Attachment of Respiratory Pathogens and Candida to Denture Base Materials—A Pilot Study
Abstract
:1. Introduction
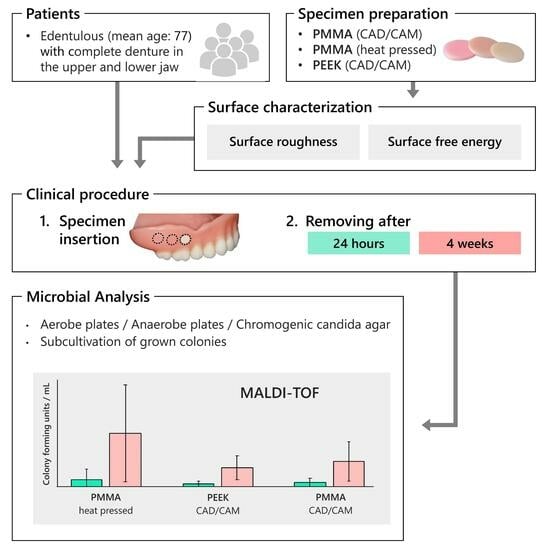
2. Materials and Methods
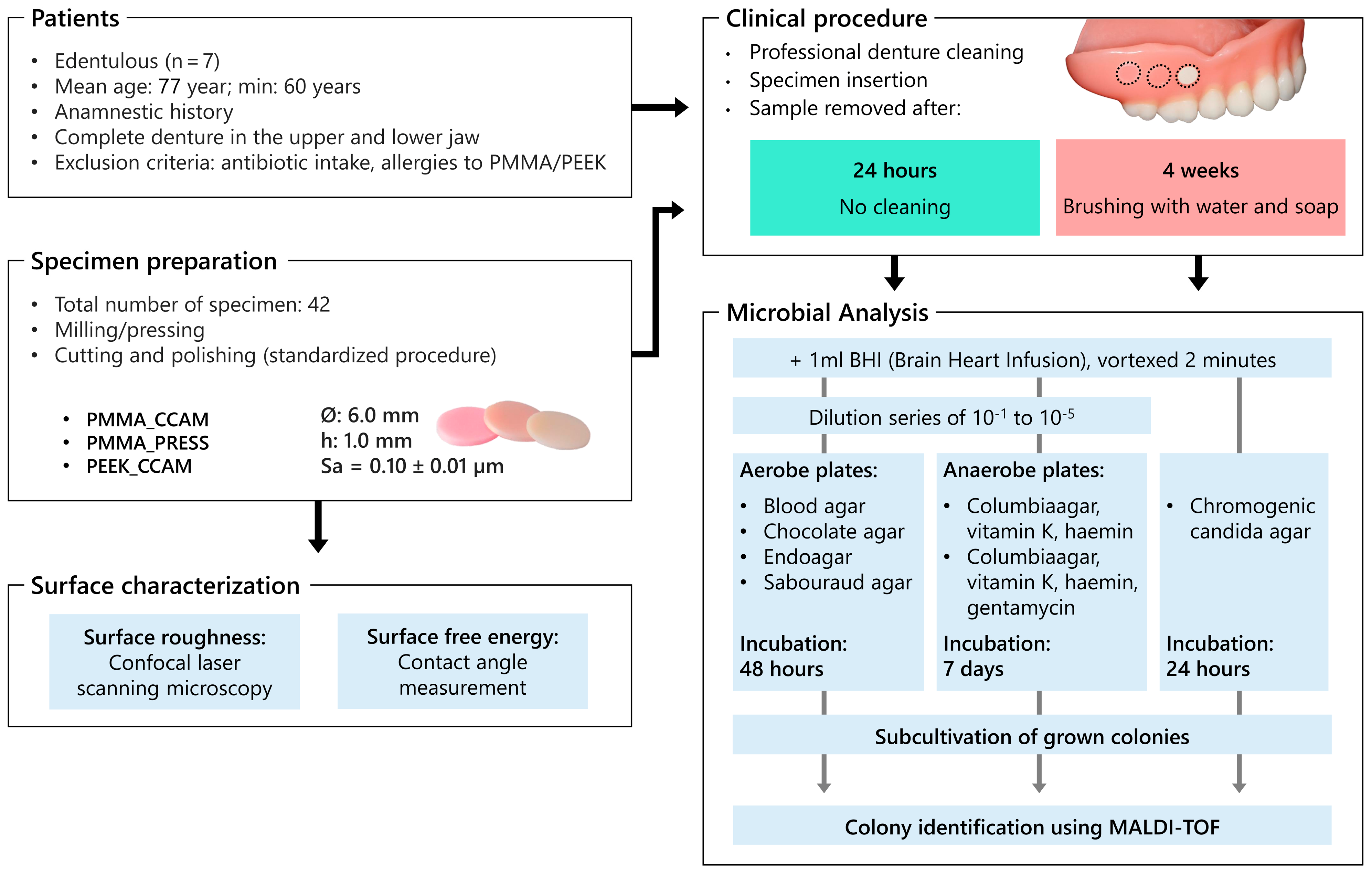
2.1. Study Population
2.2. Specimen Preparation
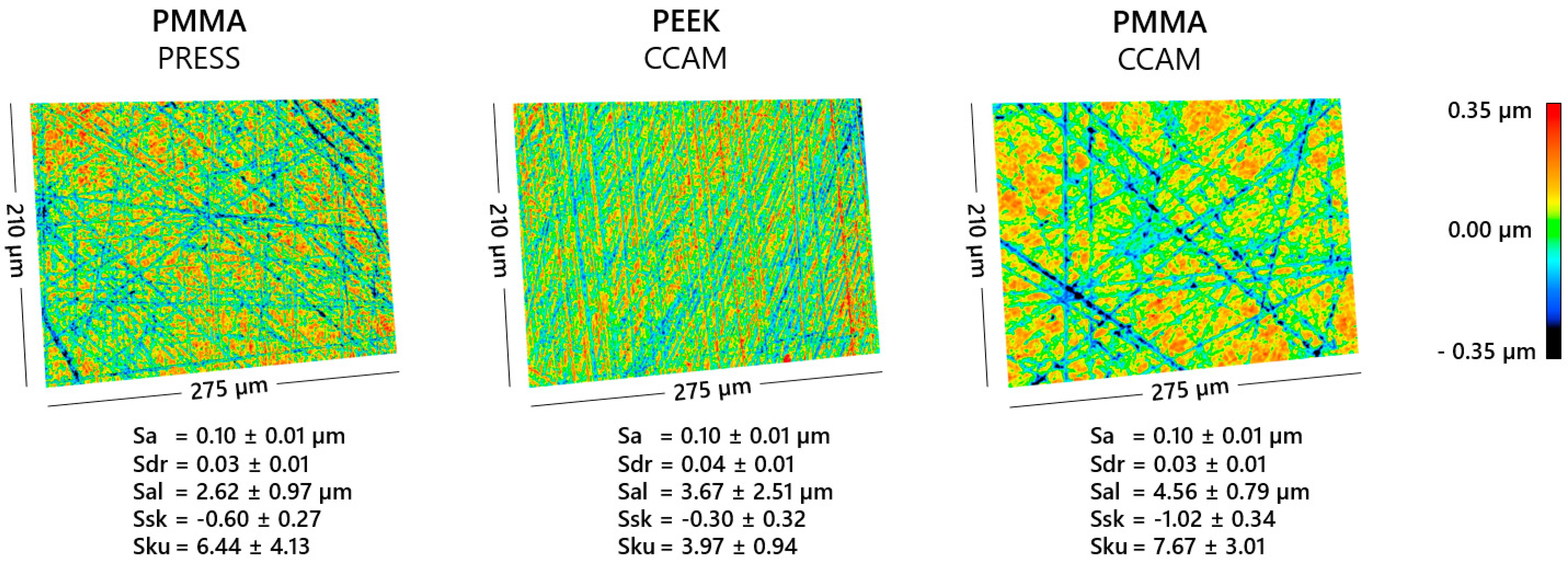
2.3. Surface Characterization
2.4. Specimen Insertion
2.5. Study Protocol
2.6. Microbial Analysis
2.7. Statistical Analysis
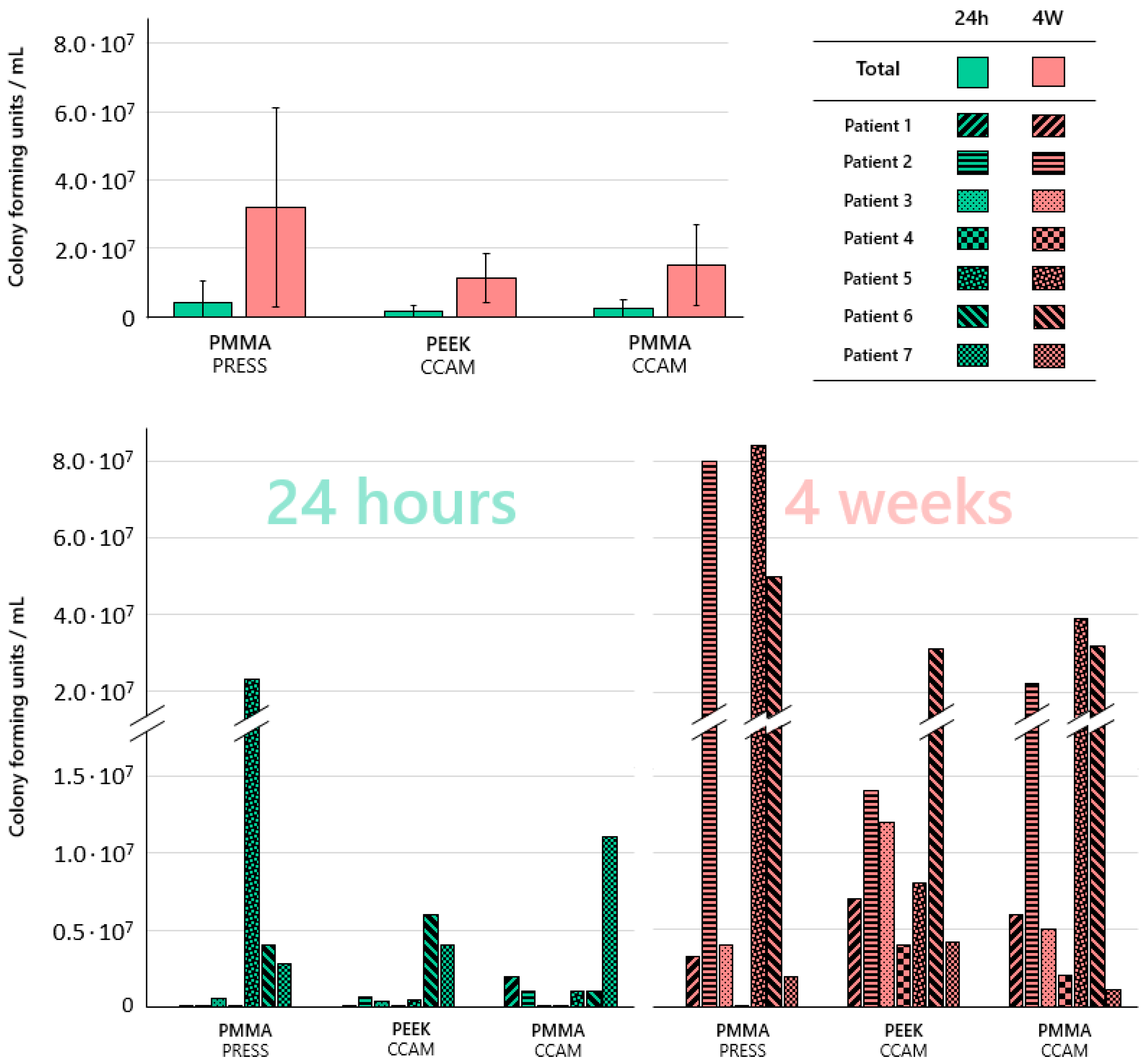
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fauroux, M.-A.; Germa, A.; Tramini, P.; Nabet, C. Prosthetic treatment in the adult French population: Prevalence and relation with demographic, socioeconomic and medical characteristics. Rev. Epidemiol. Sante Publique 2019, 67, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Nitschke, I.; Stark, H. Krankheits- und Versorgungsprävalenzen bei Älteren Senioren (75- bis 100- Jährige). In Fünfte Deutsche Mundgesundheitsstudie (DMS V); Jordan, A.R., Micheelis, W., Eds.; Deutscher Zahnärzte Verlag DÄV: Köln, Germany, 2016; pp. 517–548. ISBN 978-3-7691-0020-4. [Google Scholar]
- Guzmán, J.M.; Pawliczko, A.; Beales, S.; Till, C.; Voelcker, I. Ageing in the Twenty-First Century: Celabration and a Challenge; United Nations Population Fund; HelpAge International: New York, NY, USA; London, UK, 2012; ISBN 9780897149815. [Google Scholar]
- Schwendicke, F.; Nitschke, I.; Stark, H.; Micheelis, W.; Jordan, R.A. Epidemiological trends, predictive factors, and projection of tooth loss in Germany 1997–2030: Part II. Edentulism in seniors. Clin. Oral Investig. 2020, 24, 3997–4003. [Google Scholar] [CrossRef] [PubMed]
- Do, T.; Devine, D.; Marsh, P.D. Oral biofilms: Molecular analysis, challenges, and future prospects in dental diagnostics. Clin. Cosmet. Investig. Dent. 2013, 5, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Samaranayake, L.; Bandara, N.; Pesee, S. Oral Biofilms: What Are They? In Oral Biofilms and Modern Dental Materials: Advances Toward Bioactivity; Ionescu, A.C., Hahnel, S., Eds.; Springer International Publishing: Cham, Germany, 2021; pp. 1–7. ISBN 9783030673888. [Google Scholar]
- Günther, E.; Kommerein, N.; Hahnel, S. Biofilms on polymeric materials for the fabrication of removable dentures. DZZ Int. 2020, 2, 142–145. [Google Scholar] [CrossRef]
- Voorhis, A.; Miranda-Sanchez, F.; Dewhirst, F.E.; Mark Welch, J.; Kauffman, K.; Viala, S.; Yost, S.; Chen, T.; Wade, W.G. HOMD: Human Oral Microbiome Database. Available online: https://www.homd.org/ (accessed on 11 July 2023).
- Ghannoum, M.A.; Jurevic, R.J.; Mukherjee, P.K.; Cui, F.; Sikaroodi, M.; Naqvi, A.; Gillevet, P.M. Characterization of the oral fungal microbiome (mycobiome) in healthy individuals. PLoS Pathog. 2010, 6, e1000713. [Google Scholar] [CrossRef]
- Kilian, M.; Chapple, I.L.C.; Hannig, M.; Marsh, P.D.; Meuric, V.; Pedersen, A.M.L.; Tonetti, M.S.; Wade, W.G.; Zaura, E. The oral microbiome-an update for oral healthcare professionals. Br. Dent. J. 2016, 221, 657–666. [Google Scholar] [CrossRef]
- Gendreau, L.; Loewy, Z.G. Epidemiology and etiology of denture stomatitis. J. Prosthodont. 2011, 20, 251–260. [Google Scholar] [CrossRef]
- Budtz-Jörgensen, E. The significance of Candida albicans in denture stomatitis. Scand. J. Dent. Res. 1974, 82, 151–190. [Google Scholar] [CrossRef]
- Susewind, S.; Lang, R.; Hahnel, S. Biofilm formation and Candida albicans morphology on the surface of denture base materials. Mycoses 2015, 58, 719–727. [Google Scholar] [CrossRef]
- Bilhan, H.; Sulun, T.; Erkose, G.; Kurt, H.; Erturan, Z.; Kutay, O.; Bilgin, T. The role of Candida albicans hyphae and Lactobacillus in denture-related stomatitis. Clin. Oral Investig. 2009, 13, 363–368. [Google Scholar] [CrossRef]
- Alzamil, H.; Wu, T.T.; van Wijngaarden, E.; Mendoza, M.; Malmstrom, H.; Fiscella, K.; Kopycka-Kedzierawski, D.T.; Billings, R.J.; Xiao, J. Removable Denture Wearing as a Risk Predictor for Pneumonia Incidence and Time to Event in Older Adults. JDR Clin. Trans. Res. 2021, 8, 23800844211049406. [Google Scholar] [CrossRef] [PubMed]
- Iinuma, T.; Arai, Y.; Abe, Y.; Takayama, M.; Fukumoto, M.; Fukui, Y.; Iwase, T.; Takebayashi, T.; Hirose, N.; Gionhaku, N.; et al. Denture wearing during sleep doubles the risk of pneumonia in the very elderly. J. Dent. Res. 2015, 94, 28S–36S. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.-L.; Seon, S.-H.; Rhee, D.-K. Pneumonia and Streptococcus pneumoniae vaccine. Arch. Pharm. Res. 2017, 40, 885–893. [Google Scholar] [CrossRef]
- O’Donnell, L.E.; Smith, K.; Williams, C.; Nile, C.J.; Lappin, D.F.; Bradshaw, D.; Lambert, M.; Robertson, D.P.; Bagg, J.; Hannah, V.; et al. Dentures are a Reservoir for Respiratory Pathogens. J. Prosthodont. 2016, 25, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Kusama, T.; Aida, J.; Yamamoto, T.; Kondo, K.; Osaka, K. Infrequent Denture Cleaning Increased the Risk of Pneumonia among Community-dwelling Older Adults: A Population-based Cross-sectional Study. Sci. Rep. 2019, 9, 13734. [Google Scholar] [CrossRef]
- Marik, P.E.; Kaplan, D. Aspiration pneumonia and dysphagia in the elderly. Chest 2003, 124, 328–336. [Google Scholar] [CrossRef]
- Schmutzler, A.; Rauch, A.; Nitschke, I.; Lethaus, B.; Hahnel, S. Cleaning of removable dental prostheses-a systematic review. J. Evid. Based Dent. Pract. 2021, 21, 101644. [Google Scholar] [CrossRef]
- El-Solh, A.A. Association between pneumonia and oral care in nursing home residents. Lung 2011, 189, 173–180. [Google Scholar] [CrossRef]
- Stawarczyk, B.; Eichberger, M.; Uhrenbacher, J.; Wimmer, T.; Edelhoff, D.; Schmidlin, P.R. Three-unit reinforced polyetheretherketone composite FDPs: Influence of fabrication method on load-bearing capacity and failure types. Dent. Mater. J. 2015, 34, 7–12. [Google Scholar] [CrossRef]
- Quirynen, M. The clinical meaning of the surface roughness and the surface free energy of intra-oral hard substrata on the microbiology of the supra- and subgingival plaque: Results of in vitro and in vivo experiments. J. Dent. 1994, 22 (Suppl. S1), S13–S16. [Google Scholar] [CrossRef]
- Bürgers, R.; Krohn, S.; Wassmann, T. Surface Properties of Dental Materials and Biofilm Formation. In Oral Biofilms and Modern Dental Materials: Advances toward Bioactivity; Ionescu, A.C., Hahnel, S., Eds.; Springer International Publishing: Cham, Germany, 2021; pp. 55–69. ISBN 9783030673888. [Google Scholar]
- Hannig, C.; Hannig, M. The oral cavity—A key system to understand substratum-dependent bioadhesion on solid surfaces in man. Clin. Oral Investig. 2009, 13, 123–139. [Google Scholar] [CrossRef] [PubMed]
- Sterzenbach, T.; Helbig, R.; Hannig, C.; Hannig, M. Bioadhesion in the oral cavity and approaches for biofilm management by surface modifications. Clin. Oral Investig. 2020, 24, 4237–4260. [Google Scholar] [CrossRef]
- Schmalz, G.; Cieplik, F. Biofilms on Restorative Materials. Monogr. Oral Sci. 2021, 29, 155–194. [Google Scholar] [CrossRef] [PubMed]
- Teughels, W.; van Assche, N.; Sliepen, I.; Quirynen, M. Effect of material characteristics and/or surface topography on biofilm development. Clin. Oral Implant. Res. 2006, 17 (Suppl. S2), 68–81. [Google Scholar] [CrossRef] [PubMed]
- Koch, C.; Bürgers, R.; Hahnel, S. Candida albicans adherence and proliferation on the surface of denture base materials. Gerodontology 2013, 30, 309–313. [Google Scholar] [CrossRef] [PubMed]
- Huhtamäki, T.; Tian, X.; Korhonen, J.T.; Ras, R.H.A. Surface-wetting characterization using contact-angle measurements. Nat. Protoc. 2018, 13, 1521–1538. [Google Scholar] [CrossRef] [PubMed]
- Owens, D.K.; Wendt, R.C. Estimation of the surface free energy of polymers. J. Appl. Polym. Sci. 1969, 13, 1741–1747. [Google Scholar] [CrossRef]
- de Castro, D.T.; do Nascimento, C.; Alves, O.L.; de Souza Santos, E.; Agnelli, J.A.M.; Dos Reis, A.C. Analysis of the oral microbiome on the surface of modified dental polymers. Arch. Oral Biol. 2018, 93, 107–114. [Google Scholar] [CrossRef]
- Keijser, B.J.F.; van den Broek, T.J.; Slot, D.E.; van Twillert, L.; Kool, J.; Thabuis, C.; Ossendrijver, M.; van der Weijden, F.A.; Montijn, R.C. The Impact of Maltitol-Sweetened Chewing Gum on the Dental Plaque Biofilm Microbiota Composition. Front. Microbiol. 2018, 9, 381. [Google Scholar] [CrossRef]
- Schmidt, K.E.; Auschill, T.M.; Heumann, C.; Frankenberger, R.; Eick, S.; Sculean, A.; Arweiler, N.B. Clinical and laboratory evaluation of the effects of different treatment modalities on titanium healing caps: A randomized, controlled clinical trial. Clin. Oral Investig. 2018, 22, 2149–2160. [Google Scholar] [CrossRef]
- Murat, S.; Alp, G.; Alatalı, C.; Uzun, M. In Vitro Evaluation of Adhesion of Candida albicans on CAD/CAM PMMA-Based Polymers. J. Prosthodont. 2019, 28, e873–e879. [Google Scholar] [CrossRef] [PubMed]
- An, J.; Ding, N.; Zhang, Z. Mechanical and antibacterial properties of polymethyl methacrylate modified with zinc dimethacrylate. J. Prosthet. Dent. 2022, 128, 100.e1–100.e8. [Google Scholar] [CrossRef] [PubMed]
- Bollen, C.M.; Lambrechts, P.; Quirynen, M. Comparison of surface roughness of oral hard materials to the threshold surface roughness for bacterial plaque retention: A review of the literature. Dent. Mater. 1997, 13, 258–269. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Pan, Y.; Wang, M.; Wang, Y.; Lin, H.; Jiang, L.; Lin, D.; Cheng, H. Comparative analysis of leaching residual monomer and biological effects of four types of conventional and CAD/CAM dental polymers: An in vitro study. Clin. Oral Investig. 2022, 26, 2887–2898. [Google Scholar] [CrossRef] [PubMed]
- Waltimo, T.; Vallittu, P.; Haapasalo, M. Adherence of Candida species to newly polymerized and water-stored denture base polymers. Int. J. Prosthodont. 2001, 14, 457–460. [Google Scholar] [PubMed]
- Engler, M.L.P.D.; Güth, J.-F.; Keul, C.; Erdelt, K.; Edelhoff, D.; Liebermann, A. Residual monomer elution from different conventional and CAD/CAM dental polymers during artificial aging. Clin. Oral Investig. 2020, 24, 277–284. [Google Scholar] [CrossRef]
- Etxeberria, M.; Escuin, T.; Vinas, M.; Ascaso, C. Useful surface parameters for biomaterial discrimination. Scanning 2015, 37, 429–437. [Google Scholar] [CrossRef]
- Schubert, A.; Wassmann, T.; Holtappels, M.; Kurbad, O.; Krohn, S.; Bürgers, R. Predictability of Microbial Adhesion to Dental Materials by Roughness Parameters. Coatings 2019, 9, 456. [Google Scholar] [CrossRef]
- Prpić, V.; Schauperl, Z.; Ćatić, A.; Dulčić, N.; Čimić, S. Comparison of Mechanical Properties of 3D-Printed, CAD/CAM, and Conventional Denture Base Materials. J. Prosthodont. 2020, 29, 524–528. [Google Scholar] [CrossRef]
- Nedeljkovic, I.; de Munck, J.; Ungureanu, A.-A.; Slomka, V.; Bartic, C.; Vananroye, A.; Clasen, C.; Teughels, W.; van Meerbeek, B.; van Landuyt, K.L. Biofilm-induced changes to the composite surface. J. Dent. 2017, 63, 36–43. [Google Scholar] [CrossRef]
- Matsuo, H.; Suenaga, H.; Takahashi, M.; Suzuki, O.; Sasaki, K.; Takahashi, N. Deterioration of polymethyl methacrylate dentures in the oral cavity. Dent. Mater. J. 2015, 34, 234–239. [Google Scholar] [CrossRef] [PubMed]
| PMMA_PRESS | PEEK_CCAM | PMMA_CCAM | |
|---|---|---|---|
| Contact angle in ° | |||
| Water | 63.89 ± 3.37 | 69.36 ± 1.81 | 62.71 ± 2.54 |
| Diiodo methane | 45.42 ± 3.22 | 42.44 ± 2.43 | 51.83 ± 2.40 |
| Surface free energy in mN/m | |||
| Total | 48.17 ± 3.65 | 46.45 ± 2.20 | 46.67 ± 2.93 |
| Polar part | 11.39 ± 1.92 | 8.09 ± 0.94 | 13.42 ± 1.57 |
| Dispersive part | 36.78 ± 1.73 | 38.36 ± 1.26 | 33.25 ± 1.36 |
| After 24 h | Patient | ||||||
|---|---|---|---|---|---|---|---|
| 1 (f) | 2 (f) | 3 (m) | 4 (f) | 5 (m) | 6 (m) | 7 (f) | |
| Number of different isolated microorganisms per sample | |||||||
| PMMA_press | 16 | 6 | 12 | 12 | 13 | 6 | 12 |
| PEEK_CCAM | 14 | 7 | 13 | 11 | 14 | 9 | 13 |
| PMMA_CCAM | 11 | 5 | 11 | 11 | 13 | 9 | 15 |
| Isolated respiratory bacteria (CFU/mL) | |||||||
| Escherichia coli | |||||||
| PMMA_press | 3000 | 0 | 0 | 0 | 0 | 0 | 0 |
| PEEK_CCAM | 0 | 0 | 0 | 0 | 0 | 0 | 30,000 |
| PMMA_CCAM | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Enterobacter species | |||||||
| PMMA_press | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| PEEK_CCAM | 1000 | 0 | 0 | 0 | 0 | 0 | 0 |
| PMMA_CCAM | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Haemophilus influenzae | |||||||
| PMMA_press | 0 | 0 | 0 | 0 | 1,200,000 | 0 | 0 |
| PEEK_CCAM | 0 | 0 | 0 | 500 | 40,000 | 0 | 0 |
| PMMA_CCAM | 0 | 0 | 0 | 0 | 60,000 | 0 | 0 |
| Klebsiella species | |||||||
| PMMA_press | 0 | 0 | 0 | 0 | 1,400,000 | 100,000 | 0 |
| PEEK_CCAM | 0 | 0 | 0 | 0 | 0 | 400,000 | 0 |
| PMMA_CCAM | 0 | 0 | 0 | 0 | 0 | 300,000 | 0 |
| Staphylococcus aureus | |||||||
| PMMA_press | 0 | 0 | 0 | 0 | 7000 | 0 | 0 |
| PEEK_CCAM | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| PMMA_CCAM | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Streptococcus pneumoniae | |||||||
| PMMA_press | 4000 | 0 | 0 | 0 | 0 | 0 | 100,000 |
| PEEK_CCAM | 50,000 | 0 | 0 | 1000 | 50,000 | 0 | 0 |
| PMMA_CCAM | 120,000 | 0 | 0 | 0 | 0 | 0 | 0 |
| Candida species (CFU/mL) | |||||||
| PMMA_press | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| PEEK_CCAM | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| PMMA_CCAM | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| After Four Weeks | Patient | ||||||
|---|---|---|---|---|---|---|---|
| 1 (f) | 2 (f) | 3 (m) | 4 (f) | 5 (m) | 6 (m) | 7 (f) | |
| Number of different isolated microorganisms per sample | |||||||
| PMMA_press | 15 | 12 | 17 | 4 | 17 | 16 | 16 |
| PEEK_CCAM | 15 | 13 | 12 | 16 | 13 | 12 | 13 |
| PMMA_CCAM | 14 | 19 | 11 | 16 | 17 | 12 | 15 |
| Isolated respiratory bacteria (CFU/mL) | |||||||
| Escherichia coli | |||||||
| PMMA_press | 0 | 0 | 0 | 0 | 1,000,000 | 0 | 2000 |
| PEEK_CCAM | 0 | 0 | 0 | 2000 | 0 | 0 | 0 |
| PMMA_CCAM | 0 | 0 | 0 | 10,000 | 110,000 | 0 | 0 |
| Enterobacter species | |||||||
| PMMA_press | 0 | 0 | 0 | 0 | 0 | 0 | 20,000 |
| PEEK_CCAM | 0 | 100,000 | 0 | 10,000 | 0 | 0 | 0 |
| PMMA_CCAM | 0 | 0 | 0 | 30,000 | 1,100,000 | 0 | 0 |
| Haemophilus influenzae | |||||||
| PMMA_press | 0 | 10,000 | 0 | 0 | 0 | 0 | 0 |
| PEEK_CCAM | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| PMMA_CCAM | 0 | 0 | 0 | 0 | 150,000 | 0 | 0 |
| Klebsiella species | |||||||
| PMMA_press | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| PEEK_CCAM | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| PMMA_CCAM | 0 | 0 | 0 | 600,000 | 0 | 0 | 0 |
| Staphylococcus aureus | |||||||
| PMMA_press | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| PEEK_CCAM | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| PMMA_CCAM | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Streptococcus pneumoniae | |||||||
| PMMA_press | 100,000 | 0 | 0 | 0 | 0 | 0 | 0 |
| PEEK_CCAM | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| PMMA_CCAM | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Candida species (CFU/mL) | |||||||
| PMMA_press | 0 | 2,006,000 | 31,000 | 0 | 500,000 | 3,509,000 | 0 |
| PEEK_CCAM | 0 | 2,020,000 | 0 | 0 | 0 | 10,100,000 | 0 |
| PMMA_CCAM | 100 | 3,140,000 | 3000 | 0 | 0 | 4,700,000 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schmutzler, A.; Stingu, C.S.; Günther, E.; Lang, R.; Fuchs, F.; Koenig, A.; Rauch, A.; Hahnel, S. Attachment of Respiratory Pathogens and Candida to Denture Base Materials—A Pilot Study. J. Clin. Med. 2023, 12, 6127. https://doi.org/10.3390/jcm12196127
Schmutzler A, Stingu CS, Günther E, Lang R, Fuchs F, Koenig A, Rauch A, Hahnel S. Attachment of Respiratory Pathogens and Candida to Denture Base Materials—A Pilot Study. Journal of Clinical Medicine. 2023; 12(19):6127. https://doi.org/10.3390/jcm12196127
Chicago/Turabian StyleSchmutzler, Anne, Catalina Suzana Stingu, Elena Günther, Reinhold Lang, Florian Fuchs, Andreas Koenig, Angelika Rauch, and Sebastian Hahnel. 2023. "Attachment of Respiratory Pathogens and Candida to Denture Base Materials—A Pilot Study" Journal of Clinical Medicine 12, no. 19: 6127. https://doi.org/10.3390/jcm12196127
APA StyleSchmutzler, A., Stingu, C. S., Günther, E., Lang, R., Fuchs, F., Koenig, A., Rauch, A., & Hahnel, S. (2023). Attachment of Respiratory Pathogens and Candida to Denture Base Materials—A Pilot Study. Journal of Clinical Medicine, 12(19), 6127. https://doi.org/10.3390/jcm12196127