Association between Obstructive Sleep Apnea and Heart Failure in Adults—A Systematic Review
Abstract
:1. Introduction
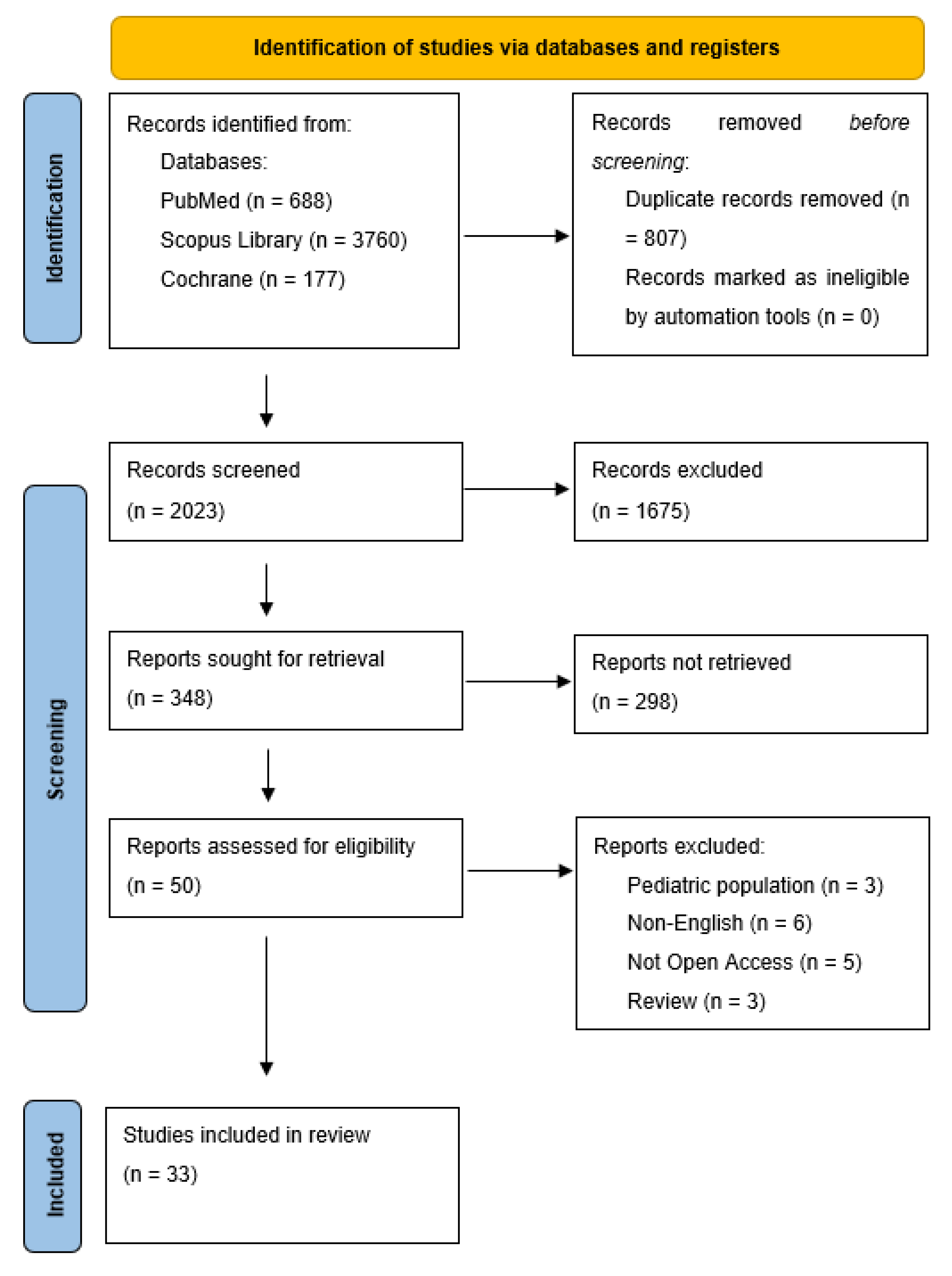
2. Materials and Methods
3. Results
3.1. The Prevalence of Obstructive Sleep Apnea in the Heart Failure Population
3.2. Does Positive Airway Pressure Play a Role in HF Patients?
3.2.1. Obstructive Sleep Apnea and Heart Failure with Preserved Ejection Fraction
3.2.2. Obstructive Sleep Apnea and Heart Failure with Reduced Ejection Fraction
3.3. Healthcare Resources Utilization in Heart Failure and Obstructive Sleep Apnea Patients
3.4. Do New Medicaments in Heart Failure Pharmacotherapy Play a Role in Sleep-Disordered Breathing Patients?
3.4.1. Sodium/Glucose Cotransporter-2 Inhibitors (SGLT2i)
3.4.2. Sacubitril/Valsartan
4. Discussion
4.1. The Prevalence of Obstructive Sleep Apnea in Heart Failure Patients
4.2. The Impact of Positive Airway Pressure Therapy in Heart Failure and Obstructive Sleep Apnea Patients
4.3. The Heart Failure Medications on Sleep Parameters: Correlation and Potential Mechanisms
4.4. OSA and HF—Clinical Relevance, Clinical Practice and Patient Care
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Heatley, E.M.; Harris, M.; Battersby, M.; McEvoy, R.D.; Chai-Coetzer, C.L.; Antic, N.A. Obstructive sleep apnoea in adults: A common chronic condition in need of a comprehensive chronic condition management approach. Sleep Med. Rev. 2013, 17, 349–355. [Google Scholar] [CrossRef]
- Epstein, L.J.; Kristo, D.; Strollo, P.J.; Friedman, N.; Malhotra, A.; Patil, S.P.; Ramar, K.; Rogers, R.; Schwab, R.J.; Weaver, E.M.; et al. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J. Clin. Sleep Med. 2009, 5, 263–276. [Google Scholar] [PubMed]
- American Academy of Sleep Medicine Task Force. Sleep-related breathing disorders in adults: Recommendations for syndrome definition and measurement techniques in clinical research. The report of an American Academy of Sleep Medicine task force. Sleep 1999, 22, 667–689. [Google Scholar] [CrossRef]
- Malhotra, A.; Heinzer, R.; Morrell, M.J.; Penzel, T.; Pepin, J.-L.; Valentine, K.; Nunez, C.; Benjafield, A. Late Breaking Abstract—European prevalence of OSA in adults: Estimation using currently available data. Eur. Respir. J. 2018, 52, OA4961. [Google Scholar] [CrossRef]
- Kapur, V.K.; Auckley, D.H.; Chowdhuri, S.; Kuhlmann, D.C.; Mehra, R.; Ramar, K.; Harrod, C.G. Clinical Practice Guideline for Diagnostic Testing for Adult Obstructive Sleep Apnea. J. Clin. Sleep Med. 2017, 1313, 479–504. [Google Scholar] [CrossRef]
- Milicic Ivanovski, D.; Milicic Stanic, B.; Kopitovic, I. Comorbidity Profile and Predictors of Obstructive Sleep Apnea Severity and Mortality in Non-Obese Obstructive Sleep Apnea Patients. Medicina 2023, 59, 873. [Google Scholar] [CrossRef]
- McKee, Z.; Auckley, D.H. A sleeping beast: Obstructive sleep apnea and stroke. Clevel. Clin. J. Med. 2019, 86, 407–415. [Google Scholar] [CrossRef]
- Redline, S. Screening for Obstructive Sleep Apnea Implications for the Sleep Health of the Population. J. Am. Med. Assoc. 2017, 317, 368–370. [Google Scholar] [CrossRef]
- McNicholas, W.T.; Bonsignore, M.R. Sleep Apnoea as an Independent Risk for Cardiovascular Disease: Current Evidence, Basic Mechanisms and Research Priorities. Eur. Respir. J. 2007, 29, 156–178. [Google Scholar] [CrossRef]
- McNicholas, W.T. Obstructive Sleep Apnea and Inflammation. Prog. Cardiovasc. Dis. 2009, 51, 392–399. [Google Scholar] [CrossRef]
- Olszewska, E.; Rogalska, J.; Brzóska, M.M. The Association of Oxidative Stress in the Uvular Mucosa with Obstructive Sleep Apnea Syndrome: A Clinical Study. J. Clin. Med. 2021, 10, 1132. [Google Scholar] [CrossRef] [PubMed]
- Bonetti, P.O.; Lerman, L.O.; Lerman, A. Endothelial Dysfunction: A Marker of Atherosclerotic Risk. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 168–175. [Google Scholar] [CrossRef] [PubMed]
- GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1789–1858. [Google Scholar]
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R.; et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2022, 79, e263–e421. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chionce, O.; et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- Oldenburg, O.; Lamp, B.; Faber, L.; Teschler, H.; Horstkotte, D.; Töpfer, V. Sleep-disordered breathing in patients with symptomatic heart failure: A contemporary study of prevalence in and characteristics of 700 patients. Eur. J. Heart Fail. 2007, 9, 251–257. [Google Scholar] [CrossRef]
- Bekfani, T.; Schöbel, C.; Pietrock, C.; Valentova, M.; Ebner, N.; Döhner, W.; Schulze, P.C.; Anker, S.D.; von Haehling, S. Heart failure and sleep-disordered breathing: Susceptibility to reduced muscle strength and preclinical congestion (SICA-HF cohort). ESC Heart Fail. 2020, 7, 2063–2070. [Google Scholar] [CrossRef]
- Holt, A.; Bjerre, J.; Zareini, B.; Koch, H.; Tønnesen, P.; Gislason, G.H.; Nielsen, O.W.; Schou, M.; Lamberts, M. Sleep apnea, the risk of developing heart failure, and potential benefits of continuous positive airway pressure (CPAP) therapy. J. Am. Heart Assoc. 2018, 7, e008684. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Wang, T.; Yu, F.C.; Wei, Q.; Chen, L.; Xu, X.; Ding, N.; Tong, J.Y. Prevalence and clinical characteristics of sleep-disordered breathing in patients with heart failure of different left ventricular ejection fractions. Sleep Breath. 2022, 27, 245–253. [Google Scholar] [CrossRef]
- Wang, T.; Yu, F.C.; Wei, Q.; Xu, X.; Xie, L.; Ding, N.; Tong, J.Y. Sleep-disordered breathing in heart failure patients with different etiologies. Clin. Cardiol. 2022, 45, 778–785. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Agrawal, S.; Goel, A.D.; Ish, P.; Chakrabarti, S.; Suri, J.C. Profile of sleep disordered breathing in heart failure with preserved ejection fraction. Monaldi Arch. Chest Dis. 2020, 90, 660–665. [Google Scholar] [CrossRef]
- Arzt, M.; Oldenburg, O.; Graml, A.; Erdmann, E.; Teschler, H.; Wegscheider, K.; Suling, A.; Woehrle, H. SchlaHF Investigators. Phenotyping of Sleep-Disordered Breathing in Patients With Chronic Heart Failure With Reduced Ejection Fraction-the SchlaHF Registry. J. Am. Heart Assoc. 2017, 6, e005899. [Google Scholar] [CrossRef] [PubMed]
- Bitter, T.; Faber, L.; Hering, D.; Langer, C.; Horstkotte, D.; Oldenburg, O. Sleep-disordered breathing in heart failure with normal left ventricular ejection fraction. Eur. J. Heart Fail. 2009, 11, 602–608. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.; Sanderson, J.; Chan, W.; Lai, C.; Choy, D.; Ho, A.; Leung, R. Prevalence of sleep-disordered breathing in diastolic heart failure. Chest 1997, 111, 1488–1493. [Google Scholar] [CrossRef] [PubMed]
- Yumino, D.; Wang, H.; Floras, J.S.; Newton, G.E.; Mak, S.; Ruttanaumpawan, P.; Parker, J.D.; Bradley, T.D. Prevalence and physiological predictors of sleep apnea in patients with heart failure and systolic dysfunction. J. Card. Fail. 2009, 15, 279–285. [Google Scholar] [CrossRef]
- Herrscher, T.E.; Akre, H.; Øverland, B.; Sandvik, L.; Westheim, A.S. High prevalence of sleep apnea in heart failure outpatients: Even in patients with preserved systolic function. J. Card. Fail. 2011, 17, 420–425. [Google Scholar] [CrossRef] [PubMed]
- Kalaydzhiev, P.; Poroyliev, N.; Somleva, D.; Ilieva, R.; Markov, D.; Kinova, E.; Goudev, A. Sleep apnea in patients with exacerbated heart failure and overweight. Sleep Med. X 2023, 5, 100065. [Google Scholar] [CrossRef]
- Arikawa, T.; Toyoda, S.; Haruyama, A.; Amano, H.; Inami, S.; Otani, N.; Sakuma, M.; Taguchi, I.; Abe, S.; Node, K.; et al. Impact of Obstructive Sleep Apnoea on Heart Failure with Preserved Ejection Fraction. Heart Lung Circ. 2016, 25, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Naito, R.; Kasai, T.; Dohi, T.; Takaya, H.; Narui, K.; Momomura, S.I. Factors Associated With the Improvement of Left Ventricular Systolic Function by Continuous Positive Airway Pressure Therapy in Patients With Heart Failure With Reduced Ejection Fraction and Obstructive Sleep Apnea. Front. Neurol. 2022, 13, 781054. [Google Scholar] [CrossRef]
- Kaneko, Y.; Floras, J.S.; Usui, K.; Plante, J.; Tkacova, R.; Kubo, T.; Ando, S.; Bradley, T.D. Cardiovascular effects of continuous positive airway pressure in patients with heart failure and obstructive sleep apnea. N. Engl. J. Med. 2003, 348, 1233–1241. [Google Scholar] [CrossRef] [PubMed]
- Mansfield, D.R.; Gollogly, N.C.; Kaye, D.M.; Richardson, M.; Bergin, P.; Naughton, M.T. Controlled trial of continuous positive airway pressure in obstructive sleep apnea and heart failure. Am. J. Respir. Crit. Care Med. 2004, 169, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Fox, H.; Bitter, T.; Sauzet, O.; Rudolph, V.; Oldenburg, O. Automatic positive airway pressure for obstructive sleep apnea in heart failure with reduced ejection fraction. Clin. Res. Cardiol. 2021, 110, 983–992. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Shim, C.Y.; Cho, Y.J.; Park, S.; Lee, C.J.; Park, J.H.; Cho, H.J.; Ha, J.W.; Hong, G.R. Continuous Positive Airway Pressure Therapy Restores Cardiac Mechanical Function in Patients With Severe Obstructive Sleep Apnea: A Randomized, Sham-Controlled Study. J. Am. Soc. Echocardiogr. 2019, 32, 826–835. [Google Scholar] [CrossRef]
- Matthew, P.; Gilman JS Floras, K.U.; Yasuyuki, K.; Richard, S.T.L.; Douglas, T.B. Continuous positive airway pressure increases heart rate variability in heart failure patients with obstructive sleep apnoea. Clin. Sci. 2008, 114, 243–249. [Google Scholar] [CrossRef]
- Servantes, D.M.; Javaheri, S.; Kravchychyn, A.C.P.; Storti, L.J.; Almeida, D.R.; de Mello, M.T.; Cintra, F.D.; Tufik, S.; Bittencourt, L. Effects of Exercise Training and CPAP in Patients With Heart Failure and OSA: A Preliminary Study. Chest 2018, 154, 808–817. [Google Scholar] [CrossRef]
- Egea, C.J.; Aizpuru, F.; Pinto, J.A.; Ayuela, J.M.; Ballester, E.; Zamarrón, C.; Sojo, A.; Montserrat, J.M.; Barbe, F.; Alonso-Gomez, A.M.; et al. Cardiac function after CPAP therapy in patients with chronic heart failure and sleep apnea: A multicenter study. Sleep Med. 2008, 9, 660–666. [Google Scholar] [CrossRef]
- Ryan, C.M.; Usui, K.; Floras, J.S.; Bradley, T.D. Effect of continuous positive airway pressure on ventricular ectopy in heart failure patients with obstructive sleep apnoea. Thorax 2005, 60, 781–785. [Google Scholar] [CrossRef]
- Cistulli, P.A.; Malhotra, A.; Cole, K.V.; Malik, A.S.; Pépin, J.L.; Sert Kuniyoshi, F.H.; Benjafield, A.V.; Somers, V.K.; medXcloud group. Positive Airway Pressure Therapy Adherence and Health Care Resource Use in Patients With Obstructive Sleep Apnea and Heart Failure With Preserved Ejection Fraction. J. Am. Heart Assoc. 2023, 8, e028733. [Google Scholar] [CrossRef]
- Abdullah, A.; Eigbire, G.; Salama, A.; Wahab, A.; Nadkarni, N.; Alweis, R. Relation of Obstructive Sleep Apnea to Risk of Hospitalization in Patients With Heart Failure and Preserved Ejection Fraction from the National Inpatient Sample. Am. J. Cardiol. 2018, 122, 612–615. [Google Scholar] [CrossRef]
- Malhotra, A.; Cole, K.V.; Malik, A.S.; Pépin, J.L.; Sert Kuniyoshi, F.H.; Cistulli, P.A.; Benjafield, A.V.; Somers, V.K.; medXcloud group. Positive Airway Pressure Adherence and Health Care Resource Utilization in Patients With Obstructive Sleep Apnea and Heart Failure with Reduced Ejection Fraction. J. Am. Heart Assoc. 2023, 12, e028732. [Google Scholar] [CrossRef] [PubMed]
- Wojeck, B.S.; Inzucchi, S.E.; Neeland, I.J.; Mancuso, J.P.; Frederich, R.; Masiukiewicz, U.; Cater, N.B.; McGuire, D.K.; Cannon, C.P.; Yaggi, H.K. Ertugliflozin and incident obstructive sleep apnea: An analysis from the VERTIS CV trial. Sleep Breath. 2023, 27, 669–672. [Google Scholar] [CrossRef] [PubMed]
- Neeland, I.J.; Eliasson, B.; Kasai, T.; Marx, N.; Zinman, B.; Inzucchi, S.E.; Wanner, C.; Zwiener, I.; Wojeck, B.S.; Yaggi, H.K.; et al. EMPA-REG OUTCOME Investigators. The Impact of Empagliflozin on Obstructive Sleep Apnea and Cardiovascular and Renal Outcomes: An Exploratory Analysis of the EMPA-REG OUTCOME Trial. Diabetes Care 2020, 43, 3007–3015. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, S.; Miyake, T.; Senba, H.; Sakai, T.; Furukawa, E.; Yamamoto, S.; Niiya, T.; Matsuura, B.; Hiasa, Y. The effectiveness of dapagliflozin for sleep-disordered breathing among Japanese patients with obesity and type 2 diabetes mellitus. Endocr. J. 2018, 65, 953–961. [Google Scholar] [CrossRef]
- Tang, Y.; Sun, Q.; Bai, X.Y.; Zhou, Y.F.; Zhou, Q.L.; Zhang, M. Effect of dapagliflozin on obstructive sleep apnea in patients with type 2 diabetes: A preliminary study. Nutr. Diabetes 2019, 9, 32. [Google Scholar] [CrossRef]
- Sawada, K.; Karashima, S.; Kometani, M.; Oka, R.; Takeda, Y.; Sawamura, T.; Fujimoto, A.; Demura, M.; Wakayama, A.; Usukura, M.; et al. Effect of sodium glucose cotransporter 2 inhibitors on obstructive sleep apnea in patients with type 2 diabetes. Endocr. J. 2018, 65, 461–467. [Google Scholar] [CrossRef]
- Xie, L.; Song, S.; Li, S.; Wei, Q.; Liu, H.; Zhao, C.; Yu, F.; Tong, J. Efficacy of dapagliflozin in the treatment of HFrEF with obstructive sleep apnea syndrome (DAHOS study): Study protocol for a multicentric, prospective, randomized controlled clinical trial. Trials 2023, 24, 318. [Google Scholar] [CrossRef]
- Owens, R.L.; Birkeland, K.; Heywood, J.T.; Steinhubl, S.R.; Dorn, J.; Grant, D.; Fombu, E.; Khandwalla, R. Sleep Outcomes from AWAKE-HF: A Randomized Clinical Trial of Sacubitril/Valsartan vs Enalapril in Patients With Heart Failure and Reduced Ejection Fraction. J. Card. Fail. 2021, 27, 1466–1471. [Google Scholar] [CrossRef]
- Pelaia, C.; Armentaro, G.; Volpentesta, M.; Mancuso, L.; Miceli, S.; Caroleo, B.; Perticone, M.; Maio, R.; Arturi, F.; Imbalzano, E.; et al. Effects of Sacubitril-Valsartan on Clinical, Echocardiographic, and Polygraphic Parameters in Patients Affected by Heart Failure with Reduced Ejection Fraction and Sleep Apnea. Front. Cardiovasc. Med. 2022, 9, 861663. [Google Scholar] [CrossRef]
- Jaffuel, D.; Nogue, E.; Berdague, P.; Galinier, M.; Fournier, P.; Dupuis, M.; Georger, F.; Cadars, M.P.; Ricci, J.E.; Plouvier, N.; et al. Sacubitril-valsartan initiation in chronic heart failure patients impacts sleep apnea: The ENTRESTO-SAS study. ESC Heart Fail. 2021, 8, 2513–2526. [Google Scholar] [CrossRef]
- Wang, Y.; Branco, R.F.; Salanitro, M.; Penzel, T.; Schöbel, C. Effects of sacubitril-valsartan on central and obstructive apneas in heart failure patients with reduced ejection fraction. Sleep Breath. 2023, 27, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Passino, C.; Sciarrone, P.; Vergaro, G.; Borrelli, C.; Spiesshoefer, J.; Gentile, F.; Emdin, M.; Giannoni, A. Sacubitril-valsartan treatment is associated with decrease in central apneas in patients with heart failure with reduced ejection fraction. Int. J. Cardiol. 2021, 330, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Khayat, R.N.; Jarjoura, D.; Porter, K.; Sow, A.; Wannemacher, J.; Dohar, R.; Pleister, A.; Abraham, W.T. Sleep disordered breathing and post-discharge mortality in patients with acute heart failure. Eur. Heart J. 2015, 36, 1463–1469. [Google Scholar] [CrossRef] [PubMed]
- Khayat, R.N.; Javaheri, S.; Porter, K.; Sow, A.; Holt, R.; Randerath, W.; Abraham, W.T.; Jarjoura, D. In-Hospital Management of Sleep Apnea During Heart Failure Hospitalization: A Randomized Controlled Trial. J. Card. Fail. 2020, 26, 705–712. [Google Scholar] [CrossRef]
- Ambrosy, A.P.; Gheorghiade, M.; Chioncel, O.; Mentz, R.J.; Butler, J. Global perspectives in hospitalized heart failure: Regional and ethnic variation in patient characteristics, management, and outcomes. Curr. Heart Fail. Rep. 2014, 11, 416–427. [Google Scholar] [CrossRef]
- Suen, C.; Wong, J.; Ryan, C.M.; Goh, S.; Got, T.; Chaudhry, R.; Lee, D.S.; Chung, F. Prevalence of Undiagnosed Obstructive Sleep Apnea Among Patients Hospitalized for Cardiovascular Disease and Associated In-Hospital Outcomes: A Scoping Review. J. Clin. Med. 2020, 9, 989. [Google Scholar] [CrossRef]
- Javaheri, S.; Brown, L.K.; Abraham, W.T.; Khayat, R. Apneas of Heart Failure and Phenotype-Guided Treatments: Part One: OSA. Chest 2020, 157, 394–402. [Google Scholar] [CrossRef]
- Kishan, S.; Rao, M.S.; Ramachandran, P.; Devasia, T.; Samanth, J. Prevalence and Patterns of Sleep-Disordered Breathing in Indian Heart Failure Population. Pulm. Med. 2021, 2021, 9978906. [Google Scholar] [CrossRef]
- Javaheri, S.; Barbe, F.; Campos-Rodriguez, F.; Dempsey, J.A.; Khayat, R.; Javaheri, S.; Malhotra, A.; Martinez-Garcia, M.A.; Mehra, R.; Pack, A.I.; et al. Sleep Apnea: Types, Mechanisms, and Clinical Cardiovascular Consequences. J. Am. Coll. Cardiol. 2017, 69, 841–858. [Google Scholar] [CrossRef]
- Javaheri, S.; Javaheri, S. Obstructive Sleep Apnea in Heart Failure: Current Knowledge and Future Directions. J. Clin. Med. 2022, 11, 3458. [Google Scholar] [CrossRef]
- Donovan, L.M.; Boeder, S.; Malhotra, A.; Patel, S.R. New developments in the use of positive airway pressure for obstructive sleep apnea. J. Thorac. Dis. 2015, 7, 1323–1342. [Google Scholar] [CrossRef] [PubMed]
- Henderson, L.A.; Fatouleh, R.H.; Lundblad, L.C.; McKenzie, D.K.; Macefield, V.G. Effects of 12 Months Continuous Positive Airway Pressure on Sympathetic Activity Related Brainstem Function and Structure in Obstructive Sleep Apnea. Front. Neurosci. 2016, 10, 90. [Google Scholar] [CrossRef] [PubMed]
- Jullian-Desayes, I.; Joyeux-Faure, M.; Tamisier, R.; Launois, S.; Borel, A.L.; Levy, P.; Pepin, J.L. Impact of obstructive sleep apnea treatment by continuous positive airway pressure on cardiometabolic biomarkers: A systematic review from sham CPAP randomized controlled trials. Sleep Med. Rev. 2015, 21, 23–38. [Google Scholar] [CrossRef] [PubMed]
- Spaak, J.; Egri, Z.J.; Kubo, T.; Yu, E.; Ando, S.; Kaneko, Y.; Usui, K.; Bradley, T.D.; Floras, J.S. Muscle sympathetic nerve activity during wakefulness in heart failure patients with and without sleep apnea. Hypertension 2005, 46, 1327–1332. [Google Scholar] [CrossRef]
- Fiedorczuk, P.; Polecka, A.; Walasek, M.; Olszewska, E. Potential Diagnostic and Monitoring Biomarkers of Obstructive Sleep Apnea-Umbrella Review of Meta-Analyses. J. Clin. Med. 2022, 12, 60. [Google Scholar] [CrossRef]
- Peker, Y.; Balcan, B. Cardiovascular outcomes of continuous positive airway pressure therapy for obstructive sleep apnea. J. Thorac. Dis. 2018, 10 (Suppl. S34), S4262–S4279. [Google Scholar] [CrossRef]
- Franco, O.H.; Peeters, A.; Bonneux, L.; de Laet, C. Blood pressure in adulthood and life expectancy with cardiovascular disease in men and women: Life course analysis. Hypertension 2005, 46, 280–286. [Google Scholar] [CrossRef]
- Reil, J.C.; Custodis, F.; Swedberg, K.; Komajda, M.; Borer, J.S.; Ford, I.; Tavazzi, L.; Laufs, U.; Bohm, M. Heart rate reduction in cardiovascular disease and therapy. Clin. Res. Cardiol. 2011, 100, 11–19. [Google Scholar] [CrossRef]
- Bohm, M.; Swedberg, K.; Komajda, M.; Borer, J.S.; Ford, I.; Dubost-Brama, A.; Lerebours, G.; Tavazzi, L.; Investigators, S. Heart rate as a risk factor in chronic heart failure (shift): The association between heart rate and outcomes in a randomised placebo-controlled trial. Lancet 2010, 376, 886–894. [Google Scholar] [CrossRef]
- Shepard, J.W., Jr.; Pevernagie, D.A.; Stanson, A.W.; Daniels, B.K.; Sheedy, P.F. Effects of changes in central venous pressure on upper airway size in patients with obstructive sleep apnea. Am. J. Respir. Crit. Care Med. 1996, 153, 250–254. [Google Scholar] [CrossRef]
- Yumino, D.; Redolfi, S.; Ruttanaumpawan, P.; Su, M.C.; Smith, S.; Newton, G.E.; Mak, S.; Bradley, T.D. Nocturnal rostral fluid shift: A unifying concept for the pathogenesis of obstructive and central sleep apnea in men with heart failure. Circulation 2010, 121, 1598–1605. [Google Scholar] [CrossRef] [PubMed]
- Carmo, J.; Araújo, I.; Marques, F.; Fonseca, C. Sleep-disordered breathing in heart failure: The state of the art after the SERVE-HF trial. Rev. Port. Cardiol. 2017, 36, 859–867. [Google Scholar] [CrossRef] [PubMed]
- Revol, B.; Jullian-Desayes, I.; Bailly, S.; Tamisier, R.; Grillet, Y.; Sapène, M.; Joyeux-Faure, M.; Pépin, J.L. Who May benefit from diuretics in OSA?: A propensity score-match observational study. Chest 2020, 158, 359–364. [Google Scholar] [CrossRef] [PubMed]
- Bucca, C.B.; Brussino, L.; Battisti, A.; Mutani, R.; Rolla, G.; Mangiardi, L.; Cicolin, A. Diuretics in obstructive sleep apnea with diastolic heart failure. Chest 2007, 132, 440–446. [Google Scholar] [CrossRef]
- Lamba, J.; Simpson, C.S.; Redfearn, D.P.; Michael, K.A.; Fitzpatrick, M.; Baranchuk, A. Cardiac resynchronization therapy for the treatment of sleep apnoea: A meta-analysis. EP Eur. 2011, 13, 1174–1179. [Google Scholar] [CrossRef]
- Bolinder, J.; Ljunggren, Ö.; Kullberg, J.; Johansson, L.; Wilding, J.; Langkilde, A.M.; Sugg, J.; Parikh, S. Effects of dapagliflozin on body weight, total fat mass, and regional adipose tissue distribution in patients with type 2 diabetes mellitus with inadequate glycemic control on metformin. J. Clin. Endocrinol. Metab. 2012, 97, 1020–1031. [Google Scholar] [CrossRef]
- Bolinder, J.; Ljunggren, Ö.; Johansson, L.; Wilding, J.; Langkilde, A.M.; Sjöström, C.D.; Sugg, J.; Parikh, S. Dapagliflozin maintains glycaemic control while reducing weight and body fat mass over 2 years in patients with type 2 diabetes mellitus inadequately controlled on metformin. Diabetes Obes. Metab. 2014, 16, 159–169. [Google Scholar] [CrossRef]
- ČertíkováChábová, V.; Zakiyanov, O. Sodium glucose cotransporter-2 inhibitors: Spotlight on favorable effects on clinical outcomes beyond diabetes. Int. J. Mol. Sci. 2022, 23, 2812. [Google Scholar] [CrossRef]
- Yokono, M.; Takasu, T.; Hayashizaki, Y.; Mitsuoka, K.; Kihara, R.; Muramatsu, Y.; Miyoshi, S.; Tahara, A.; Kurosaki, E.; Li, Q.; et al. SGLT2 selective inhibitor ipragliflozin reduces body fat mass by increasing fatty acid oxidation in high-fat diet-induced obese rats. Eur. J. Pharmacol. 2014, 15, 66–74. [Google Scholar] [CrossRef]
- Liang, Y.; Arakawa, K.; Ueta, K.; Matsushita, Y.; Kuriyama, C.; Martin, T.; Du, F.; Liu, Y.; Xu, J.; Conway, B.; et al. Effect of canagliflozin on renal threshold for glucose, glycemia, and body weight in normal and diabetic animal models. PLoS ONE 2012, 7, e30555. [Google Scholar] [CrossRef]
- Suzuki, M.; Takeda, M.; Kito, A.; Fukazawa, M.; Yata, T.; Yamamoto, M.; Nagata, T.; Fukuzawa, T.; Yamane, M.; Honda, K.; et al. Tofogliflozin, a sodium/glucose cotransporter 2 inhibitor, attenuates body weight gain and fat accumulation in diabetic and obese animal models. Nutr. Diabetes 2014, 4, e125. [Google Scholar] [CrossRef] [PubMed]
- Ferrannini, E.; Muscelli, E.; Frascerra, S.; Baldi, S.; Mari, A.; Heise, T.; Broedl, U.C.; Woerle, H.J. Metabolic response to sodium–glucose cotransporter 2 inhibition in type 2 diabetic patients. J. Clin. Investig. 2014, 124, 499–508. [Google Scholar] [CrossRef] [PubMed]
- Itani, T.; Ishihara, T. Efficacy of canagliflozin against nonalcoholic fatty liver disease: A prospective cohort study. Obes. Sci. Pract. 2018, 4, 477–482. [Google Scholar] [CrossRef] [PubMed]
- Raj, H.; Durgia, H.; Palui, R.; Kamalanathan, S.; Selvarajan, S.; Kar, S.S.; Sahoo, J. SGLT-2 inhibitors in non-alcoholic fatty liver disease patients with type 2 diabetes mellitus: A systematic review. World J. Diabetes 2019, 10, 114–132. [Google Scholar] [CrossRef]
- Choi, D.H.; Jung, C.H.; Mok, J.O.; Kim, C.H.; Kang, S.K.; Kim, B.Y. Effect of dapagliflozin on alanine aminotransferase improvement in type 2 diabetes mellitus with non-alcoholic fatty liver disease. Endocrinol. Metab. 2018, 33, 387–394. [Google Scholar] [CrossRef]
- Shimizu, M.; Suzuki, K.; Kato, K.; Jojima, T.; Iijima, T.; Murohisa, T.; Iijima, M.; Takekawa, H.; Usui, I.; Hiraishi, H.; et al. Evaluation of the effects of dapagliflozin, a sodium–glucose co-transporter-2 inhibitor, on hepatic steatosis and fibrosis using transient elastography in patients with type 2 diabetes and non-alcoholic fatty liver disease. Diabetes Obes. Metab. 2019, 21, 285–292. [Google Scholar] [CrossRef]
- Omori, K.; Nakamura, A.; Miyoshi, H.; Takahashi, K.; Kitao, N.; Nomoto, H.; Kameda, H.; Cho, K.Y.; Takagi, R.; Hatanaka, K.C.; et al. Effects of dapagliflozin and/or insulin glargine on beta cell mass and hepatic steatosis in db/db mice. Metabolism 2019, 98, 27–36. [Google Scholar] [CrossRef]
- Yagi, S.; Hirata, Y.; Ise, T.; Kusunose, K.; Yamada, H.; Fukuda, D.; Salim, H.M.; Maimaituxun, G.; Nishio, S.; Takagawa, Y.; et al. Canagliflozin reduces epicardial fat in patients with type 2 diabetes mellitus. Diabetol. Metab. Syndr. 2017, 4, 78. [Google Scholar] [CrossRef]
- Shimabukuro, M.; Hirata, Y.; Tabata, M.; Dagvasumberel, M.; Sato, H.; Kurobe, H.; Fukuda, D.; Soeki, T.; Kitagawa, T.; Takanashi, S.; et al. Epicardial adipose tissue volume and adipocytokine imbalance are strongly linked to human coronary atherosclerosis. Arter. Thromb. Vasc. Biol. 2013, 33, 1077–1084. [Google Scholar] [CrossRef]
- Mahabadi, A.A.; Lehmann, N.; Kälsch, H.; Robens, T.; Bauer, M.; Dykun, I.; Budde, T.; Moebus, S.; Jöckel, K.H.; Erbel, R.; et al. Association of epicardial adipose tissue with progression of coronary artery calcification is more pronounced in the early phase of atherosclerosis: Results from the Heinz Nixdorf recall study. JACC Cardiovasc. Imaging 2014, 7, 909–916. [Google Scholar] [CrossRef]
- Avogaro, A.; Fadini, G.P. Counterpoint to the hypothesis that SGLT2 inhibitors protect the heart by antagonizing leptin. Diabetes Obes. Metab. 2018, 20, 1367–1368. [Google Scholar] [CrossRef]
- Packer, M. Do sodium–glucose co-transporter-2 inhibitors prevent heart failure with a preserved ejection fraction by counterbalancing the effects of leptin? A novel hypothesis. Diabetes Obes. Metab. 2018, 20, 1361–1366. [Google Scholar] [CrossRef]
- Pan, W.; Kastin, A.J. Leptin: A biomarker for sleep disorders? Sleep Med. Rev. 2014, 18, 283–290. [Google Scholar] [CrossRef]
- Berger, S.; Polotsky, V.Y. Leptin and leptin resistance in the pathogenesis of obstructive sleep apnea: A possible link to oxidative stress and cardiovascular complications. Oxidative Med. Cell. Longev. 2018, 2018, 5137947. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Wen, W.; Li, J.; Xu, J.; Zhao, M.; Chen, H.; Sun, J. Systematic review and meta-analysis of randomized controlled trials on the effect of SGLT2 inhibitor on blood leptin and adiponectin level in patients with type 2 diabetes. Horm. Metab. Res. 2019, 51, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Imayama, I.; Prasad, B. Role of Leptin in Obstructive Sleep Apnea. Ann. Am. Thorac. Soc. 2017, 14, 1607–1621. [Google Scholar] [CrossRef]
- Selvaraj, S.; Claggett, B.; Pozzi, A.; McMurray, J.J.; Jhund, P.S.; Packer, M.; Desai, A.S.; Lewis, E.F.; Vaduganathan, M.; Lefkowitz, M.P.; et al. Prognostic implications of congestion on physical examination among contemporary patients with heart failure and reduced ejection fraction: PARADIGM-HF. Circulation 2019, 140, 1369–1379. [Google Scholar] [CrossRef] [PubMed]
- Giannoni, A.; Raglianti, V.; Taddei, C.; Borrelli, C.; Chubuchny, V.; Vergaro, G.; Mirizzi, G.; Valleggi, A.; Cameli, M.; Pasanisi, E.; et al. Cheyne-Stokes respiration related oscillations in cardiopulmonary hemodynamics in patients with heart failure. Int. J. Cardiol. 2019, 289, 76–82. [Google Scholar] [CrossRef]
- Bayard, G.; Da Costa, A.; Pierrard, R.; Roméyer-Bouchard, C.; Guichard, J.B.; Isaaz, K. Impact of sacubitril/valsartan on echo parameters in heart failure patients with reduced ejection fraction a prospective evaluation. IJC Heart Vasc. 2019, 25, 100418. [Google Scholar] [CrossRef]
- Romano, G.; Vitale, G.; Ajello, L.; Agnese, V.; Bellavia, D.; Caccamo, G.; Corrado, E.; Di Gesaro, G.; Falletta, C.; La Franca, E.; et al. The effects of sacubitril/valsartan on clinical, biochemical and echocardiographic parameters in patients with heart failure with reduced ejection fraction: The “hemodynamic recovery”. J. Clin. Med. 2019, 8, 2165. [Google Scholar] [CrossRef]
- Giannoni, A.; Raglianti, V.; Mirizzi, G.; Taddei, C.; Del Franco, A.; Iudice, G.; Bramanti, F.; Aimo, A.; Pasanisi, E.; Emdin, M.; et al. Influence of central apneas and chemoreflex activation on pulmonary artery pressure in chronic heart failure. Int. J. Cardiol. 2016, 202, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Spiesshoefer, J.; Aries, J.; Giannoni, A.; Emdin, M.; Fox, H.; Boentert, M.; Bitter, T.; Oldenburg, O. APAP therapy does not improve impaired sleep quality and sympatho-vagal balance: A randomized trial in patients with obstructive sleep apnea and systolic heart failure. Sleep Breath. 2020, 24, 211–219. [Google Scholar] [CrossRef] [PubMed]
- White, L.H.; Bradley, T.D. Role of nocturnal rostral fluid shift in the pathogenesis of obstructive and central sleep apnoea. J. Physiol. 2013, 591, 1179–1193. [Google Scholar] [CrossRef] [PubMed]
- Fox, H.; Bitter, T.; Horstkotte, D.; Oldenburg, O. Resolution of Cheyne-Stokes respiration after treatment of heart failure with sacubitril/valsartan: A first case report. Cardiology 2017, 137, 96–99. [Google Scholar] [CrossRef] [PubMed]
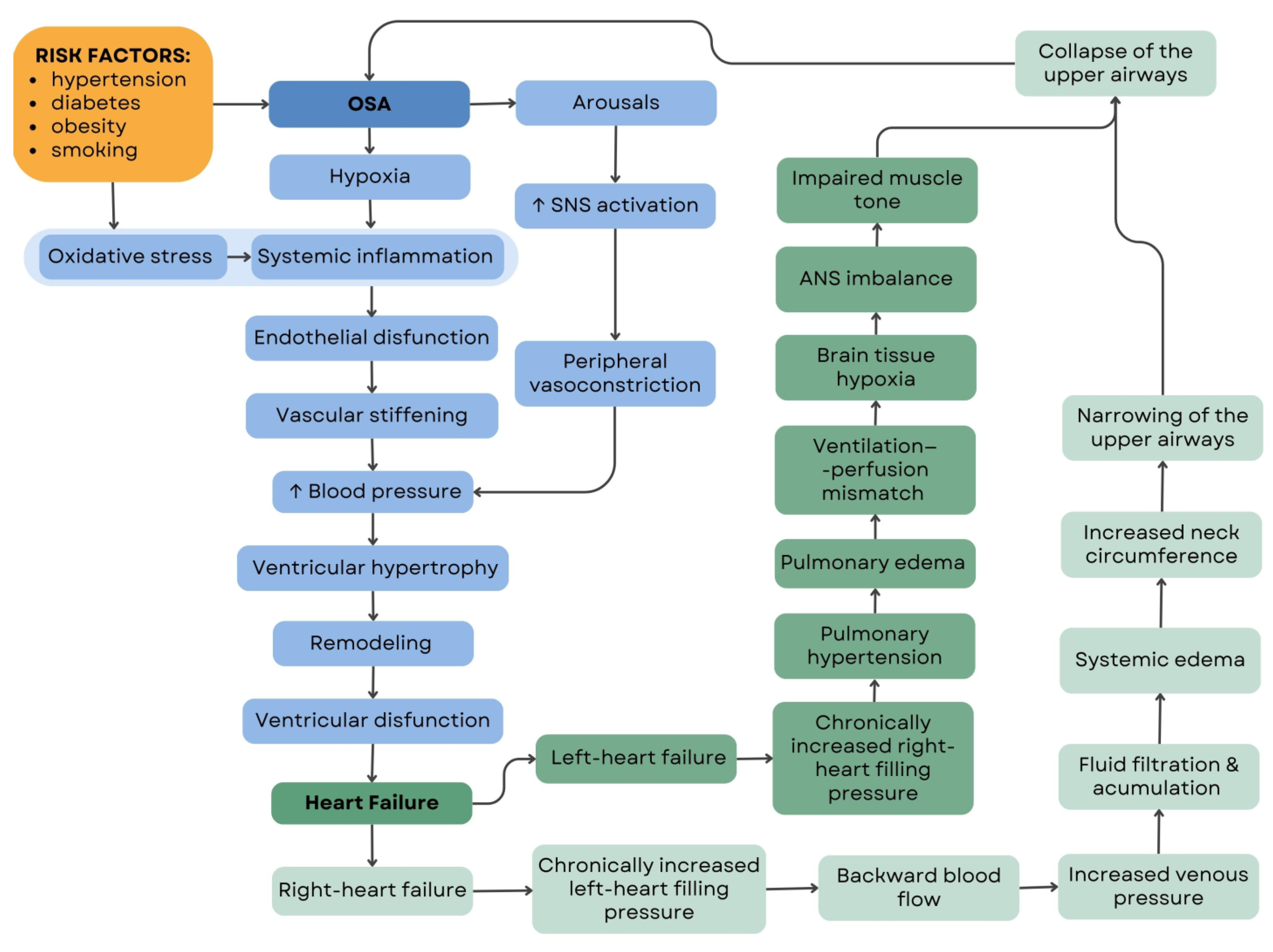

| What Is the Prevalence of OSA in HF Patients?/How Do Sleep and Cardiac Parameters Change after PAP Therapy in These Patients?/Does New Cardiological Pharmacotherapy (SGLT2i and Sacubitril/Valsartan) Play a Role in the Treatment of OSA? | |
|---|---|
| The population | Patients with OSA and HF (OSA + HF)/patients with OSA and SGLT2i or sacubitril/valsartan in treatment |
| The indicator | AHI, LVEF, NT-proBNP concentration. |
| The control | Groups of patients without OSA or patients with other SDB/ patients without PAP treatment/patients without SGLT2i or sacubitril/valsartan in the treatment |
| The outcome | The difference in AHI/LVEF/NT-proBNP/BNP concentration |
| The study design | Peer-reviewed English articles. Adult (>18 years) human subjects. Case–control studies, randomized control trials, and observational studies. |
| Author, Year | N | Sex, M/F | Age, Years | BMI, kg/m2 | EF, % | AHI | OSA Prevalence, % | Overall OSA Prevalence, % | SDB Prevalence, % |
|---|---|---|---|---|---|---|---|---|---|
| Kalaydzhiev et al., 2023 [28] | 100 screened; 61 SDB; 50 OSA; 11 CSA | 32/29 | 66.2 ± 9.1 OSA; 66.1 ± 11.9 CSA | 38.5 ± 7.1 OSA; 31.9 ± 4.5 CSA | 49.6 ± 8.5 OSA; 41.8 ± 11.4 CSA | 41.8 ± 23.2 OSA; 37.7 ± 12.6 CSA | 81.97 (50/61) | 50 (50/100) | 61 (61/100) |
| Wang et al., 2022 [20] | 252, 36 r; 43 mr; 173 p | 134/118 | 70.1, 68.3 ± 12.6 r; 65.0 ± 14.7 mr; 71.8 ± 11.8 p | 24.8 24.0 (21.1, 27.3)r; 24.8 (23.1, 27.0)mr; 24.5(22.0, 26.9)p | <40 r; 40–50 mr; ≥50 p | 26.4 ± 16.2 r; 26.1 ± 15.2 mr; 14.4 ± 15.6 p *◊ | 42 (15/36)r; 47 (20/43)mr; 49 (85/173)p | 48 (120/252) | 86 (31/252) r; 86 (37/252) mr; 62 (108/252) p *◊ |
| Wang et al., 2022 [21] | 248, 89 I; 43 H; 36 M; 27 V; 53 A | 132/116 | 70.4 ± 12.4, I: 73.0 (66.0–81.5); H: 75.0 (68.0–82.0); M: 67.0 (56.0–75.0); V: 73.0 (62.0–82.0); A: 70.0 (61.5–79.0) | 24.5, I: 24.0 (21.9–26.7); H: 25.5 (22.9–28.7); M: 24.3 (21.2–27.2); V: 23.9 (20.1–25.8); A: 24.8 (22.2–27.8) | ND | I: 18.3 (5.0–31.4); H: 12.8 (6.1–28.0); M: 20.3 (9.3–34.5); V: 6.6 (1.7–22.5) ◊; A: 6.9 (3.6–20.5) | ND | 47.6, 38 (42.7%)I; 31 (72.1%)H; 13 (36.1%)M; 10 (37.0%)V; 26 (49.1%)A | 70.6 (175/248) |
| Gupta et al., 2020 [22] | 50 (25P/25C) | 40/10 | 58.4 + 9.8 OSA; 60 + 10 CSA | 27.9 + 1.6 OSA; 29.4 + 0.6 CSA | 55.84 + 2.01 ◊ SDB; 52.08 + 3.24 ◊ noSDB | 9.9 + 4.2 ◊ SDB; 3.7 + 1.1 ◊ noSDB | 52 (13/25) | 26 (13/50) | 32 (16/50) |
| Arzt et al., 2017 [23] | 9221screened, 1557SDB; 452 OSA; 624 OSA + CSA; 481 CSA | 1353/204 | 66 ± 11 OSA; 69 ± 10 OSA + CSA; 69 ± 10 CSA | 31 ± 6 OSA; 29 ± 5 OSA + CSA; 28 ± 4 CSA | 35 ± 8 OSA; 34 ± 8 OSA + CSA; 32 ± 8 CSA | 37 ± 19 OSA; 36 ± 16 OSA + CSA; 38 ± 15 CSA | 29 (452/1557) | 4.90 (452/9221) | 16.89 (1557/9221) |
| Herrscher et al., 2011 [27] | 115, 62 OSA, 31 CSA, 22 noSDB | 91/24 | 62.0 ± 9.7; 62.46 ± 9.2 OSA; 62.26 ± 10.6 CSA; 60.26 ± 10.0 noSDB | 30.2 ± 6.0 OSA; 28.4 ± 4.2 CSA; 27.1 ± 4.8 noSDB | 40.4 ± 13.2 OSA; 34.0 ± 12.5 CSA; 37.3 ± 12.0 noSDB | 25.06 ± 21.7 OSA #◊; 26.86 ± 13.1 CSA #◊; 2.36 ± 1.5 noSDB | 66.67 (62/93) | 53.91 (62/115) | 80.87 (93/115) |
| Bitter et al., 2009 [24] | 244, 72 CSA;97 OSA;75 noSDB | 157/87 | 65.3 ± 1.4; 66.9 ± 2.4 CSA ‡; 66.8 ± 1.9 OSA †; 61.6 ± 3.3 noSDB | 29.3 ± 0.9 CSA ‡; 29.3 ± 1.1 OSA†; 26.42 ± 1 noSDB | >55 | impaired relaxation: 15.0 ± 3.6; pseudonormal: 20.0 ± 3.3 †; restrictive: 23.4 ± 6.2 ‡ | 57.4 (97/169) | 39.75 (97/244) | 69.3 (169/244) |
| Yumino et al., 2009 [26] | 218, 56 OSA; 45 CSA;117 M-NSA | 168/50 | 55.66 ± 12.7 56.36 ± 12.1 OSA; 60.46 ± 8.9 CSA; 53.46 ± 13.6 M-NSA | 29.26 ± 5.3 31.0 ± 5.0 ^ OSA; 27.8 ± 5.4 CSA **; 28.9 ± 5.3 M-NSA | 25.76 ± 9.1 OSA; 21.36 ± 9.5 CSA; 25.56 ± 10.3 M-NSA | 33.6 ± 14.5 ^ OSA; 34.8 ± 15.6 ‘ CSA; 6.8 ± 3.9 M-NSA | 55.45 (56/101) | 25.69 (56/218) | 46.33 (101/218) |
| Oldenburg et al., 2007 [16] | 700, 253 OSA; 278 CSA; 169 noSDB | 139/561 | 65.02 ± 9.5 OSA a; 65.86 ± 10.5 CSAa; 61.45 ± 11.0 noSDB | 27.84 ± 4.7 OSAa; 26.30 ± 4.1 CSA b; 25.77 ± 3.7 noSDB | 29.3 ± 2.6 OSA; 27.4 ± 6.6 CSA a; 28.2 ± 7.3 noSDB | 18.45 ± 13.3 a OSA; 30.15 ± 15.2 a,b CSA; 2.28 ± 1.6 noSDB | 48.21 (256/531) | 36 (256/700) | 76 (531/700) |
| Chan et al., 1997 [25] | 20, 11 SDB, 9 noSDB | 7/13 | 65 ± 6.0 7.3 ± 1.3 SDB; 7.2 ± 0.8 noSDB | ND | 28 ± 3.2 29.1 ± 4.2 SDB; 27.6 ± 1.3 noSDB | 19.5 ± 10.8 SDB ◊; 3.9 ± 3.5 noSDB ◊ | 63.64 (7/11) | 35 (7/20) | 55 (11/20) |
| Author, Year | N | Sex, M/F | Age, Years | EF, % Pre | EF, % Post | OSA Prevalence, % | CPAP Adherence | AHI Pre | AHI Post | BNP Pre, pg/mL | BNP Post, pg/mL |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Arikawa et al., 2016 [29] | 58 | 31/19 | 66 ± 15 (OSA) 65 ± 11 (nOSA) | 61 ± 5 (OSA) 63 ± 9 (nOSA) | ND | 67% (39/58) | ND | ND | ND | 444 (233–752) (OSA) 316 (218–703) (nOSA) | 1 m: 302 (202–350) (OSA) 212 (180–405) (nOSA) 6 m: 222 (137–324) ◊ (OSA) 76 (38–96) ◊ (nOSA) 12 m: 123 (98–197) ◊ (OSA) 52 (38–76) ◊ (nOSA) 36 m: 115 (64–174) ◊ (OSA) 56 (25–74) ◊ (nOSA) |
| Author, Year | N | Sex, M/F | Age, Years | Ef, % Pre | EF, % Post | CPAP Duration | AHI pre | AHI Post | NT-proBNP Pre | NT-proBNP Post |
|---|---|---|---|---|---|---|---|---|---|---|
| Naito et al., 2022 [30] | 55 OSA | 52/3 | 60.7 ± 12.2 | 37.2 ± 9.8 ◊ | 43.2 ± 11.7 ◊ | 1 m | 45.3 ± 16.1 ◊ | 5.4 ± 4.1 ◊ | ND | ND |
| Kaneko et al., 2003 [31] | 24 12 c/12 p | 21/3 | 55.2 ± 3.6 c 55.9 ± 2.5 p | 28.5 ± 1.8 c 25.0 ± 2.8 p | ND | 1 m | 45.2 ± 5.3 c 37.1 ± 6.4 p◊ | 44.7 ± 6.8 c 8.3 ± 2.8 p◊ | ND | ND |
| Mansfield et al., 2004 [32] | 55 27 c/28 p | 52/3 | 57.5 ± 1.6 c 57.2 ± 1.7 p | 33.6 ± 2.6 c 37.6 ± 2.5 p Δ1.5 ± 1.4 ◊ | 35.1 ± 3.1 c 42.6 ± 0.3 p Δ5.0 ± 1.0 ◊ | 3 m | 26.6 ± 4.5 c 25.0 ± 4.1 p Δ−8.4 ± 3.6 ◊ | 18.2 ± 2.8 c 2.9 ± 0.8 p Δ−21.1 ± 3.8 ◊ | ND | ND |
| Fox et al., 2023 [33] | 58 33 c/25 pa | 51/7 | 64.9 ± 10.1 c 67.4 ± 9.8 pa | 36.31 ± 6.91 c 39.29 ± 6.51 p◊ | 39.23 ± 9.41 c 44.35 ± 8.96 p◊ | 6 m | 35 ± 13 c 34 ± 17 p◊ | 33 ± 20 c 9 ± 8 p◊ | ND | ND |
| Kim et al., 2019 [34] | 52 26 c/26 p | 48/4 | 48.8 ± 10.7 c 49.1 ± 11.4 p | 64 ± 6 c 66 ± 5 p | 64 ± 6 c 65 ± 6 p | 3 m | 53.4 ± 20.5 c 64.2 ± 20.5 p | ND | ND | ND |
| Gilman et al., 2008 [35] | 19 7 c/12 p | 17/2 | 58.1 ± 7.1 c 56.7 ± 8.0 p | 30.4 ± 10.5 c 26.4 ± 10.3 p◊ | 29.5 ± 6.3 c 34.8 ± 8.3 p◊ | 1 m | 41 ± 13 c 30 ± 15 p◊ | 37 ± 18 c 7 ± 6 p◊ | ND | ND |
| Servantes et al., 2018 [36] | 65 18 c/17 e/15 p/ 15 e,p | 43/65 | 57 ± 8 c 51 ± 9 e 57 ± 7 p 53 ± 10 e,p | 29 ± 6 c 31 ± 5 e 31 ± 6 p 33 ± 5 e,p | ND | 3 m | 29 ± 17 c 28 ± 17 e◊ 32 ± 25 p◊ 25 ± 15 e,p◊ | 31 ± 14 c 18 ± 12 e◊ 8 ± 11 p◊ 10 ± 16 e,p◊ | ND | ND |
| Egea et al., 2008 [37] | 60 32 c/28 p | 56/60 | 63 ± 1.6 c 64 ± 0.9 p | 28.1 ± 1.5 c 28.0 ± 0.5 p◊ | 28.1 ± 1.7 c 30.5 ± 0.8 p◊ | 3 m | 35.3 ± 3.1 c 43.7 ± 4.4 p◊ | 28.0 ± 4.6 c 10.8 ± 2.2 p◊ | ND | ND |
| Ryan et al., 2005 [38] | 18 8 c/10 p | 16/2 | 60.3 ± 4.1 c 57.6 ± 2.2 p | 34.1 ± 3.0 c 27.6 ± 3.4 p◊ | 29.6 ± 3.1 c 34.3 ± 2.8 p◊ | 1 m | 57.9 ± 5.50 c 29.3 ± 4.8 p◊ | 56.2 ± 5.3 c 6.1 ± 1.1 p◊ | ND | ND |
| Author, Year | N | Sex, M/F | Age, Years | PAP Adherence | PAP Usage | Effect of PAP Adherence on Hospitalizations and ER Visits |
|---|---|---|---|---|---|---|
| Cistulli et al., 2023 [39] | 4237 | 1950/2287 | 64.1 | 64.1% Adherent (n = 1701) Intermediate (n = 1250) Nonadherent (n = 1286) | Hours per day: A: 6.8 ± 1.5 ◊ I: 2.9 ± 1.4 ◊ N: 0.4 ± 0.6 ◊ Days per week: A: 6.6 ± 0.5 ◊ I: 3.8 ± 1.7 ◊ N: 0.9 ± 1.2 ◊ Hours per use day: A: 7.2 ± 1.4 ◊ I: 5.4 ± 1.3 ◊ N: 2.9 ± 1.7 ◊ | Composite: A: 1.22 ± 2.06; I: 1.88 ± 3.12; N: 1.99 ± 3.21 A-N ◊ A-I ◊ I-N ER: A: 0.89 ± 1.66; I: 1.37 ± 2.54, N: 1.41 ± 2.68 A-N ◊ A-I ◊ I-N All-cause hospitalization: A: 0.33 ± 0.84; I: 0.51 ± 1.23, N: 0.59 ± 1.17 A-N ◊ A-I ◊ I-N ◊ Cardiovascular hospitalization: A: 0.06 ± 0.27, I: 0.13 ± 0.61, N: 0.13 ± 0.47 A-N ◊ A-I ◊ I-N |
| Malhotra et al., 2023 [41] | 3182 | 2223/959 | 59.7 ± 11.2 | Adherent 39%, (n = 1252); Intermediate 29%, (n = 935); Nonadherent 31%, (n = 995) | Hours per day: A: 6.6 ± 1.5 ◊ I: 2.8 ± 1.4 ◊ N: 0.4 ± 0.6 ◊ Days per week: A: 6.6 ± 0.5 ◊ I: 3.8 ± 1.7 ◊ N: 0.9 ± 1.1◊ Hours per use day: A: 7.1 ± 1.4 ◊ I: 5.4 ± 1.3 ◊ N: 2.9 ± 1.6 ◊ | Composite: A: 1.00 ± 1.73; I: 1.30 ± 2.09; N: 1.37 ± 2.56 A-N ◊ A-I ◊ I-N ER: A: 0.71 ± 1.38; I: 0.91 ± 1.65; N: 1.00 ± 2.06 A-N ◊ A-I ◊ I-N All-cause hospitalization: A: 0.29 ± 0.77; I: 0.38 ± 0.93; N: 0.37 ± 0.99 A-N A-I ◊ I-N Cardiovascular hospitalization: A: 0.10 ± 0.43; I: 0.12 ± 0.47; N: 0.12 ± 0.47 A-N A-I I-N |
| Abdullah et al., 2018 [40] | 12,608,637, OSA 653,762; nOSA 11,954,875 | 5,442,091/7,166,546 | OSA 62.5 ± 13.7 nOSA 58.6 ± 20.8 | ND | ND | ND |
| Author, Year | N | Sex, M/F | Age, Years | Rate/1000 Patient- Years | 3P-MACE | CV Death | HHF | All-Case Mortality | Incident or Worsening Nephropaty | Changes in Sleep Parame- Ters |
|---|---|---|---|---|---|---|---|---|---|---|
| Wojeck et al., 2023 [42] | 5126 E, 2557 P | 69.2(%) | 64.3 | 1.4 E 2.6 P ◊ | ND | ND | ND | ND | ND | ND |
| Neeland et al., 2020 [43] | 7020 w/OSA: 4421 Em, 2208 P; 391 OSA: 266 Em, 125 P | 5016/2004 | 63.1 ± 8.6 w/OSA, Em; 63.2 ± 8.9 w/OSA, P; 63.7 ± 7.7 OSA, Em; 63.7 ± 7.3 OSA, P | 2.2 E; 4.6 P ◊ | 490/4687 Em; 282/2333 P ◊ | 172/4687 Em; 137/2333 P ◊ | 126/4687 Em; 95/2333P ◊ | 269/4687 Em; 194/2333 P ◊ | 525/4687 Em; 388/2333 P ◊; 459/4687 Em; 330/2333 P ◊ | ND |
| Furukawa et al., 2018 [44] | 30, 24 mSDB; 6 m-sSDB | 20/10 | 59.0 ± 10.7 mSDB; 58.3 ± 11.7 m-sSDB | ND | ND | ND | ND | ND | ND | 3% ODI, baseline: 25.0 ± 3.8; follow-up: 18.5 ± 6.1 ◊ |
| Tang et al., 2019 [45] | 36, 18 dapa; 18 w/dapa | 22/14 | 56.10 ± 7.2 dapa; 57.8 ± 10.07 w/dapa | ND | ND | ND | ND | ND | ND | AHI dapa: baseline 37.45 ± 6.04 vs. follow-up 26.72 ± 4.69 ◊; w/dapa: baseline38.11 ± 6.27 vs. follow-up 36.1 ± 4.50; LSpO2: dapa: baseline 84.06 ± 14.58 vs. follow-up 87.16 ± 13.56 ◊; w/dapa: baseline 83.72 ± 13.77 follow-up 84.12 ± 13.83 |
| Sawada et al., 2018 [46] | 18 | 14/4 | 64 ± 13 | ND | ND | ND | ND | ND | ND | AHI baseline: 31.9 ± 18.0; follow-up 18.8 ± 11.5 ◊ |
| Author, Year | N | Sex, M/F | Age, Years | Rate/1000 Patient- Years | AHI Pre | AHI Post | N-TproBNP | MOS | ODI |
|---|---|---|---|---|---|---|---|---|---|
| Owens et al., 2021 [48] | 140, 70 S/; 70 E | 108/32 | 62.3 ± 8.8 S/V; 64.2 ± 11.6 E | ND | 16.3 ± 14.2 S/V; 16.8 ± 14.3 E | 15.2 ± 15.6 S/V; 17.6 ± 16.3 E | ND | ND | ND |
| Pelaia et al., 2022 [49] | 132 | 107/25 | 67.0 ± 9.8 | ND | 26.5 ± 10.4 | 21.7 ± 8.3 ◊ | baseline: 1840 (886.0–3378); follow-up: 970.0 (571.3–2870) ◊ | baseline: 91.3 ± 1.9; follow-up: 92.0 ± 2.0 ◊ | baseline: 18.0 ± 3.7; follow-up: 13.5 ± 4.9 ◊ |
| Jaffuel et al., 2021 [50] | 118, 49G1; 27G2; 42G3 | 96/22 | 66.00 (56.00–73.00) | ND | 24.20 (16.40–43.50) G1 + G2; 22.90 (16.00–43.50) G1; 30.10 (26.40–47.60) G2 | 20.40 (12.70–31.10) G1 + G2 ◊; 19.20 (12.70–31.10) G1◊; 22.75 (14.60–36.90) G2 | G1 baseline: 1811.00 (987.00; 3958.00), follow-up: 1104.00 (391.00; 3075.00) ◊; G2 baseline: 2043.00 (845.0; 3445.00), follow-up: 1351.00 (44.00; 2164.00) ◊; G3 baseline: 852.00 (244.0; 2102.0), follow-up: 591.50 (205.0; 1128.5) ◊ | G1 + G2 baseline: 92.30 (91.35–94.55), follow up: 93.05 (91.60–94.70); G1 baseline: 93.00 (91.80–94.60), follow-up: 93.40 (92.20–94.90) G2 baseline: 91.3 (90.00–93.00), follow-up: 91.80 (91.00–92.10) | G1 + G2 baseline: −6.32 ( ± 15.79), follow-up:−6.20 (−12.70 to 0.90) ◊; G1 baseline: 11.90 (7.10–14.65), follow-up: 7.65 (4.90–13.65); G2 baseline: 31.00 (15.30–55.90), follow-up: 24.00 (11.00–45.90) |
| Wang et al., 2023 [51] | 18, 9 OSA, 7 CSA, 2 NB | 15/3 | 66.7 ± 10.7 | ND | overall population 20 ± 23 *◊; OSA 14 ± 6 *◊; CSA 36 ± 32 ◊* | overall population 7 ± 7 ◊*; OSA 7 ± 7 ◊*; CSA 7 ± 8 ◊* | baseline 1792.1 ± 1271.3; Three months follow-up 876.9 ± 984.2 ◊ | ND | ND |
| Passino et al., 2021 [52] | 51, 15 OSA, 33 CSA | 39/12 | 65 ± 9 | ND | overall population: daytime 7 (2–20); nighttime 19 (7–37); 24 h 13 (5–26); OSA: daytime 6 (2–12); nighttime 18 (10–30); 24 h 13 (5–16); CSA: daytime 10 (2–22); nighttime 23 (9–41); 24 h 14 (6–31) | overall population: daytime 3 (0–7) ◊; nighttime 16 (7–23) ◊; 24 h 8 (3–14) ◊; OSA: daytime 1 (0–3) ◊; nighttime 15 (9–27); 24 h 7 (3–8) ◊; CSA: daytime 3 (1–10) ◊; nighttime 16 (7–23) ◊; 24 h 7 (3–16) ◊ | baseline 1439 (701–3015); Six months follow-up 604 (320–1268) | ND | ND |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Polecka, A.; Olszewska, N.; Danielski, Ł.; Olszewska, E. Association between Obstructive Sleep Apnea and Heart Failure in Adults—A Systematic Review. J. Clin. Med. 2023, 12, 6139. https://doi.org/10.3390/jcm12196139
Polecka A, Olszewska N, Danielski Ł, Olszewska E. Association between Obstructive Sleep Apnea and Heart Failure in Adults—A Systematic Review. Journal of Clinical Medicine. 2023; 12(19):6139. https://doi.org/10.3390/jcm12196139
Chicago/Turabian StylePolecka, Agnieszka, Natalia Olszewska, Łukasz Danielski, and Ewa Olszewska. 2023. "Association between Obstructive Sleep Apnea and Heart Failure in Adults—A Systematic Review" Journal of Clinical Medicine 12, no. 19: 6139. https://doi.org/10.3390/jcm12196139
APA StylePolecka, A., Olszewska, N., Danielski, Ł., & Olszewska, E. (2023). Association between Obstructive Sleep Apnea and Heart Failure in Adults—A Systematic Review. Journal of Clinical Medicine, 12(19), 6139. https://doi.org/10.3390/jcm12196139






