Progression of Non-Significant Mitral and Tricuspid Regurgitation after Surgical Aortic Valve Replacement for Aortic Regurgitation
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Outcomes
2.2. Procedural Aspects
2.3. Echocardiographic Assessment
2.4. Data Collection
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics of the Study Cohort
3.2. Procedural Aspects
3.3. Outcomes
3.4. Correlates of the Primary Outcome
3.5. Predictors of the Primary Outcome
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AR | Aortic regurgitation |
| AV | Aortic valve |
| HF | Heart failure |
| MR | Mitral regurgitation |
| SAVR | Surgical aortic valve replacement |
| TR | Tricuspid regurgitation |
References
- Yang, L.-T.; Enriquez-Sarano, M.; Scott, C.G.; Padang, R.; Maalouf, J.F.; Pellikka, P.A.; Michelena, H.I. Concomitant Mitral Regurgitation in Patients with Chronic Aortic Regurgitation. J. Am. Coll. Cardiol. 2020, 76, 233–246. [Google Scholar] [CrossRef] [PubMed]
- MacHaalany, J.; Bertrand, O.F.; Voisine, P.; O’Connor, K.; Bernier, M.; Dubois-Sénéchal, I.-N.; Jacques, P.-O.; Viel, I.; Dubois, M.; Sénéchal, M. Outcomes Following Surgical Correction of Pure Aortic Regurgitation in Presence or Absence of Significant Functional Mitral Regurgitation. Echocardiography 2014, 31, 689–698. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.-T.; Enriquez-Sarano, M.; Scott, C.G.; Padang, R.; Maalouf, J.F.; Pellikka, P.A.; Michelena, H.I. Staging Cardiac Damage in Patients with Aortic Regurgitation. Int. J. Cardiovasc. Imaging 2022, 38, 2645–2653. [Google Scholar] [CrossRef]
- Silva, G.; Queirós, P.; Silva, M.; Saraiva, F.; Barros, A.; Ribeiro, J.; Fontes-Carvalho, R.; Sampaio, F. 2020 Focused Update of the 2017 ACC Expert Consensus Decision Pathway on the Management of Mitral Regurgitation: A Report of the American College of Cardiology Solution Set Oversight Committee. J. Am. Coll. Cardiol. 2020, 75, 2236–2270. [Google Scholar] [CrossRef]
- Writing Committee Members; Otto, C.M.; Nishimura, R.A.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P., III.; Gentile, F.; Toly, C. 2020 ACC/AHA Guideline for the Management of Patients with Valvular Heart Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2021, 77, 450–500. [Google Scholar] [CrossRef] [PubMed]
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. ESC/EACTS Scientific Document Group, ESC National Cardiac Societies. 2021 ESC/EACTS Guidelines for the Management of Valvular Heart Disease: Developed by the Task Force for the Management of Valvular Heart Disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2022, 43, 561–632. [Google Scholar] [CrossRef] [PubMed]
- Pai, R.G.; Varadarajan, P. Prognostic Implications of Mitral Regurgitation in Patients with Severe Aortic Regurgitation. Circulation. 2010, 122, S43–S47. [Google Scholar] [CrossRef]
- Lim, J.Y.; Jung, S.H.; Kim, J.B.; Chung, C.H.; Lee, J.W.; Song, H.; Choo, S.-J. Management of Concomitant Mild to Moderate Functional Mitral Regurgitation During Aortic Valve Surgery for Severe Aortic Insufficiency. J. Thorac. Cardiovasc. Surg. 2014, 148, 441–446. [Google Scholar] [CrossRef][Green Version]
- McCarthy, F.H.; Desai, N.D.; Fox, Z.; George, J.; Moeller, P.; Vallabhajosyula, P.; Szeto, W.Y.; Bavaria, J.E. Moderate Mitral Regurgitation in Aortic Root Replacement Surgery: Comparing Mitral Repair with No Mitral Repair. J. Thorac. Cardiovasc. Surg. 2014, 147, 938–941. [Google Scholar] [CrossRef][Green Version]
- Chancellor, W.Z.; Mehaffey, J.H.; Beller, J.P.; Hawkins, R.B.; Speir, A.M.; Quader, M.A.; Yarboro, L.T.; Teman, N.R.; Ailawadi, G. Impact of Tricuspid Regurgitation with and without Repair During Aortic Valve Replacement. J. Thorac. Cardiovasc. Surg. 2021, 162, 44–50.e2. [Google Scholar] [CrossRef]
- Bustamante-Munguira, J.; Alvarez, P.; Romero, B.; Muñoz-Guijosa, C.; Camara, M.; Vallejo, N.; Lopez-Ayerbe, J.; Coca, A.; Figuerola-Tejerina, A. Impact of Tricuspid Regurgitation Severity and Repair on Aortic Valve Replacement. Ann. Thorac. Surg. 2022, 114, 767–775. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Voigt, J.U. Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 233–271. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, C.; Rahko, P.S.; Blauwet, L.A.; Canaday, B.; Finstuen, J.A.; Foster, M.C.; Horton, K.; Ogunyankin, K.O.; Palma, R.A.; Velazquez, E.J. Guidelines for Performing a Comprehensive Transthoracic Echocardiographic Examination in Adults: Recommendations from the American Society of Echocardiography. J. Am. Soc. Echocardiogr. 2019, 32, 1–64. [Google Scholar] [CrossRef] [PubMed]
- Zoghbi, W.A.; Adams, D.; Bonow, R.O.; Enriquez-Sarano, M.; Foster, E.; Grayburn, P.A.; Hahn, R.T.; Han, Y.; Hung, J.; Lang, R.M.; et al. Recommendations for Noninvasive Evaluation of Native Valvular Regurgitation: A Report from the American Society of Echocardiography Developed in Collaboration with the Society for Cardiovascular Magnetic Resonance. J. Am. Soc. Echocardiogr. 2017, 30, 303–371. [Google Scholar] [CrossRef]
- Doherty, J.U.; Kort, S.; Mehran, R.; Schoenhagen, P.; Soman, P.; Amin, Z.; Bashore, T.M.; Boyle, A.; Calnon, D.A.; Carabello, B.; et al. ACC/AATS/AHA/ASE/ASNC/HRS/SCAI/SCCT/SCMR/STS 2017 Appropriate Use Criteria for Multimodality Imaging in Valvular Heart Disease: A Report of the American College of Cardiology Appropriate Use Criteria Task Force, American Association for Thoracic Surgery, American Heart Association, American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance, and Society of Thoracic Surgeons. J. Am. Soc. Echocardiogr. 2018, 31, 381–404. [Google Scholar] [CrossRef]
- Lancellotti, P.; Pibarot, P.; Chambers, J.; La Canna, G.; Pepi, M.; Dulgheru, R.; Dweck, M.; Delgado, V.; Garbi, M.; Vannan, M.A.; et al. Scientific Document Committee of the European Association of Cardiovascular Imaging. Multi-modality imaging assessment of native valvular regurgitation: An EACVI and ESC Council of Valvular Heart Disease Position Paper. Eur. Heart J. Cardiovasc. Imaging 2022, 23, e171–e232. [Google Scholar] [CrossRef]
- Siani, A.; Perone, F.; Costantini, P.; Rodolfi, S.; Muscogiuri, G.; Sironi, S.; Carriero, S.; Pavon, A.G.; van der Bilt, I.; van Rosendael, P.; et al. Aortic regurgitation: A multimodality approach. J. Clin. Ultrasound 2022, 50, 1041–1050. [Google Scholar] [CrossRef]
- Kato, N.; Thaden, J.J.; Miranda, W.R.; Scott, C.G.; Sarano, M.E.; Greason, K.L.; Pellikka, P.A. Impact of Aortic Valve Replacement for Severe Aortic Stenosis on Organic and Functional Mitral Regurgitation. ESC Heart Fail. 2021, 8, 5482–5492. [Google Scholar] [CrossRef]
- Witberg, G.; Codner, P.; Landes, U.; Schwartzenberg, S.; Barbanti, M.; Valvo, R.; De Backer, O.; Ooms, J.F.; Islas, F.; Marroquin, L.; et al. Effect of Transcatheter Aortic Valve Replacement on Concomitant Mitral Regurgitation and Its Impact on Mortality. J. Am. Coll. Cardiol. Interv. 2021, 14, 1181–1192. [Google Scholar] [CrossRef]
- Topilsky, Y.; Nkomo, V.T.; Vatury, O.; Michelena, H.I.; Letourneau, T.; Suri, R.M.; Pislaru, S.; Park, S.; Mahoney, D.W.; Biner, S.; et al. Clinical Outcome of Isolated Tricuspid Regurgitation. J. Am. Coll. Cardiol. Imaging 2014, 7, 1185–1194. [Google Scholar] [CrossRef]
- Chorin, E.; Rozenbaum, Z.; Topilsky, Y.; Konigstein, M.; Ziv-Baran, T.; Richert, E.; Keren, G.; Banai, S. Tricuspid Regurgitation and Long-Term Clinical Outcomes. Eur. Heart J. Cardiovasc. Imaging 2020, 21, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Offen, S.; Playford, D.; Strange, G.; Stewart, S.; Celermajer, D.S. Adverse Prognostic Impact of Even Mild or Moderate Tricuspid Regurgitation: Insights from the National Echocardiography Database of Australia. J. Am. Soc. Echocardiogr. 2022, 35, 810–817. [Google Scholar] [CrossRef] [PubMed]
- Cremer, P.C.; Wang, T.K.M.; Rodriguez, L.L.; Lindman, B.R.; Zhang, Y.; Zajarias, A.; Hahn, R.T.; Lerakis, S.; Malaisrie, S.C.; Douglas, P.S.; et al. PARTNER II Investigators. Incidence and Clinical Significance of Worsening Tricuspid Regurgitation Following Surgical or Transcatheter Aortic Valve Replacement: Analysis from the PARTNER IIA Trial. Circ. Cardiovasc. Interv. 2021, 14, e010437. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Guo, R.; Liu, D.; Hou, S.; Qiao, C.; Zhang, X. The Fate of Concomitant Mild Mitral Regurgitation in Aortic Insufficiency: A Neglected Subject. Front. Cardiovasc. Med. 2023, 9, 1035490. [Google Scholar] [CrossRef]
- Girdauskas, E.; Disha, K.; Espinoza, A.; Misfeld, M.; Reichenspurner, H.; Borger, M.A.; Kuntze, T. Mitral Regurgitation After Previous Aortic Valve Surgery for Bicuspid Aortic Valve Insufficiency. J. Cardiovasc. Surg. 2017, 58, 473–480. [Google Scholar] [CrossRef]
- Deferm, S.; Bertrand, P.B.; Verbrugge, F.H.; Verhaert, D.; Rega, F.; Thomas, J.D.; Vandervoort, P.M. Atrial Functional Mitral Regurgitation: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2019, 73, 2465–2476. [Google Scholar] [CrossRef]
- Mesi, O.; Gad, M.M.; Crane, A.D.; Ramchand, J.; Puri, R.; Layoun, H.; Miyasaka, R.; Gillinov, M.A.; Wierup, P.; Griffin, B.P.; et al. Severe Atrial Functional Mitral Regurgitation: Clinical and Echocardiographic Characteristics, Management and Outcomes. J. Am. Coll. Cardiol. Imaging 2021, 14, 797–808. [Google Scholar] [CrossRef]
- Authors/Task Force Members; McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J. ESC Scientific Document Group. 2021 ESC Guidelines for the Diagnosis and Treatment of Acute and Chronic Heart Failure: Developed by the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure of the European Society of Cardiology (ESC). With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. J. Heart Fail. 2022, 24, 4–131. [Google Scholar] [CrossRef]
- Perone, F.; Peruzzi, M.; Conte, E.; Sciarra, L.; Frati, G.; Cavarretta, E.; Pingitore, A. An Overview of Sport Participation and Exercise Prescription in Mitral Valve Disease. J. Cardiovasc. Dev. Dis. 2023, 10, 304. [Google Scholar] [CrossRef]
- Writing Committee Members; Isselbacher, E.M.; Preventza, O.; Iii, J.H.B.; Augoustides, J.G.; Beck, A.W.; Bolen, M.A.; Braverman, A.C.; Bray, B.E.; Brown-Zimmerman, M.M.; et al. 2022 ACC/AHA Guideline for the Diagnosis and Management of Aortic Disease: A Report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2022, 80, e223–e393. [Google Scholar] [CrossRef]
- Wawak, M.; Tekieli, Ł.; Badacz, R.; Pieniążek, P.; Maciejewski, D.; Trystuła, M.; Przewłocki, T.; Kabłak-Ziembicka, A. Clinical Characteristics and Outcomes of Aortic Arch Emergencies: Takayasu Disease, Fibromuscular Dysplasia, and Aortic Arch Pathologies: A Retrospective Study and Review of the Literature. Biomedicines 2023, 11, 2207. [Google Scholar] [CrossRef] [PubMed]
- Dreyfus, G.D.; Corbi, P.J.; Chan, K.M.; Bahrami, T. Secondary Tricuspid Regurgitation or Dilatation: Which Should be the Criteria for Surgical Repair? Ann. Thorac. Surg. 2005, 79, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Chikwe, J.; Itagaki, S.; Anyanwu, A.; Adams, D.H. Impact of Concomitant Tricuspid Annuloplasty on Tricuspid Regurgitation, Right Ventricular Function, and Pulmonary Artery Hypertension After Repair of Mitral Valve Prolapse. J. Am. Coll. Cardiol. 2015, 65, 1931–1938. [Google Scholar] [CrossRef] [PubMed]
- Badhwar, V.; Rankin, J.S.; He, M.; Jacobs, J.P.; Furnary, A.P.; Fazzalari, F.L.; O’brien, S.; Gammie, J.S.; Shahian, D.M. Performing Concomitant Tricuspid Valve Repair at the Time of Mitral Valve Operations Is Not Associated with Increased Operative Mortality. Ann. Thorac. Surg. 2017, 103, 587–593. [Google Scholar] [CrossRef] [PubMed]
- Brescia, A.A.; Ward, S.T.; Watt, T.M.F.; Rosenbloom, L.M.; Baker, M.; Khan, S.; Ziese, E.; Romano, M.A.; Bolling, S.F.; Michigan Mitral Research Group (MMRG). Outcomes of Guideline-Directed Concomitant Annuloplasty for Functional Tricuspid Regurgitation. Ann. Thorac. Surg. 2020, 109, 1227–1232. [Google Scholar] [CrossRef]


| Total Cohort (n = 184) | Primary Outcome (n = 36) | No Primary Outcome (n = 148) | p-Value | |
|---|---|---|---|---|
| Demographic Data | ||||
| Age | ||||
| Median (years) | 64 (55–74) | 70 (57–76) | 62 (54–73) | 0.112 |
| ≥65 years | 89 (48.4) | 21 (58.3) | 68 (45.9) | 0.182 |
| Sex Male | 141 (76.6) | 24 (66.7) | 117 (79.1) | 0.115 |
| Comorbidities | ||||
| Body Surface Area, Mosteller Formula (m2) | 1.9 (1.8–2.1) | 1.9 (1.7–2.0) | 1.9 (1.8–2.1) | 0.207 |
| Body Mass Index (kg/m2) | 27.8 (24.5–30.3) | 28.1 (24.0–29.3) | 27.6 (24.6–31.1) | 0.845 |
| Obesity | 57 (33.9) | 8 (24.2) | 49 (36.3) | 0.190 |
| Hypertension | 122 (70.1) | 25 (69.4) | 97 (70.3) | 0.921 |
| Diabetes Mellitus | 53 (30.6) | 11 (30.6) | 42 (30.7) | 0.991 |
| Dyslipidemia | 135 (78.0) | 29 (80.6) | 106 (77.4) | 0.681 |
| Smoking History | 35 (20.2) | 5 (13.9) | 30 (21.9) | 0.287 |
| Estimated Glomerular Filtration Rate, Cockcroft Formula (mL/kg/min) | 86.7 (67.9–113.5) | 78.6 (60.9–112.3) | 89.1 (70.1–114.2) | 0.298 |
| Stage ≥ III Chronic Kidney Disease | 29 (18.8) | 7 (23.3) | 22 (17.7) | 0.482 |
| Ischemic Heart Disease | 64 (36.8) | 18 (50.0) | 46 (33.3) | 0.065 |
| Prior Stroke/Transient Ischemic Attack | 23 (14.6) | 8 (25.0) | 15 (11.9) | 0.088 |
| Atrial Fibrillation/Flutter | 65 (38.2) | 22 (61.1) | 43 (32.1) | 0.001 |
| Cardiac Implantable Electronic Device | 20 (11.8) | 6 (16.7) | 14 (10.5) | 0.381 |
| Marfan Syndrome | 1 (0.6) | 0 (0.0) | 1 (0.7) | 1.000 |
| Symptomatic Status | ||||
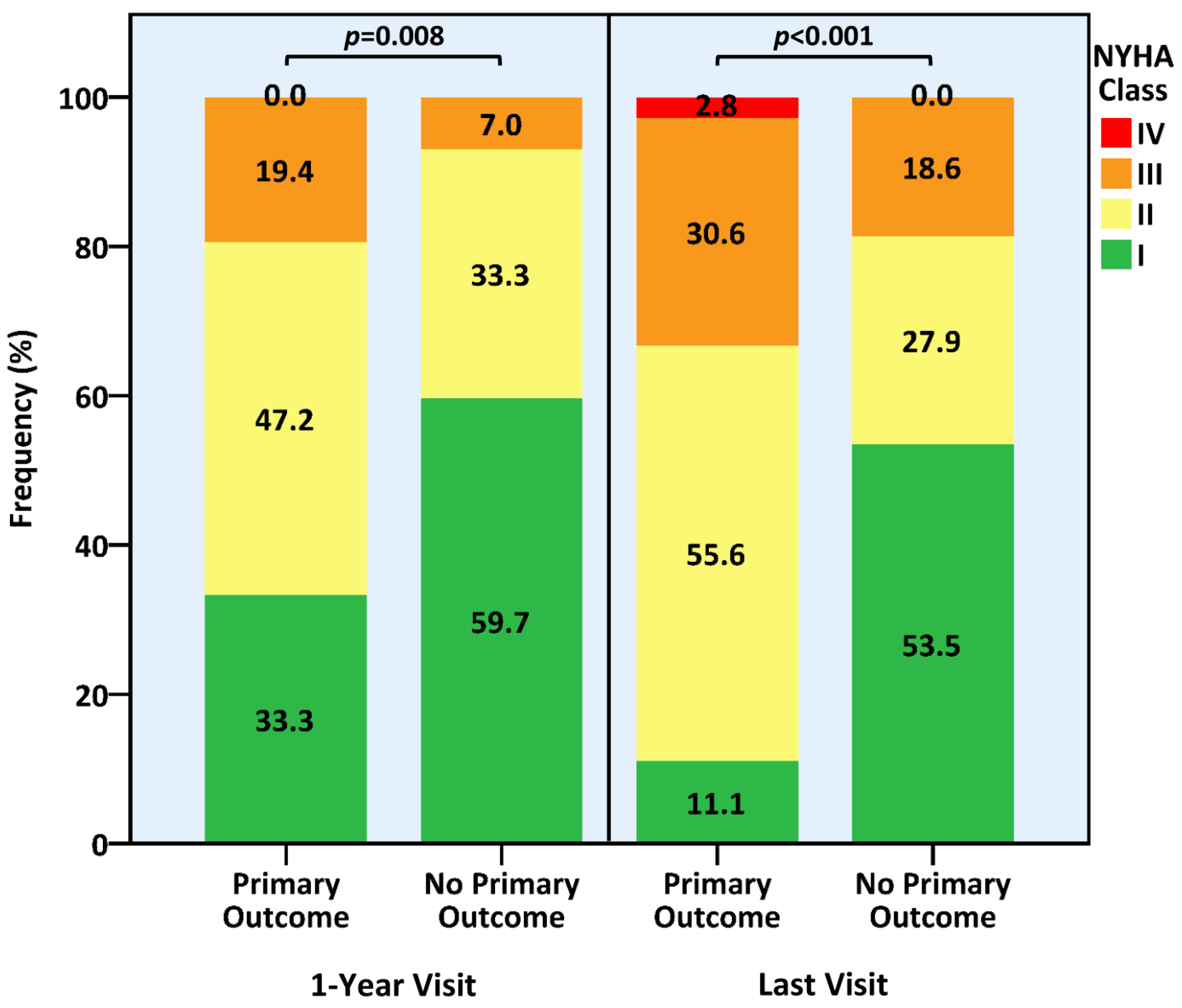
| New York Heart Association Class | 0.012 | |||
| I | 85 (47.0) | 9 (25.0) | 76 (52.4) | |
| II | 73 (40.3) | 21 (58.3) | 52 (35.9) | |
| III | 23 (12.7) | 6 (16.7) | 17 (11.7) | |
| ≥II | 96 (53.0) | 27 (75.0) | 69 (47.6) | 0.005 |
| Total Cohort (n = 184) | Primary Outcome (n = 36) | No Primary Outcome (n = 148) | p-Value | |
|---|---|---|---|---|
| Study Time Prior to Surgery (days) | 52 (13–192) | 51 (18–182) | 48 (11–208) | 0.563 |
| Aortic Valve | ||||
| Pure Aortic Regurgitation | 134 (72.8) | 30 (83.3) | 104 (70.3) | 0.114 |
| Aortic Regurgitation Severity | 0.511 | |||
| Moderate-to-Severe | 101 (54.9) | 18 (50.0) | 83 (56.1) | |
| Severe | 83 (45.1) | 18 (50.0) | 65 (43.9) | |
| Aortic Regurgitation Etiology | 0.421 | |||
| Annular Dilatation | 79 (65.8) | 16 (64.0) | 63 (66.3) | |
| Leaflet Prolapse/Flail | 14 (11.7) | 4 (16.0) | 10 (10.5) | |
| Leaflet Restriction | 10 (8.3) | 3 (12.0) | 7 (7.4) | |
| Endocarditis | 15 (12.5) | 1 (4.0) | 14 (14.7) | |
| Aortic Dissection | 2 (1.7) | 1 (4.0) | 1 (1.1) | |
| Moderate and Above Aortic Stenosis | 54 (29.3) | 6 (16.7) | 48 (32.4) | 0.062 |
| Bicuspid Aortic Valve | 60 (34.1) | 7 (20.0) | 53 (37.6) | 0.049 |
| Aorta | ||||
| Aortic Root Diameter (cm) | 3.6 (3.0–4.1) | 3.5 (2.9–4.3) | 3.6 (3.0–4.1) | 0.754 |
| Ascending Aortic Diameter | ||||
| Median (cm) | 4.1 (3.6–4.7) | 4.2 (3.9–4.9) | 4.0 (3.5–4.6) | 0.065 |
| ≥4 cm | 96 (57.1) | 21 (70.0) | 75 (54.3) | 0.116 |
| ≥4.5 cm | 50 (29.8) | 11 (36.7) | 39 (28.3) | 0.361 |
| Mitral and Tricuspid Valves | ||||
| Mitral Valve Anomalies | ||||
| Rheumatic Changes | 14 (7.6) | 3 (8.3) | 11 (7.4) | 0.739 |
| Annular Dilatation | 1 (0.5) | 0 (0.0) | 1 (0.7) | 0.621 |
| Annular Calcification | 8 (4.3) | 6 (16.7) | 2 (1.4) | 0.001 |
| Leaflet Prolapse/Flail | 44 (32.8) | 13 (43.3) | 31 (29.8) | 0.165 |
| Leaflet Restriction | 8 (4.3) | 4 (11.1) | 4 (2.7) | 0.048 |
| Leaflet Tethering/Retraction | 5 (2.7) | 4 (11.1) | 1 (0.7) | 0.005 |
| Diastolic Mitral Regurgitation | 1 (0.5) | 0 (0.0) | 1 (0.7) | 0.621 |
| Mitral and Tricuspid Regurgitation Grade | ||||
| Mitral | 0.7 ± 0.5 | 0.9 ± 0.3 | 0.6 ± 0.5 | <0.001 |
| Tricuspid | 0.4 ± 0.5 | 0.5 ± 0.5 | 0.4 ± 0.5 | 0.164 |
| Mild-to-Moderate Mitral or Tricuspid Regurgitation | ||||
| Mitral | 41 (22.3) | 15 (41.7) | 26 (17.6) | 0.002 |
| Tricuspid | 14 (7.7) | 6 (17.1) | 8 (5.4) | 0.030 |
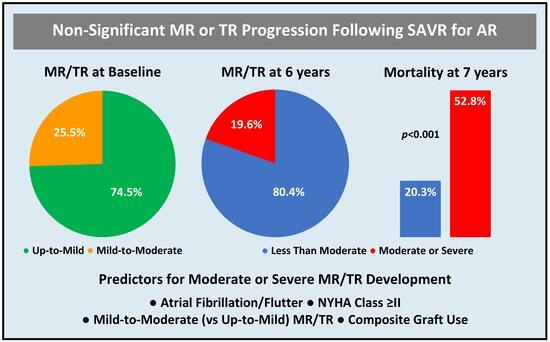
| Either | 47 (25.5) | 16 (44.4) | 31 (20.9) | 0.004 |
| Left Heart Chambers | ||||
| Left Ventricular Ejection Fraction | ||||
| Median (%) | 60 (45–60) | 50 (41–60) | 60 (50–60) | 0.005 |
| <50% | 52 (28.9) | 16 (44.4) | 36 (25.0) | 0.021 |
| Regional Wall Motion Abnormality | 19 (10.3) | 5 (13.9) | 14 (9.5) | 0.540 |
| Left Ventricular Diastolic Dysfunction | ||||
| Any | 66 (75.9) | 7 (87.5) | 59 (74.7) | 0.673 |
| Grade ≥2 | 14 (16.1) | 1 (12.5) | 13 (16.5) | 0.772 |
| Left Ventricular End-Systolic Diameter (cm) | 3.8 (3.3–4.5) | 4.1 (3.4–4.9) | 3.8 (3.3–4.4) | 0.213 |
| Left Ventricular End-Diastolic Diameter (cm) | 5.7 (5.2–6.3) | 6.1 (5.1–6.4) | 5.7 (5.2–6.1) | 0.210 |
| Left Atrial Diameter (cm) | 4.2 (3.8–4.6) | 4.4 (3.9–4.7) | 4.1 (3.8–4.6) | 0.361 |
| Left Atrial Area (cm2) | 23.8 (20.0–27.0) | 25.5 (21.5–29.0) | 23.0 (19.5–26.5) | 0.072 |
| Right Heart Chambers | ||||
| Right Ventricular Dysfunction | 8 (4.5) | 3 (8.8) | 5 (3.5) | 0.184 |
| Right Ventricular Dilatation | 3 (1.7) | 1 (2.9) | 2 (1.4) | 0.477 |
| Tricuspid Annular Systolic Plane Excursion (mm) | 22.5 (16.8–26.8) | 15.0 (13.0–16.0) | 23.0 (19.0–27.0) | 0.250 |
| Pulmonary Arterial Systolic Pressure | ||||
| Median (mmHg) | 27 (21–35) | 32 (22–39) | 26 (21–33) | 0.103 |
| >40 mmHg | 8 (5.7) | 4 (12.9) | 4 (3.6) | 0.070 |
| Total Cohort (n = 184) | Primary Outcome (n = 36) | No Primary Outcome (n = 148) | p-Value | |
|---|---|---|---|---|
| Urgent Surgery | 9 (4.9) | 2 (5.6) | 7 (4.8) | 0.850 |
| Aortic Valve Prosthesis Type | 0.295 | |||
| Biologic | 130 (70.7) | 28 (77.8) | 102 (68.9) | |
| Mechanical | 54 (29.3) | 8 (22.2) | 46 (31.1) | |
| Concomitant Aortic Vascular Intervention | ||||
| Any | 46 (25.0) | 11 (30.6) | 35 (23.6) | 0.391 |
| Composite Graft Implantation | 35 (21.6) | 11 (37.9) | 24 (18.0) | 0.018 |
| Concomitant Coronary Artery Bypass Grafting | 32 (17.5) | 8 (22.2) | 24 (16.3) | 0.404 |
| Total Cohort (n = 184) | Primary Outcome (n = 36) | No Primary Outcome (n = 148) | p-Value | |
|---|---|---|---|---|
| Study Time After Surgery (years) | 5.8 (2.8–11.0) | 5.6 (2.7–10.8) | 5.8 (2.9–11.1) | 0.724 |
| Aortic Valve | ||||
| Residual Aortic Regurgitation Severity | ||||
| Up-to-Mild | 180 (97.8) | 34 (94.4) | 146 (98.6) | 0.172 |
| Moderate | 3 (1.6) | 2 (5.6) | 1 (0.7) | 0.098 |
| Above-Moderate | 1 (0.5) | 0 (0.0) | 1 (0.7) | 1.000 |
| Residual Aortic Regurgitation Grade | 0.2 ± 0.5 | 0.4 ± 0.6 | 0.2 ± 0.5 | 0.081 |
| Moderate and Above Aortic Stenosis | 1 (0.5) | 0 (0.0) | 1 (0.7) | 1.000 |
| Mitral and Tricuspid Valves | ||||
| Moderate or Severe Mitral or Tricuspid Regurgitation | <0.001 | |||
| Mitral | 20 (10.9) | 20 (55.6) | 0 (0.0) | |
| Tricuspid | 25 (13.6) | 25 (69.4) | 0 (0.0) | |
| Either | 36 (19.7) | 36 (100.0) | 0 (0.0) | |
| Both | 9 (4.9) | 9 (25.0) | 0 (0.0) | |
| Severe Mitral or Tricuspid Regurgitation | 26 (14.1) | 26 (72.2) | 0 (0.0) | <0.001 |
| Mitral Regurgitation Grade | ||||
| Median | 0.8 ± 0.9 | 2.0 (±1.3) | 0.5 ± 0.5 | <0.001 |
| Change from Baseline | 0.2 ± 0.9 | 1.1 ± 1.2 | −0.1 ± 0.6 | <0.001 |
| Tricuspid Regurgitation Grade | ||||
| Median | 0.8 ± 1.0 | 2.3 ± 1.3 | 0.5 ± 0.5 | <0.001 |
| Change from Baseline | 0.4 ± 1.0 | 1.7 ± 1.1 | 0.1 ± 0.6 | <0.001 |
| Left Heart Chambers | ||||
| Left Ventricular Ejection Fraction | ||||
| Median (%) | 60 (50–60) | 55 (31–60) | 60 (50–60) | 0.010 |
| Change from Baseline (%) | ||||
| Absolute | 0 (−5–5) | 0 (−12–5) | 0 (−4–5) | 0.172 |
| Relative | 0.0 (−8.3–10.0) | 0.0 (−25.0–11.9) | 0.0 (−7.5–10.0) | 0.150 |
| <50% | 41 (22.8) | 16 (44.4) | 25 (17.4) | 0.001 |
| Left Ventricular End-Systolic Diameter | ||||
| Median (cm) | 3.1 (2.7–3.7) | 3.4 (2.8–4.9) | 3.0 (2.7–3.6) | 0.011 |
| Change from Baseline | ||||
| Absolute (cm) | −0.7 (−1.3–0.0) | −0.5 (−1.3–1.6) | −0.7 (−1.3–[−0.1]) | 0.097 |
| Relative (%) | −18.7 (−30.3–0.0) | −12.5 (−31.3–12.7) | −19.4 (−30.2–[−2.7]) | 0.163 |
| Left Atrial Diameter | ||||
| Median (cm) | 4.3 (3.7–4.9) | 5.0 (4.5–5.4) | 4.2 (3.7–4.7) | <0.001 |
| Change from Baseline | ||||
| Absolute (cm) | 0.1 (−0.4–0.8) | 0.8 (−0.2–1.3) | 0.1 (−0.5–0.7) | 0.010 |
| Relative (%) | 2.8 (−10.1–19.4) | 18.6 (−3.7–30.2) | 2.1 (−11.1–17.0) | 0.013 |
| Right Heart Chambers | ||||
| Right Ventricular Dysfunction | 20 (11.8) | 13 (40.6) | 7 (5.1) | <0.001 |
| Right Ventricular Dilatation | 22 (12.9) | 10 (30.3) | 12 (8.7) | 0.001 |
| Pulmonary Arterial Systolic Pressure | ||||
| Median (mmHg) | 29 (22–34) | 35 (30–46) | 26 (22–32) | <0.001 |
| Change from Baseline | ||||
| Absolute (mmHg) | 0 (−8–8) | 4 (−6–18) | −1 (−9–6) | 0.117 |
| Relative (%) | −4.3 (−26.6–26.8) | 10.5 (−17.2–63.6) | −8.0 (−27.3–20.3) | 0.063 |
| >40 mmHg | 17 (12.7) | 10 (31.3) | 7 (6.9) | 0.001 |
| OR (95% CI) | p-Value | |
|---|---|---|
| Age (Continuous) | 0.99 (0.94–1.04) | 0.599 |
| Ischemic Heart Disease | 1.22 (0.41–3.61) | 0.724 |
| Prior Stroke/Transient Ischemic Attack | 2.39 (0.66–8.58) | 0.182 |
| Atrial Fibrillation/Flutter | 3.30 (1.10–9.85) | 0.033 |
| New York Heart Association Class ≥ II | 7.42 (3.47–14.82) | 0.004 |
| Bicuspid Aortic Valve | 0.37 (0.09–1.50) | 0.163 |
| Mild-to-Moderate Mitral or Tricuspid Regurgitation | 4.17 (1.35–12.91) | 0.013 |
| Left Ventricular Ejection Fraction (continuous) | 0.98 (0.93–1.03) | 0.446 |
| Composite Graft Use | 4.20 (1.29–13.61) | 0.017 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kazum, S.; Vaturi, M.; Yedidya, I.; Schwartzenberg, S.; Morelli, O.; Skalsky, K.; Ofek, H.; Sharony, R.; Kornowski, R.; Shapira, Y.; et al. Progression of Non-Significant Mitral and Tricuspid Regurgitation after Surgical Aortic Valve Replacement for Aortic Regurgitation. J. Clin. Med. 2023, 12, 6280. https://doi.org/10.3390/jcm12196280
Kazum S, Vaturi M, Yedidya I, Schwartzenberg S, Morelli O, Skalsky K, Ofek H, Sharony R, Kornowski R, Shapira Y, et al. Progression of Non-Significant Mitral and Tricuspid Regurgitation after Surgical Aortic Valve Replacement for Aortic Regurgitation. Journal of Clinical Medicine. 2023; 12(19):6280. https://doi.org/10.3390/jcm12196280
Chicago/Turabian StyleKazum, Shirit, Mordehay Vaturi, Idit Yedidya, Shmuel Schwartzenberg, Olga Morelli, Keren Skalsky, Hadas Ofek, Ram Sharony, Ran Kornowski, Yaron Shapira, and et al. 2023. "Progression of Non-Significant Mitral and Tricuspid Regurgitation after Surgical Aortic Valve Replacement for Aortic Regurgitation" Journal of Clinical Medicine 12, no. 19: 6280. https://doi.org/10.3390/jcm12196280
APA StyleKazum, S., Vaturi, M., Yedidya, I., Schwartzenberg, S., Morelli, O., Skalsky, K., Ofek, H., Sharony, R., Kornowski, R., Shapira, Y., & Shechter, A. (2023). Progression of Non-Significant Mitral and Tricuspid Regurgitation after Surgical Aortic Valve Replacement for Aortic Regurgitation. Journal of Clinical Medicine, 12(19), 6280. https://doi.org/10.3390/jcm12196280