Significance of Hypocapnia in the Risk Assessment of Patients with Pulmonary Hypertension
Abstract
:1. Introduction
- PH Group 1—Pulmonary arterial hypertension (PAH);
- PH Group 2—Pulmonary hypertension associated with left heart disease (PH−LHD);
- PH Group 3—Pulmonary hypertension associated with lung diseases and/or hypoxia; pulmonary hypertension associated with chronic lung disease (PH−CLD);
- PH Group 4—Chronic thromboembolic pulmonary hypertension (CTEPH);
- PH Group 5—Pulmonary hypertension with unclear and/or multifactorial mechanisms.
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Data Collection and Assessments
2.4. Statistical Analysis
3. Results
3.1. Participants
3.2. Hypocapnia and Its Correlates
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maron, B.A. Revised Definition of Pulmonary Hypertension and Approach to Management: A Clinical Primer. J. Am. Heart Assoc. 2023, 12, e029024. [Google Scholar] [CrossRef]
- Hoeper, M.M.; Humbert, M.; Souza, R.; Idrees, M.; Kawut, S.M.; Sliwa-Hahnle, K.; Jing, Z.C.; Gibbs, J.S. A global view of pulmonary hypertension. Lancet Respir. Med. 2016, 4, 306–322. [Google Scholar] [CrossRef]
- Lam, C.S.; Borlaug, B.A.; Kane, G.C.; Enders, F.T.; Rodeheffer, R.J.; Redfield, M.M. Age-associated increases in pulmonary artery systolic pressure in the general population. Circulation 2009, 119, 2663–2670. [Google Scholar] [CrossRef]
- Simonneau, G.; Montani, D.; Celermajer, D.S.; Denton, C.P.; Gatzoulis, M.A.; Krowka, M.; Williams, P.G.; Souza, R. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur. Respir. J. 2019, 53, 1801913. [Google Scholar] [CrossRef]
- Hoeper, M.M.; Ghofrani, H.A.; Grünig, E.; Klose, H.; Olschewski, H.; Rosenkranz, S. Pulmonary Hypertension. Dtsch. Ärzteblatt Int. 2017, 114, 73–84. [Google Scholar] [CrossRef]
- Kovacs, G.; Olschewski, H. Debating the new haemodynamic definition of pulmonary hypertension: Much ado about nothing? Eur. Respir. J. 2019, 54, 1901278. [Google Scholar] [CrossRef]
- Tanyeri, S.; Akbal, O.Y.; Keskin, B.; Hakgor, A.; Karagoz, A.; Tokgoz, H.C.; Dogan, C.; Bayram, Z.; Kulahcioglu, S.; Erdogan, E.; et al. Impact of the updated hemodynamic definitions on diagnosis rates of pulmonary hypertension. Pulm. Circ. 2020, 10, 2045894020931299. [Google Scholar] [CrossRef]
- Maron, B.A.; Hess, E.; Maddox, T.M.; Opotowsky, A.R.; Tedford, R.J.; Lahm, T.; Joynt, K.E.; Kass, D.J.; Stephens, T.; Stanislawski, M.A.; et al. Association of Borderline Pulmonary Hypertension with Mortality and Hospitalization in a Large Patient Cohort: Insights From the Veterans Affairs Clinical Assessment, Reporting, and Tracking Program. Circulation 2016, 133, 1240–1248. [Google Scholar] [CrossRef]
- Assad, T.R.; Maron, B.A.; Robbins, I.M.; Xu, M.; Huang, S.; Harrell, F.E.; Farber-Eger, E.H.; Wells, Q.S.; Choudhary, G.; Hemnes, A.R.; et al. Prognostic Effect and Longitudinal Hemodynamic Assessment of Borderline Pulmonary Hypertension. JAMA Cardiol. 2017, 2, 1361–1368. [Google Scholar] [CrossRef]
- Kolte, D.; Lakshmanan, S.; Jankowich, M.D.; Brittain, E.L.; Maron, B.A.; Choudhary, G. Mild Pulmonary Hypertension Is Associated With Increased Mortality: A Systematic Review and Meta-Analysis. J. Am. Heart Assoc. 2018, 7, e009729. [Google Scholar] [CrossRef]
- Humbert, M.; Kovacs, G.; Hoeper, M.M.; Badagliacca, R.; Berger, R.M.F.; Brida, M.; Carlsen, J.; Coats, A.J.S.; Escribano-Subias, P.; Ferrari, P.; et al. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur. Heart J. 2022, 43, 3618–3731. [Google Scholar] [CrossRef]
- Rosenkranz, S. 2022 ESC/ERS guidelines on the diagnostics and treatment of pulmonary hypertension: A focussed review. Herz 2023, 48, 23–30. [Google Scholar] [CrossRef]
- Douschan, P.; Kovacs, G.; Avian, A.; Foris, V.; Gruber, F.; Olschewski, A.; Olschewski, H. Mild Elevation of Pulmonary Arterial Pressure as a Predictor of Mortality. Am. J. Respir. Crit. Care Med. 2018, 197, 509–516. [Google Scholar] [CrossRef]
- Hoeper, M.M.; Humbert, M. The new haemodynamic definition of pulmonary hypertension: Evidence prevails, finally! Eur. Respir. J. 2019, 53, 1900038. [Google Scholar] [CrossRef]
- Kovacs, G.; Avian, A.; Tscherner, M.; Foris, V.; Bachmaier, G.; Olschewski, A.; Olschewski, H. Characterization of patients with borderline pulmonary arterial pressure. Chest 2014, 146, 1486–1493. [Google Scholar] [CrossRef]
- Coghlan, J.G.; Wolf, M.; Distler, O.; Denton, C.P.; Doelberg, M.; Harutyunova, S.; Marra, A.M.; Benjamin, N.; Fischer, C.; Grünig, E. Incidence of pulmonary hypertension and determining factors in patients with systemic sclerosis. Eur. Respir. J. 2018, 51, 1701197. [Google Scholar] [CrossRef]
- Boucly, A.; Weatherald, J.; Savale, L.; Jaïs, X.; Cottin, V.; Prevot, G.; Picard, F.; de Groote, P.; Jevnikar, M.; Bergot, E.; et al. Risk assessment, prognosis and guideline implementation in pulmonary arterial hypertension. Eur. Respir. J. 2017, 50, 1700889. [Google Scholar] [CrossRef]
- Hoeper, M.M.; Pittrow, D.; Opitz, C.; Gibbs, J.S.R.; Rosenkranz, S.; Grünig, E.; Olsson, K.M.; Huscher, D. Risk assessment in pulmonary arterial hypertension. Eur. Respir. J. 2018, 51, 1702606. [Google Scholar] [CrossRef]
- Rich, S.; Dantzker, D.R.; Ayres, S.M.; Bergofsky, E.H.; Brundage, B.H.; Detre, K.M.; Fishman, A.P.; Goldring, R.M.; Groves, B.M.; Koerner, S.K.; et al. Primary pulmonary hypertension. A national prospective study. Ann. Intern. Med. 1987, 107, 216–223. [Google Scholar] [CrossRef]
- Mélot, C.; Naeije, R. Pulmonary vascular diseases. Compr. Physiol. 2011, 1, 593–619. [Google Scholar] [CrossRef]
- Hoeper, M.M.; Pletz, M.W.; Golpon, H.; Welte, T. Prognostic value of blood gas analyses in patients with idiopathic pulmonary arterial hypertension. Eur. Respir. J. 2007, 29, 944–950. [Google Scholar] [CrossRef]
- Harbaum, L.; Fuge, J.; Kamp, J.C.; Hennigs, J.K.; Simon, M.; Sinning, C.; Oqueka, T.; Grimminger, J.; Olsson, K.M.; Hoeper, M.M.; et al. Blood carbon dioxide tension and risk in pulmonary arterial hypertension. Int. J. Cardiol. 2020, 318, 131–137. [Google Scholar] [CrossRef]
- Moutchia, J.; McClelland, R.L.; Al-Naamani, N.; Appleby, D.H.; Blank, K.; Grinnan, D.; Holmes, J.H.; Mathai, S.C.; Minhas, J.; Ventetuolo, C.E.; et al. Minimal Clinically Important Difference in the 6-minute-walk Distance for Patients with Pulmonary Arterial Hypertension. Am. J. Respir. Crit. Care Med. 2023, 207, 1070–1079. [Google Scholar] [CrossRef]
- Weatherald, J.; Boucly, A.; Montani, D.; Jaïs, X.; Savale, L.; Humbert, M.; Sitbon, O.; Garcia, G.; Laveneziana, P. Gas Exchange and Ventilatory Efficiency During Exercise in Pulmonary Vascular Diseases. Arch. Bronconeumol. 2020, 56, 578–585. [Google Scholar] [CrossRef]
- Khirfan, G.; Ahmed, M.K.; Faulx, M.D.; Dakkak, W.; Dweik, R.A.; Tonelli, A.R. Gasometric gradients between blood obtained from the pulmonary artery wedge and pulmonary artery positions in pulmonary arterial hypertension. Respir. Res. 2019, 20, 6. [Google Scholar] [CrossRef]
- Al-Naamani, N.; Palevsky, H.I.; Lederer, D.J.; Horn, E.M.; Mathai, S.C.; Roberts, K.E.; Tracy, R.P.; Hassoun, P.M.; Girgis, R.E.; Shimbo, D.; et al. Prognostic Significance of Biomarkers in Pulmonary Arterial Hypertension. Ann. Am. Thorac. Soc. 2016, 13, 25–30. [Google Scholar] [CrossRef]
- Frantz, R.P.; Farber, H.W.; Badesch, D.B.; Elliott, C.G.; Frost, A.E.; McGoon, M.D.; Zhao, C.; Mink, D.R.; Selej, M.; Benza, R.L. Baseline and Serial Brain Natriuretic Peptide Level Predicts 5-Year Overall Survival in Patients With Pulmonary Arterial Hypertension: Data From the REVEAL Registry. Chest 2018, 154, 126–135. [Google Scholar] [CrossRef]
- Nagaya, N.; Nishikimi, T.; Uematsu, M.; Satoh, T.; Kyotani, S.; Sakamaki, F.; Kakishita, M.; Fukushima, K.; Okano, Y.; Nakanishi, N.; et al. Plasma brain natriuretic peptide as a prognostic indicator in patients with primary pulmonary hypertension. Circulation 2000, 102, 865–870. [Google Scholar] [CrossRef]
- Casserly, B.; Klinger, J.R. Brain natriuretic peptide in pulmonary arterial hypertension: Biomarker and potential therapeutic agent. Drug Des. Devel. Ther. 2009, 3, 269–287. [Google Scholar] [CrossRef]
- Valerio, C.J.; Schreiber, B.E.; Handler, C.E.; Denton, C.P.; Coghlan, J.G. Borderline mean pulmonary artery pressure in patients with systemic sclerosis: Transpulmonary gradient predicts risk of developing pulmonary hypertension. Arthritis Rheum. 2013, 65, 1074–1084. [Google Scholar] [CrossRef]
| Characteristics | Participants (n = 157) |
|---|---|
| Age, years | 70 ± 11 |
| Male sex, n (%) | 65 (41) |
| Smoking status | |
| Smoker, n (%) | 23 (15) |
| Ex−smoker, n (%) | 55 (35) |
| Smoking pack−years | 40 |
| Pulmonary hypertension group 1, n (%) | |
| 1 | 48 (30) |
| 2 | 45 (29) |
| 3 | 44 (28) |
| 4 | 16 (10) |
| Comorbidities, n (%) | |
| Heart failure with reduced ejection fraction | 5 (3) |
| Heart failure with mid−range ejection fraction | 21 (13) |
| Heart failure with preserved ejection fraction | 106 (68) |
| Arterial hypertension | 113 (72) |
| Atrial fibrillation | 50 (32) |
| Coronary heart disease | 42 (27) |
| Valvular cardiomyopathy | 8 (5) |
| Chronic obstructive pulmonary disease | 54 (35) |
| Asthma | 13 (8) |
| Interstitial lung disease | 32 (20) |
| Diabetes mellitus | 55 (35) |
| Systemic sclerosis | 13 (8) |
| Connective tissue disease | 15 (10) |
| Obstructive sleep apnea syndrome | 30 (19) |
| Pulmonary embolism | 52 (33) |
| History of lung cancer | 6 (4) |
| Dyslipidemia | 77 (49) |
| Obesity | 52 (33) |
| Chronic renal insufficiency | 53 (34) |
| Medications n (%) | |
| β−blockers | 73 (47) |
| Angiotensin converting enzyme inhibitors | 50 (32) |
| Angiotensin receptor blockers | 33 (21) |
| Calcium channel blockers | 28 (18) |
| Thiazide diuretics | 36 (23) |
| Mineralocorticoid receptor antagonist | 44 (28) |
| Long−acting β−agonists | 64 (41) |
| Long−acting muscarinic antagonists | 62 (40) |
| Inhaled corticosteroids | 37 (24) |
| Phosphodiesterase−5 inhibitor | 61 (39) |
| Riociguat | 14 (9) |
| Endothelin receptor antagonists | 37 (24) |
| Prostanoids | 5 (3) |
| Highly dosed calcium channel blocker by proven reversibility | 4 (3) |
| Laboratory tests | |
| Hemoglobin, g/dL | 13.27 ± 2.08 |
| Creatinine, mg/dL | 1.20 ± 0.72 |
| Aspartate aminotransferase, U/L | 29.4 ± 14.0 |
| Alanine aminotransferase, U/L | 26.7 ± 21.2 |
| NT−pro−BNP, pg/mL | 3428.8 ± 5079.7 |
| Characteristics | Pulmonary Hypertension | ||
|---|---|---|---|
| With Hypocapnia (n = 62) | Without Hypocapnia (n = 95) | p-Value | |
| Age, years | 69.7 ± 9.5 | 70.2 ± 12.1 | 0.998 |
| Hemoglobin, g/dL | 13.31 ± 2.3 | 13.29 ± 1.95 | 0.998 |
| Creatinine, mg/dL | 1.23 ± 0.57 | 1.19 ± 0.81 | 0.994 |
| HFpEF, n (%) | 37 (24) | 67 (43) | |
| Arterial hypertension, n (%) | 39 (25) | 71 (45) | |
| Atrial fibrillation, n (%) | 14 (9) | 35 (22) | |
| Coronary heart disease, n (%) | 21 (13) | 19 (12) | |
| COPD, n (%) | 17 (11) | 36 (23) | |
| Interstitial lung disease, n (%) | 11 (7) | 17 (11) | |
| Diabetes mellitus, n (%) | 20 (13) | 33 (21) | |
| Connective tissue disease, n (%) | 4 (3) | 11 (7) | |
| Systemic sclerosis, n (%) | 9 (6) | 4 (3) | |
| Dyslipidemia, n (%) | 31 (20) | 44 (28) | |
| Chronic renal insufficiency, n (%) | 20 (123) | 33 (21) | |
| At diagnosis/baseline | |||
| Right atrial pressure | 9.5 ± 4.7 | 10.3 ± 4.9 | 0.145 |
| Cardiac index | 2.5 ± 0.7 | 2.7 ± 1.0 | 0.096 |
| Stroke volume index | 0.034 ± 0.011 | 0.036 ± 0.016 | 0.217 |
| Venous oxygen saturation | 62.6 ± 10.3 | 60.4 ± 9.0 | 0.12 |
| WHO functional class | 3.1 ± 0.7 | 2.9 ± 0.7 | 0.188 |
| NT−pro−BNP | 4252.6 ± 5404.7 | 2926.7 ± 4845.6 | 0.089 |
| At 3− to 6−month follow−up | |||
| WHO functional class | 2.4 ± 0.8 | 2.4 ± 0.8 | 0.444 |
| NT−pro−BNP | 3342.3 ± 10,333.9 | 1173.1 ± 1729.7 | 0.065 |
| At 7− to 12−month follow−up | |||
| WHO functional class | 2.4 ± 1.0 | 2.5 ± 0.9 | 0.512 |
| NT−pro−BNP | 1427.0 ± 1975.7 | 1634.8 ± 2571.9 | 0.374 |
| NT−Pro−BNP | Pulmonary Artery Hypertension | ||
|---|---|---|---|
| With Hypocapnia | Without Hypocapnia | p-Value | |
| At diagnosis/baseline | |||
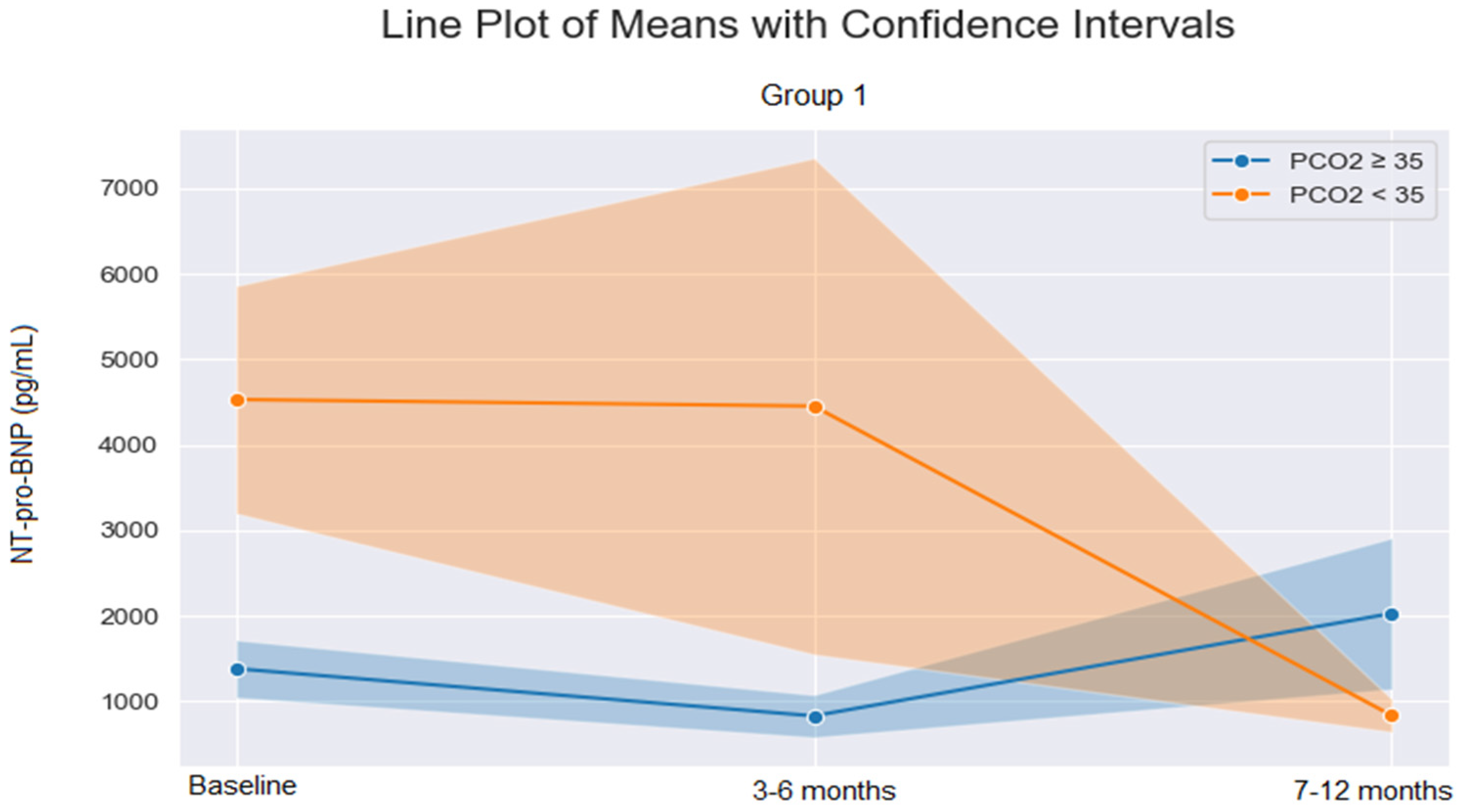
| PH Group 1 | 4529 ± 5646 | 1380 ± 1429 | 0.026 |
| PH Group 2 | 5711 ± 6363 | 3220 ± 4393 | 0.31 |
| PH Group 3 | 3617 ± 5433 | 1917 ± 3442 | 0.31 |
| PH Group 4 | 1433 ± 1550 | 12,821 ± 11,093 | 0.21 |
| At 3− to 6−month follow−up | |||
| PH Group 1 | 4452 ± 13,623 | 827 ± 963 | 0.33 |
| PH Group 2 | 3802 ± 3819 | 2545 ± 3723 | 0.51 |
| PH Group 3 | 1591 ± 1506 | 847 ± 953 | 0.36 |
| PH Group 4 | 873 ± 824 | 2750 ± 3524 | 0.37 |
| At 7− to 12−month follow−up | |||
| PH Group 1 | 832 ± 810 | 2026 ± 3428 | 0.23 |
| PH Group 2 | 3795 ± 4426 | 2865 ± 1579 | 0.64 |
| PH Group 3 | 3288 ± 585 | 645 ± 786 | 0.07 |
| PH Group 4 | 601 ± 250 | 1806 ± 340 | 0.18 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aetou, M.; Wahab, L.; Dreher, M.; Daher, A. Significance of Hypocapnia in the Risk Assessment of Patients with Pulmonary Hypertension. J. Clin. Med. 2023, 12, 6307. https://doi.org/10.3390/jcm12196307
Aetou M, Wahab L, Dreher M, Daher A. Significance of Hypocapnia in the Risk Assessment of Patients with Pulmonary Hypertension. Journal of Clinical Medicine. 2023; 12(19):6307. https://doi.org/10.3390/jcm12196307
Chicago/Turabian StyleAetou, Maria, Lora Wahab, Michael Dreher, and Ayham Daher. 2023. "Significance of Hypocapnia in the Risk Assessment of Patients with Pulmonary Hypertension" Journal of Clinical Medicine 12, no. 19: 6307. https://doi.org/10.3390/jcm12196307
APA StyleAetou, M., Wahab, L., Dreher, M., & Daher, A. (2023). Significance of Hypocapnia in the Risk Assessment of Patients with Pulmonary Hypertension. Journal of Clinical Medicine, 12(19), 6307. https://doi.org/10.3390/jcm12196307









