large animal model (lamb) | Pulmonary artery replacement by Hoerstrup et al. [113]. Hoerstrup et al. [113] used large diameter TEVG fabricated from PGA scaffold, seeded with lamb ECs and fibroblasts; 14 subjects were only followed for 100 weeks, appropriately functioning graft during this time. | |
| Large vessel replacement | Patient with congenital heart malformation | Biosynthetic, biodegradable TEVG fabricated from PLCL-PGA scaffold, seeded with BM-MNCs in a 3-year-old female patient; appropriately functioning during a follow-up period of 11 years [114]. | |
| Large vessel replacement | Canine model | Large vessel TEVG fabricated (L’Heureux et al. [116]) through VSMC and fibroblast sheet rolling, with EC seeding; short term implantation in canines (6 days), appropriate biomechanical characteristics and handling. | |
| Large vessel replacement | Renal dialysis patients | Large vessel TEVGs, also fabricated through cell-sheet rolling, exhibited 60% patency at 6 months, when transplanted in 6 patients (L’Heureux et al. [117]); additional interventions due to restenosis and occlusion were required; one patient exhibited dangerous hemorrhage [114]. | |
| Small vessel replacement | Rat model | Biodegradable TEVG fabricated from natural polymer (alginate) containing human VSMCs, ECs through 3D printing; implanted as part of the rat aorta [118]. | |
| Large vessel replacement | Artegraft | Decellularized bovine carotid artery graft, commercially available, described by some as having similar quality to ePTFE grafts [114,119]. | |
| Experimental evaluation of immunogenicity | Rat model | Decellularized porcine aorta seeded with human ECs and myofibroblasts; immunogenicity evaluated through implantation in a rat model; exhibiting appropriate integration and host reaction was observed [120]. | |
| Experimental evaluation of biomechanical characteristics | In vitro | Biomechanical characteristics of a decellularized human aorta were tested by Aldridge et al.; no cell seeding stages [121]. | |
| AT-1001 (larazotide acetate) | Cell–cell adhesion (ZO-1, Claudin-5) | Murine model (TAA) | TJ sealing agent (preserves distribution of cell-adhesion proteins); tested in both ascending and descending thoracic aortic sections (Yang et al. [125]) Also assessed for the treatment of inflammatory bowel disease (IBD) [125]. |
| Batimastat, Marimastat | MMP (such as MMP-2, MMP-3, MMP-9) | Phase III Clinical Trials (2020) (AAA) | MMPi (peptidomimetic) with low bioavailability, musculoskeletal side effects; proposed nanoparticle delivery systems to improve side effects and bioavailability [126]. |
| Disulfiram | MMP (MMP-2, MMP-9), Type IV collagenase | Phase II Clinical Trials (2020) (AAA) | MMPi (non-peptide) [126] Already in use, for treatment of chronic alcohol intoxication (FDA-approved) [126] Antitumor effects [126] |
| XL784 | MMP (High specificity for MMP-2) | Murine Models (AAA) Phase II Clinical Trials (2020) (AAA) | MMPi (non-peptide, synthetic molecule); fewer musculoskeletal symptoms compared to peptidomimetic MMPis [127]. |
| miR-21 | AKT signaling pathway | Murine Models (AAA) Human aortic samples (AAA) | Augmentation mitigates aneurysm progression (interference with VSMC proliferation and apoptosis pathways); delivery through exosomes or bound to proteins (i.e., Argonautes) (Maegdefessel et al. [131]). |
| miR-133a | Fibroblast | Murine Models (TAA) | Overexpression halts aneurysm progression by affecting fibroblast function; delivery through exosomes or bound to proteins (i.e., Argonautes) (Akerman et al. [132]). |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stougiannou, T.M.; Christodoulou, K.C.; Georgakarakos, E.; Mikroulis, D.; Karangelis, D. Promising Novel Therapies in the Treatment of Aortic and Visceral Aneurysms. J. Clin. Med. 2023, 12, 5878. https://doi.org/10.3390/jcm12185878
Stougiannou TM, Christodoulou KC, Georgakarakos E, Mikroulis D, Karangelis D. Promising Novel Therapies in the Treatment of Aortic and Visceral Aneurysms. Journal of Clinical Medicine. 2023; 12(18):5878. https://doi.org/10.3390/jcm12185878
Chicago/Turabian StyleStougiannou, Theodora M., Konstantinos C. Christodoulou, Efstratios Georgakarakos, Dimitrios Mikroulis, and Dimos Karangelis. 2023. "Promising Novel Therapies in the Treatment of Aortic and Visceral Aneurysms" Journal of Clinical Medicine 12, no. 18: 5878. https://doi.org/10.3390/jcm12185878







