Muscle Strength and Efficiency of Muscle Activities Recovery Using Single-Joint Type Hybrid Assistive Limb in Knee Rehabilitation after Anterior Cruciate Ligament Reconstruction
Abstract
:1. Introduction
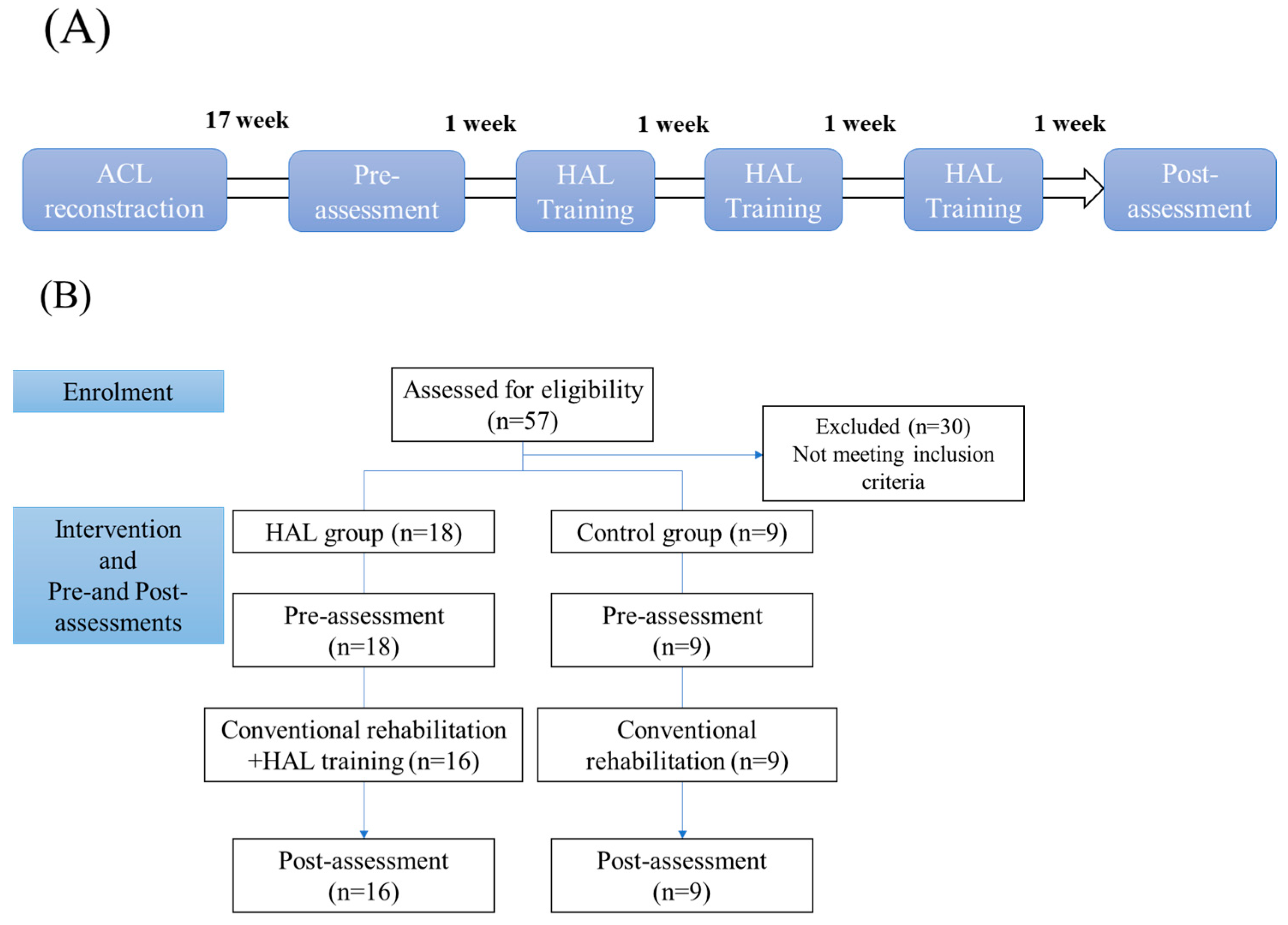
2. Materials and Methods
2.1. Patients
2.2. Knee HAL Single-Joint Training
2.3. Measurements
2.4. Statistical Analysis
2.5. Sample Size
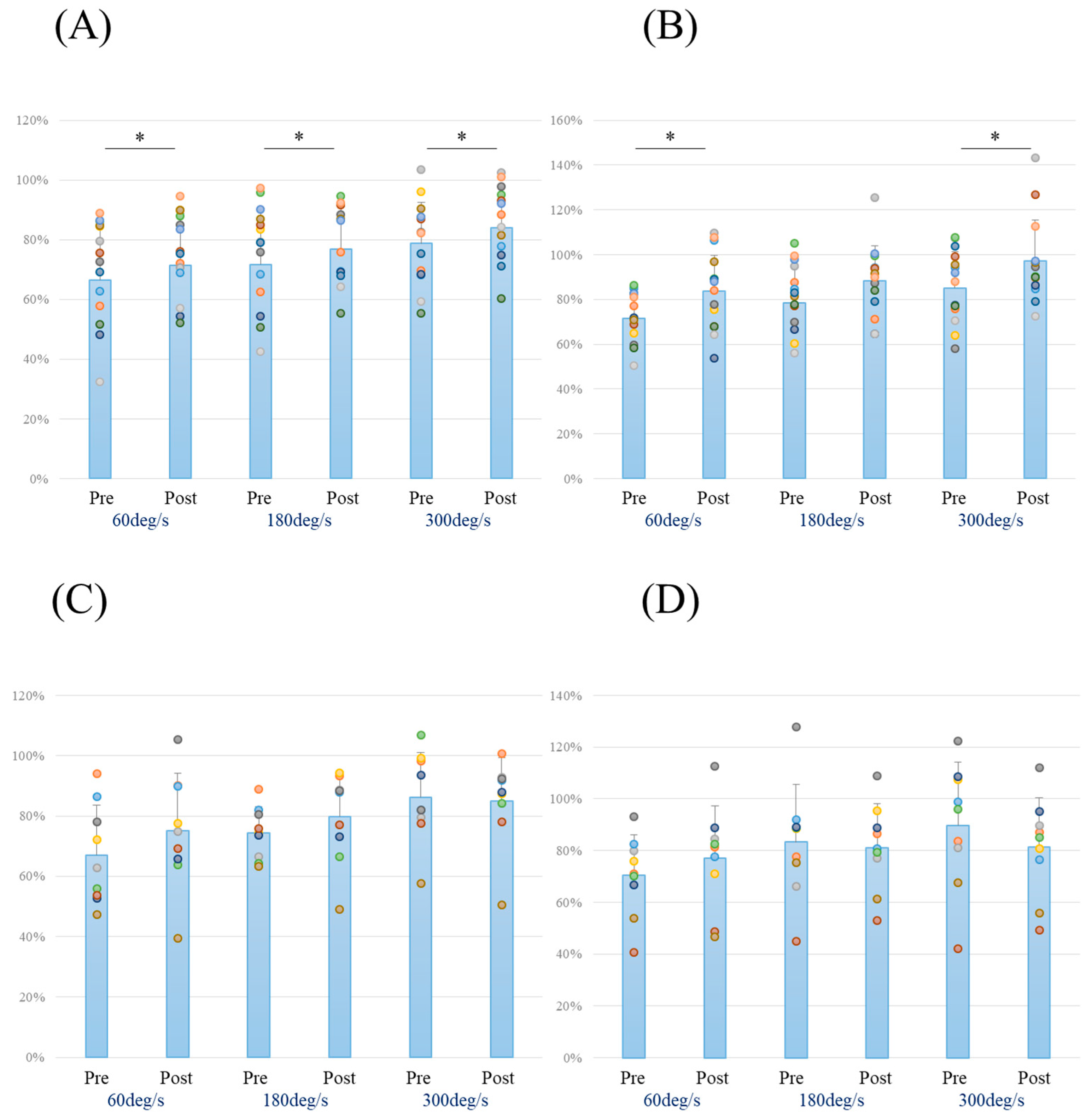
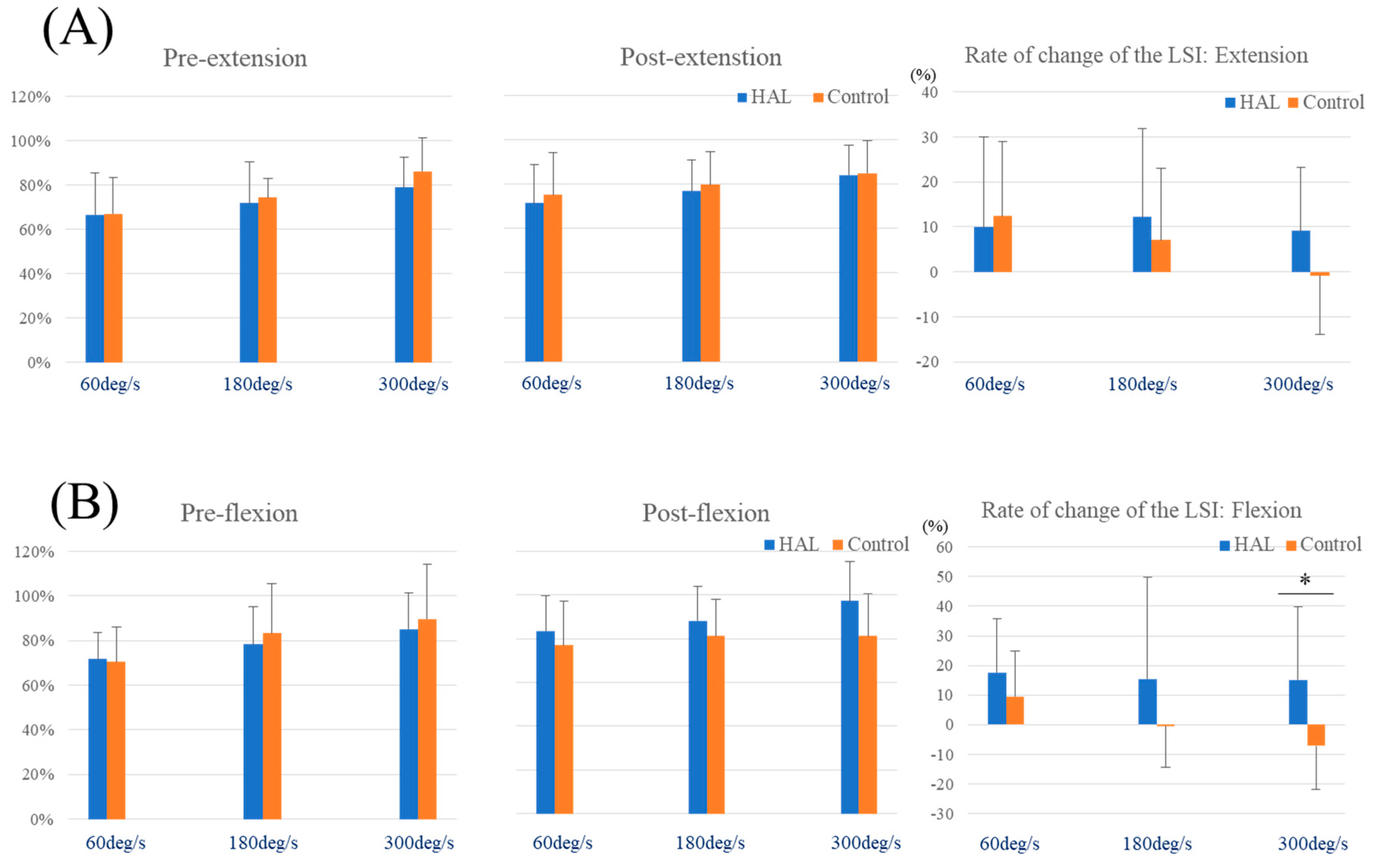
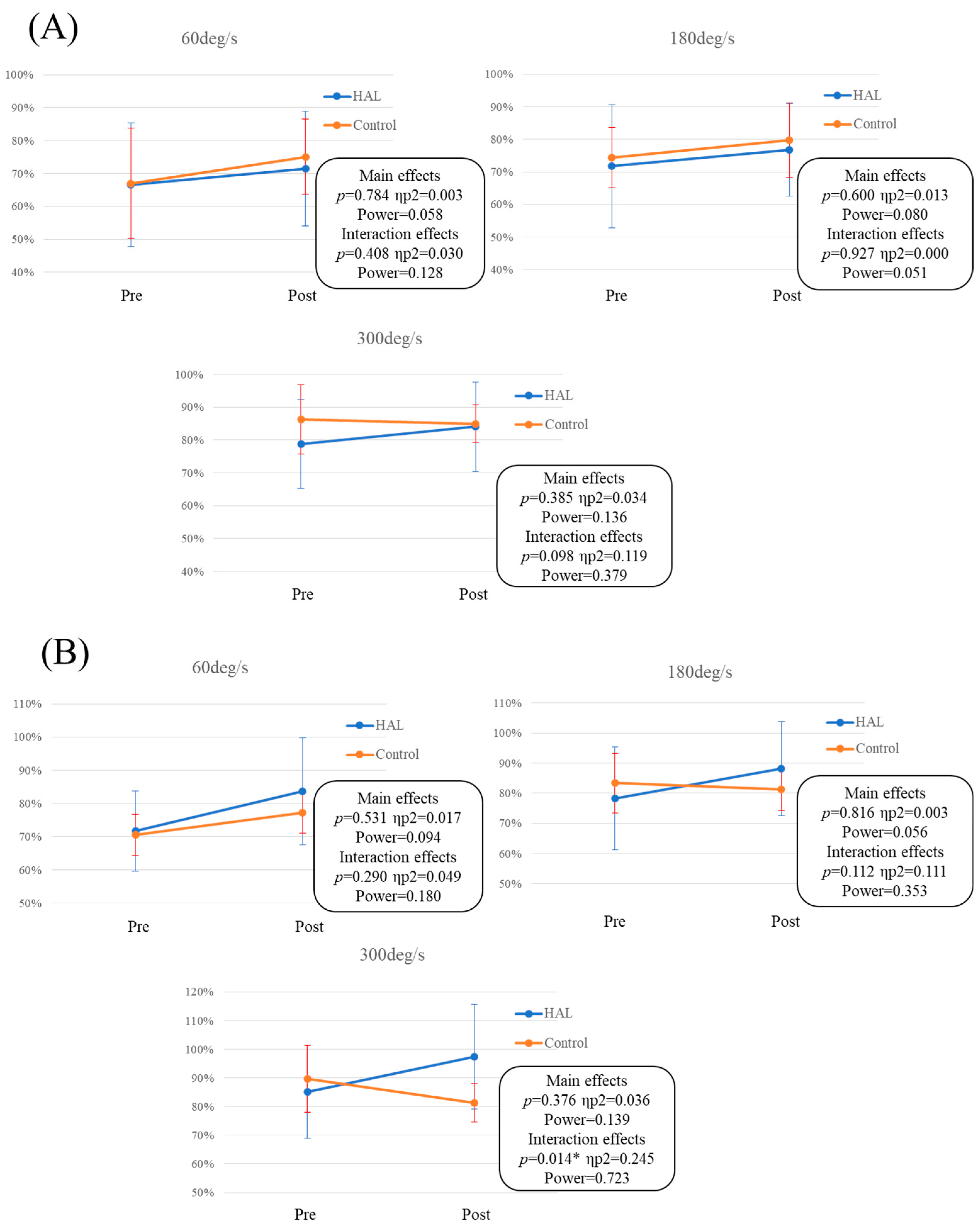
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Filbay, S.R.; Ackerman, I.N.; Russell, T.G.; Macri, E.M.; Crossley, K.M. Health-related quality of life after anterior cruciate ligament reconstruction: A systematic review. Am. J. Sports Med. 2014, 42, 1247–1255. [Google Scholar] [CrossRef]
- Kaneko, F.; Onari, K.; Kawaguchi, K.; Tsukisaka, K.; Roy, S.H. Electromechanical delay after ACL reconstruction: An innovative method for investigating central and peripheral contributions. J. Orthop. Sports Phys. Ther. 2002, 32, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, Y.; Totsuka, M.; Sato, T.; Fukuda, Y.; Hirota, K. Effect of disuse on the ultrastructure of the achilles tendon in rats. Eur. J. Appl. Physiol. Occup. Physiol. 1989, 59, 239–242. [Google Scholar] [CrossRef]
- Karpakka, J.; Vaananen, K.; Orava, S.; Takala, T.E. The effects of preimmobilization training and immobilization on collagen synthesis in rat skeletal muscle. Int. J. Sports Med. 1990, 11, 484–488. [Google Scholar] [CrossRef]
- Widrick, J.J.; Fitts, R.H. Peak force and maximal shortening velocity of soleus fibers after non-weight-bearing and resistance exercise. J. Appl. Physiol. 1997, 82, 189–195. [Google Scholar] [CrossRef] [PubMed]
- van Melick, N.; van Cingel, R.E.; Brooijmans, F.; Neeter, C.; van Tienen, T.; Hullegie, W.; Nijhuis-van der Sanden, M.W. Evidence-based clinical practice update: Practice guidelines for anterior cruciate ligament rehabilitation based on a systematic review and multidisciplinary consensus. Br. J. Sports Med. 2016, 50, 1506–1515. [Google Scholar] [CrossRef]
- Decker, L.M.; Moraiti, C.; Stergiou, N.; Georgoulis, A.D. New insights into anterior cruciate ligament deficiency and reconstruction through the assessment of knee kinematic variability in terms of nonlinear dynamics. Knee Surg. Sports Traumatol. Arthrosc. 2011, 19, 1620–1633. [Google Scholar] [CrossRef]
- Sonnery-Cottet, B.; Saithna, A.; Quelard, B.; Daggett, M.; Borade, A.; Ouanezar, H.; Thaunat, M.; Blakeney, W.G. Arthrogenic muscle inhibition after ACL reconstruction: A scoping review of the efficacy of interventions. Br. J. Sports Med. 2019, 53, 289–298. [Google Scholar] [CrossRef]
- Kubota, S.; Abe, T.; Kadone, H.; Shimizu, Y.; Funayama, T.; Watanabe, H.; Marushima, A.; Koda, M.; Hada, Y.; Sankai, Y.; et al. Hybrid assistive limb (HAL) treatment for patients with severe thoracic myelopathy due to ossification of the posterior longitudinal ligament (OPLL) in the postoperative acute/subacute phase: A clinical trial. J. Spinal Cord. Med. 2019, 42, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Lafitte, M.N.; Kadone, H.; Kubota, S.; Shimizu, Y.; Tan, C.K.; Koda, M.; Hada, Y.; Sankai, Y.; Suzuki, K.; Yamazaki, M. Alteration of muscle activity during voluntary rehabilitation training with single-joint hybrid Assistive Limb (HAL) in patients with shoulder elevation dysfunction from cervical origin. Front. Neurosci. 2022, 16, 817659. [Google Scholar] [CrossRef]
- Soma, Y.; Mutsuzaki, H.; Yoshioka, T.; Kubota, S.; Shimizu, Y.; Kanamori, A.; Yamazaki, M. Single-joint type hybrid assistive limb in knee rehabilitation after ACL reconstruction: An open-label feasibility and safety trial. Prog. Rehabil. Med. 2022, 7, 20220036. [Google Scholar] [CrossRef] [PubMed]
- Aglietti, P.; Giron, F.; Losco, M.; Cuomo, P.; Ciardullo, A.; Mondanelli, N. Comparison between single-and double-bundle anterior cruciate ligament reconstruction: A prospective, randomized, single-blinded clinical trial. Am. J. Sports Med. 2010, 38, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Goradia, V.K.; Rochat, M.C.; Kida, M.; Grana, W.A. Natural history of a hamstring tendon autograft used for anterior cruciate ligament reconstruction in a sheep model. Am. J. Sports Med. 2000, 28, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Fukaya, T.; Mutsuzaki, H.; Yoshikawa, K.; Sano, A.; Mizukami, M.; Yamazaki, M. The training effect of early intervention with a hybrid assistive limb after total knee arthroplasty. Case Rep. Orthop. 2017, 2017, 6912706. [Google Scholar] [CrossRef] [PubMed]
- Thomeé, R.; Neeter, C.; Gustavsson, A.; Thomeé, P.; Augustsson, J.; Eriksson, B.; Karlsson, J. Variability in leg muscle power and hop performance after anterior cruciate ligament reconstruction. Knee Surg. Sports Traumatol. Arthrosc. 2012, 20, 1143–1151. [Google Scholar] [CrossRef]
- Tegner, Y.; Lysholm, J. Rating systems in the evaluation of knee ligament injuries. Clin. Orthop. Relat. Res. 1985, 198, 43–49. [Google Scholar] [CrossRef]
- Hefti, F.; Müller, W.; Jakob, R.P.; Stäubli, H.U. Evaluation of knee ligament injuries with the IKDC form. Knee Surg. Sports Traumatol. Arthrosc. 1993, 1, 226–234. [Google Scholar] [CrossRef]
- Ochi, M.; Iwasa, J.; Uchio, Y.; Adachi, N.; Kawasaki, K. Induction of somatosensory evoked potentials by mechanical stimulation in reconstructed anterior cruciate ligaments. J. Bone Jt. Surg. Br. 2002, 84, 761–766. [Google Scholar] [CrossRef]
- Ochi, M.; Iwasa, J.; Uchio, Y.; Adachi, N.; Sumen, Y. The regeneration of sensory neurones in the reconstruction of the anterior cruciate ligament. J. Bone Jt. Surg. Br. 1999, 81, 902–906. [Google Scholar] [CrossRef]
- Petschnig, R.; Baron, R.; Albrecht, M. The relationship between isokinetic quadriceps strength test and hop tests for distance and one-legged vertical jump test following anterior cruciate ligament reconstruction. J. Orthop. Sports Phys. Ther. 1998, 28, 23–31. [Google Scholar] [CrossRef]
- Rudolph, K.S.; Axe, M.J.; Snyder-Mackler, L. Dynamic stability after ACL injury: Who can hop? Knee Surg. Sports Traumatol. Arthrosc. 2000, 8, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Sueyoshi, T.; Nakahata, A.; Emoto, G.; Yuasa, T. Single-leg hop test performance and isokinetic knee strength after anterior cruciate ligament reconstruction in athletes. Orthop. J. Sports Med. 2017, 5, 2325967117739811. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.H.; Yoo, J.C.; Yang, H.S.; Kim, J.H.; Wang, J.H. Second-look arthroscopic findings of 208 patients after ACL reconstruction. Knee Surg. Sports Traumatol. Arthrosc. 2007, 15, 242–248. [Google Scholar] [CrossRef]
- Kawamoto, H.; Sankai, Y. Power assist method based on Phase Sequence and muscle force condition for HAL. Adv. Robot. 2005, 19, 717–734. [Google Scholar] [CrossRef]
- Sczesny-Kaiser, M.; Höffken, O.; Aach, M.; Cruciger, O.; Grasmücke, D.; Meindl, R.; Schildhauer, T.A.; Schwenkreis, P.; Tegenthoff, M. HAL® exoskeleton training improves walking parameters and normalizes cortical excitability in primary somatosensory cortex in spinal cord injury patients. J. Neuroeng. Rehabil. 2015, 12, 68. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, J.B. How we walk: Central control of muscle activity during human walking. Neuroscientist 2003, 9, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Konishi, Y.; Aihara, Y.; Sakai, M.; Ogawa, G.; Fukubayashi, T. Gamma loop dysfunction in the quadriceps femoris of patients who underwent anterior cruciate ligament reconstruction remains bilaterally. Scand. J. Med. Sci. Sports 2007, 17, 393–399. [Google Scholar] [CrossRef]
- Boon, A. Ultrasonography and electrodiagnosis: Are they complementary techniques? PM R 2013, 5, S100–S106. [Google Scholar] [CrossRef]
- Serpell, B.G.; Scarvell, J.M.; Pickering, M.R.; Ball, N.B.; Newman, P.; Perriman, D.; Warmenhoven, J.; Smith, P.N. Medial and lateral hamstrings and quadriceps co-activation affects knee joint kinematics and ACL elongation: A pilot study. BMC Musculoskelet. Disord. 2015, 16, 348. [Google Scholar] [CrossRef]
| HAL Group | Control Group | p-Value | |
|---|---|---|---|
| Age (year) | 23.4 ± 7.0 | 20.2 ± 1.7 | 0.202 |
| Sex (male/female) | 10/8 | 3/6 | 0.411 |
| Height (cm) | 168.0 ± 8.9 | 162.7 ± 10.0 | 0.205 |
| Weight (kg) | 66.7 ± 13.0 | 64.9 ± 10.7 | 0.588 |
| BMI (kg/m2) | 23.5 ± 3.5 | 24.6 ± 4.2 | 0.655 |
| Injured side (right/left) | 6/12 | 4/5 | 0.671 |
| Graft materials (Single bundle/double bundle) | 16/2 | 9/0 | 0.520 |
| Sports level (Competitive/Recreational) | 9/9 | 8/1 | 0.088 |
| HAL Group | Control Group | p-Value | Effect Size (d) | Power | ||||
|---|---|---|---|---|---|---|---|---|
| The LSI (%) | Mean | SD | Mean | SD | ||||
| Pre-extension | 60 deg/s | 66.6 | 18.8 | 67.1 | 16.5 | 0.951 | 0.026 | 0.050 |
| 180 deg/s | 71.7 | 18.9 | 74.4 | 8.6 | 0.635 | 0.166 | 0.066 | |
| 300 deg/s | 78.8 | 13.6 | 86.3 | 14.9 | 0.215 | 0.532 | 0.232 | |
| Pre-flexion | 60 deg/s | 71.7 | 12.0 | 70.4 | 15.7 | 0.638 | 0.092 | 0.055 |
| 180 deg/s | 78.3 | 17.1 | 83.3 | 22.4 | 0.479 | 0.263 | 0.093 | |
| 300 deg/s | 85.2 | 16.2 | 89.8 | 24.3 | 0.155 | 0.237 | 0.085 | |
| Post-extension | 60 deg/s | 71.5 | 17.4 | 75.1 | 19.0 | 0.638 | 0.199 | 0.074 |
| 180 deg/s | 76.7 | 14.2 | 79.8 | 14.9 | 0.621 | 0.212 | 0.078 | |
| 300 deg/s | 84.1 | 13.6 | 85.0 | 14.4 | 0.871 | 0.069 | 0.053 | |
| Post-flexion | 60 deg/s | 83.6 | 16.2 | 77.1 | 20.2 | 0.388 | 0.367 | 0.135 |
| 180 deg/s | 88.2 | 15.7 | 81.2 | 16.9 | 0.315 | 0.433 | 0.169 | |
| 300 deg/s | 97.3 | 18.2 | 81.3 | 19.2 | 0.052 | 0.865 | 0.512 | |
| The rate of change of the LSI (%) | ||||||||
| Extension | 60 deg/s | 9.98 | 19.96 | 12.41 | 16.50 | 0.759 | 0.129 | 0.060 |
| 180 deg/s | 12.15 | 19.59 | 7.08 | 15.98 | 0.519 | 0.276 | 0.097 | |
| 300 deg/s | 9.16 | 14.06 | −0.83 | 13.06 | 0.098 | 0.728 | 0.388 | |
| Flexion | 60 deg/s | 17.58 | 18.29 | 9.53 | 15.43 | 0.277 | 0.464 | 0.187 |
| 180 deg/s | 15.44 | 34.32 | −0.39 | 14.12 | 0.204 | 0.552 | 0.246 | |
| 300 deg/s | 15.07 | 24.68 | −7.21 | 14.79 | 0.023 * | 1.031 | 0.659 | |
| Hamstring/quadriceps ratio (%) | ||||||||
| Pre-assessment | 60 deg/s | 57.8 | 14.5 | 52.7 | 11.6 | 0.383 | 0.370 | 0.136 |
| 180 deg/s | 61.2 | 11.2 | 62.5 | 15.1 | 0.812 | 0.100 | 0.056 | |
| 300 deg/s | 72.4 | 11.8 | 68.8 | 17.1 | 0.244 | 0.499 | 0.209 | |
| Post-assessment | 60 deg/s | 58.6 | 21.8 | 52.9 | 11.4 | 0.478 | 0.301 | 0.107 |
| 180 deg/s | 64.5 | 14.9 | 58.9 | 13.2 | 0.358 | 0.396 | 0.149 | |
| 300 deg/s | 74.0 | 13.1 | 60.5 | 17.3 | 0.043 * | 0.905 | 0.548 | |
| HAL Group | p-Value | Effect Size (d) | Control Group | p-Value | Effect Size | ||||
|---|---|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | ||||||
| Active ROM (°) | Extension | −5.3 ± 3.5 | −1.9 ± 2.8 | 0.006 * | 1.644 | −6.3 ± 2.6 | −5.3 ± 2.0 | 0.234 | 0.500 |
| Flexion | 129.3 ± 5.2 | 133.7 ± 4.5 | 0.000 * | 1.288 | 130.3 ± 12.5 | 132.0 ± 5.3 | 0.613 | 0.202 | |
| Passive ROM (°) | Extension | −2.1 ± 2.6 | −0.3 ± 1.1 | 0.000 * | 1.633 | −1.7 ± 2.0 | −1.5 ± 2.1 | 0.793 | 0.113 |
| Flexion | 134.7 ± 4.5 | 139.6 ± 4.8 | 0.011 * | 0.761 | 135.8 ± 12.1 | 138.3 ± 6.4 | 0.361 | 0.410 | |
| Pivot shift test result | - | - | - | - | |||||
| Lachman’s test result | - | - | - | - | |||||
| Tegner activity scale score | 5.0 ± 0.8 | 5.7 ± 0.7 | 0.001 * | 1.097 | 5.1 ± 0.7 | 6.0 ± 1.4 | 0.111 | 0.705 | |
| Lysholm knee questionnaire score | 68.9 ± 6.6 | 82.5 ± 10.4 | 0.003 * | 0.918 | 73.1 ± 10.7 | 83.1 ± 8.7 | 0.011 * | 1.361 | |
| IKDC subjective knee form score | A | A | A | A | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soma, Y.; Mutsuzaki, H.; Yoshioka, T.; Kubota, S.; Iwai, K.; Shimizu, Y.; Kanamori, A.; Yamazaki, M. Muscle Strength and Efficiency of Muscle Activities Recovery Using Single-Joint Type Hybrid Assistive Limb in Knee Rehabilitation after Anterior Cruciate Ligament Reconstruction. J. Clin. Med. 2023, 12, 6117. https://doi.org/10.3390/jcm12196117
Soma Y, Mutsuzaki H, Yoshioka T, Kubota S, Iwai K, Shimizu Y, Kanamori A, Yamazaki M. Muscle Strength and Efficiency of Muscle Activities Recovery Using Single-Joint Type Hybrid Assistive Limb in Knee Rehabilitation after Anterior Cruciate Ligament Reconstruction. Journal of Clinical Medicine. 2023; 12(19):6117. https://doi.org/10.3390/jcm12196117
Chicago/Turabian StyleSoma, Yuichiro, Hirotaka Mutsuzaki, Tomokazu Yoshioka, Shigeki Kubota, Koichi Iwai, Yukiyo Shimizu, Akihiro Kanamori, and Masashi Yamazaki. 2023. "Muscle Strength and Efficiency of Muscle Activities Recovery Using Single-Joint Type Hybrid Assistive Limb in Knee Rehabilitation after Anterior Cruciate Ligament Reconstruction" Journal of Clinical Medicine 12, no. 19: 6117. https://doi.org/10.3390/jcm12196117






