Feasibility, Acceptability, and Efficacy of Home-Based Transcranial Direct Current Stimulation on Pain in Older Adults with Alzheimer’s Disease and Related Dementias: A Randomized Sham-Controlled Pilot Clinical Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Design
2.2. Participants
2.3. tDCS and Sham Conditions
2.4. Data Collection
2.5. Clinical Pain Intensity
2.6. Behavioral and Psychological Symptoms of Dementia (BPSD)
2.7. Feasibility
2.8. Acceptability
2.9. Statistical Methods
3. Results
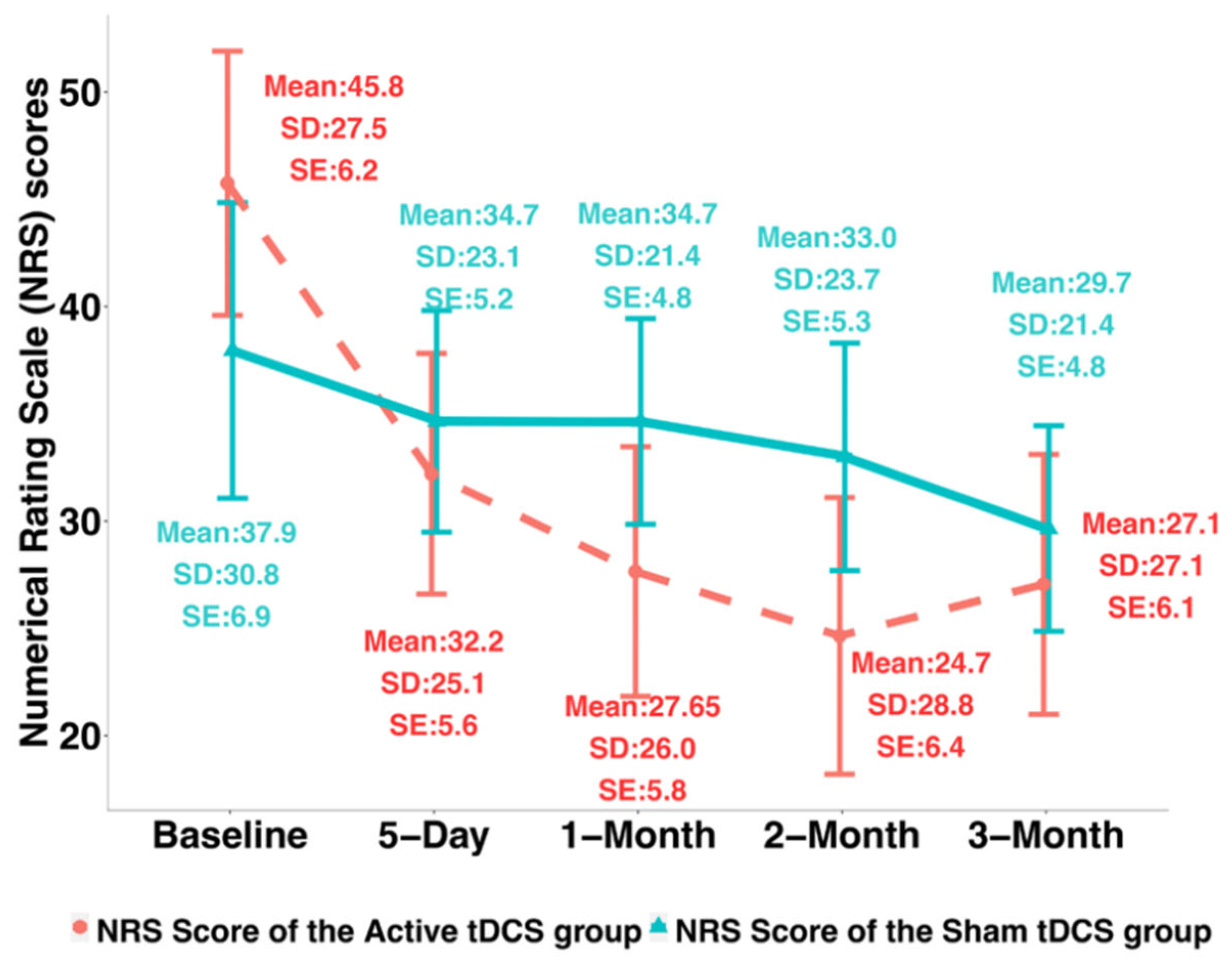
3.1. Clinical Pain Intensity
Behavioral and Psychological Symptoms of Dementia (BPSD)
3.2. Feasibility and Acceptability
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Goodman, R.A.; Lochner, K.A.; Thambisetty, M.; Wingo, T.S.; Posner, S.F.; Ling, S.M. Prevalence of dementia subtypes in United States Medicare fee-for-service beneficiaries, 2011–2013. Alzheimers Dement. 2017, 13, 28–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alzheimer’s Association: 2016 Alzheimer’s disease facts and figures. Alzheimers Dement. 2016, 12, 459–509. [CrossRef]
- Matthews, K.A.; Xu, W.; Gaglioti, A.H.; Holt, J.B.; Croft, J.B.; Mack, D.; McGuire, L.C. Racial and ethnic estimates of Alzheimer’s disease and related dementias in the United States (2015–2060) in adults aged ≥ 65 years. Alzheimers Dement. 2019, 15, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Corbett, A.; Husebo, B.; Malcangio, M.; Staniland, A.; Cohen-Mansfield, J.; Aarsland, D.; Ballard, C. Assessment and treatment of pain in people with dementia. Nat. Rev. Neurol. 2012, 8, 264–274. [Google Scholar] [CrossRef]
- Patel, K.V.; Guralnik, J.M.; Dansie, E.J.; Turk, D.C. Prevalence and impact of pain among older adults in the United States: Findings from the 2011 National Health and Aging Trends Study. Pain 2013, 154, 2649–2657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Kooten, J.; Binnekade, T.T.; van der Wouden, J.C.; Stek, M.L.; Scherder, E.J.; Husebo, B.S.; Smalbrugge, M.; Hertogh, C.M. A Review of Pain Prevalence in Alzheimer’s, Vascular, Frontotemporal and Lewy Body Dementias. Dement. Geriatr. Cogn. Disord. 2016, 41, 220–232. [Google Scholar] [CrossRef]
- Beerens, H.C.; Sutcliffe, C.; Renom-Guiteras, A.; Soto, M.E.; Suhonen, R.; Zabalegui, A.; Bokberg, C.; Saks, K.; Hamers, J.P. RightTimePlaceCare C: Quality of life and quality of care for people with dementia receiving long term institutional care or professional home care: The European Right Time Place Care study. J. Am. Med. Dir. Assoc. 2014, 15, 54–61. [Google Scholar] [CrossRef]
- Achterberg, W.; Lautenbacher, S.; Husebo, B.; Erdal, A.; Herr, K. Pain in dementia. Pain Rep. 2020, 5, e803. [Google Scholar] [CrossRef]
- Ahn, H.; Horgas, A. The relationship between pain and disruptive behaviors in nursing home resident with dementia. BMC Geriatr. 2013, 13, 14. [Google Scholar] [CrossRef] [Green Version]
- Ahn, H.; Garvan, C.; Lyon, D. Pain and Aggression in Nursing Home Residents With Dementia: Minimum Data Set 3.0 Analysis. Nurs. Res. 2015, 64, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Volicer, L.; Frijters, D.H.; Van der Steen, J.T. Relationship between symptoms of depression and agitation in nursing home residents with dementia. Int. J. Geriatr. Psychiatry 2011, 27, 749–754. [Google Scholar] [CrossRef]
- Scherder, E.J.; Eggermont, L.; Plooij, B.; Oudshoorn, J.; Vuijk, P.J.; Pickering, G.; Lautenbacher, S.; Achterberg, W.; Oosterman, J. Relationship between Chronic Pain and Cognition in Cognitively Intact Older Persons and in Patients with Alzheimer’s Disease. Gerontology 2008, 54, 50–58. [Google Scholar] [CrossRef]
- Lin, P.-C.; Lin, L.-C.; Shyu, Y.-I.L.; Hua, M.-S. Predictors of pain in nursing home residents with dementia: A cross-sectional study. J. Clin. Nurs. 2011, 20, 1849–1857. [Google Scholar] [CrossRef]
- Van Dalen-Kok, A.H.; Pieper, M.J.C.; De Waal, M.W.M.; Lukas, A.; Husebo, B.S.; Achterberg, W.P. Association between pain, neuropsychiatric symptoms, and physical function in dementia: A systematic review and meta-analysis. BMC Geriatr. 2015, 15, 19. [Google Scholar] [CrossRef] [Green Version]
- Reid, M.C.; Henderson, C.R.; Jr Papaleontiou, M.; Amanfo, L.; Olkhovskaya, Y.; Moore, A.A.; Parikh, S.S.; Turner, B.J. Characteristics of older adults receiving opioids in primary care: Treatment duration and outcomes. Pain Med. 2010, 11, 1063–1071. [Google Scholar] [CrossRef] [Green Version]
- Karafin, M.S.; Chen, G.; Wandersee, N.J.; Brandow, A.M.; Hurley, R.W.; Simpson, P.; Ward, D.; Li, S.-J.; Field, J.J. Chronic pain in adults with sickle cell disease is associated with alterations in functional connectivity of the brain. PLoS ONE 2019, 14, e0216994. [Google Scholar] [CrossRef]
- Gwilym, S.E.; Keltner, J.R.; Warnaby, C.E.; Carr, A.J.; Chizh, B.; Chessell, I.; Tracey, I. Psychophysical and functional imaging evidence supporting the presence of central sensitization in a cohort of osteoarthritis patients. Arthritis Rheum. 2009, 61, 1226–1234. [Google Scholar] [CrossRef]
- Hiramatsu, T.; Nakanishi, K.; Yoshimura, S.; Yoshino, A.; Adachi, N.; Okamoto, Y.; Yamawaki, S.; Ochi, M. The dorsolateral prefrontal network is involved in pain perception in knee osteoarthritis patients. Neurosci. Lett. 2014, 581, 109–114. [Google Scholar] [CrossRef] [Green Version]
- Sofat, N.; Smee, C.; Hermansson, M.; Howard, M.; Baker, E.H.; Howe, F.A.; Barrick, T.R. Functional MRI demonstrates pain perception in hand osteoarthritis has features of central pain processing. J. Biomed. Graph. Comput. 2013, 3, 20. [Google Scholar] [CrossRef] [Green Version]
- Kindler, L.L.; Bennett, R.; Jones, K.D. Central Sensitivity Syndromes: Mounting Pathophysiologic Evidence to Link Fibromyalgia with Other Common Chronic Pain Disorders. Pain Manag. Nurs. 2011, 12, 15–24. [Google Scholar] [CrossRef]
- Kumaradev, S.; Fayosse, A.; Dugravot, A.; Dumurgier, J.; Roux, C.; Kivimäki, M.; Singh-Manoux, A.; Sabia, S. Timeline of pain before dementia diagnosis: A 27-year follow-up study. Pain 2020, 162, 1578–1585. [Google Scholar] [CrossRef]
- Wang, K.; Liu, H. Association between widespread pain and dementia, Alzheimer’s disease and stroke: A cohort study from the Framingham Heart Study. Reg. Anesth. Pain Med. 2021, 46, 879–885. [Google Scholar] [CrossRef]
- Defrin, R.; Amanzio, M.; de Tommaso, M.; Dimova, V.; Filipovic, S.; Finn, D.P.; Gimenez-Llort, L.; Invitto, S.; Jensen-Dahm, C.; Lautenbacher, S.; et al. Experimental pain processing in individuals with cognitive impairment: Current state of the science. Pain 2015, 156, 1396–1408. [Google Scholar] [CrossRef]
- Anderson, A.R.; Iversen, W.L.; Carter, M.A.; Moss, K.O.; Cowan, R.L.; Monroe, T.B. Experimentally evoked pain in Alzheimer’s disease. J. Am. Assoc. Nurse Prac. 2021, 34, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Cole, L.J.; Farrell, M.; Duff, E.; Barber, J.B.; Egan, G.; Gibson, S.J. Pain sensitivity and fMRI pain-related brain activity in Alzheimer’s disease. Brain 2006, 129, 2957–2965. [Google Scholar] [CrossRef] [PubMed]
- Beach, P.A.; Huck, J.T.; Zhu, D.C.; Bozoki, A.C. Altered Behavioral and Autonomic Pain Responses in Alzheimer’s Disease Are Associated with Dysfunctional Affective, Self-Reflective and Salience Network Resting-State Connectivity. Front. Aging Neurosci. 2017, 9, 297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monroe, T.B.; Beach, P.A.; Bruehl, S.P.; Dietrich, M.S.; Rogers, B.P.; Gore, J.C.; Atalla, S.W.; Cowan, R.L. The Impact of Alzheimer’s Disease on the Resting State Functional Connectivity of Brain Regions Modulating Pain: A Cross Sectional Study. J. Alzheimers Dis. 2017, 57, 71–83. [Google Scholar] [CrossRef]
- Cole, L.J.; Gavrilescu, M.; Johnston, L.A.; Gibson, S.J.; Farrell, M.J.; Egan, G.F. The impact of Alzheimer’s disease on the functional connectivity between brain regions underlying pain perception. Eur. J. Pain 2011, 15, e561–e568. [Google Scholar]
- Bunk, S.; Zuidema, S.; Koch, K.; Lautenbacher, S.; De Deyn, P.P.; Kunz, M. Pain processing in older adults with dementia-related cognitive impairment is associated with frontal neurodegeneration. Neurobiol. Aging 2021, 106, 139–152. [Google Scholar] [CrossRef] [PubMed]
- Lefaucheur, J.-P.; Antal, A.; Ayache, S.S.; Benninger, D.H.; Brunelin, J.; Cogiamanian, F.; Cotelli, M.; De Ridder, D.; Ferrucci, R.; Langguth, B.; et al. Evidence-based guidelines on the therapeutic use of transcranial direct current stimulation (tDCS). Clin. Neurophysiol. 2016, 128, 56–92. [Google Scholar] [CrossRef]
- Woods, A.J.; Antal, A.; Bikson, M.; Boggio, P.S.; Brunoni, A.R.; Celnik, P.; Cohen, L.G.; Fregni, F.; Herrmann, C.S.; Kappenman, E.S.; et al. A technical guide to tDCS, and related non-invasive brain stimulation tools. Clin. Neurophysiol. 2016, 127, 1031–1048. [Google Scholar] [CrossRef] [PubMed]
- Kessler, S.K.; Turkeltaub, P.E.; Benson, J.G.; Hamilton, R.H. Differences in the experience of active and sham transcranial direct current stimulation. Brain Stimul. 2012, 5, 155–162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- United States Food and Drug Administration: Medical Device Regulatory Framework: Part812-Investigational Device Exemptions; The Food and Drug Administration (FDA): Silver Spring, MD, USA, 2015.
- Fregni, F.; Nitsche, M.A.; Loo, C.; Brunoni, A.R.; Marangolo, P.; Leite, J.; Carvalho, S.; Bolognini, N.; Caumo, W.; Paik, N.-J.; et al. Regulatory considerations for the clinical and research use of transcranial direct current stimulation (tDCS): Review and recommendations from an expert panel. Clin. Res. Regul. Aff. 2014, 32, 22–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahn, H.; Woods, A.J.; Kunik, M.E.; Bhattacharjee, A.; Chen, Z.; Choi, E.; Fillingim, R.B. Efficacy of transcranial direct current stimulation over primary motor cortex (anode) and contralateral supraorbital area (cathode) on clinical pain severity and mobility performance in persons with knee osteoarthritis: An experimenter- and participant-blinded, randomized, sham-controlled pilot clinical study. Brain Stimul. 2017, 10, 902–909. [Google Scholar] [CrossRef] [PubMed]
- Ahn, H.; Suchting, R.; Woods, A.J.; Miao, H.; Green, C.; Cho, R.Y.; Choi, E.; Fillingim, R. Bayesian analysis of the effect of transcranial direct current stimulation on experimental pain sensitivity in older adults with knee osteoarthritis: Randomized sham-controlled pilot clinical study. J. Pain Res. 2018, 11, 2071–2082. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suchting, R.; Colpo, G.D.; Rocha, N.P.; Ahn, H. The Effect of Transcranial Direct Current Stimulation on Inflammation in Older Adults With Knee Osteoarthritis: A Bayesian Residual Change Analysis. Biol. Res. Nurs. 2019, 22, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Ahn, H.; Sorkpor, S.; Miao, H.; Zhong, C.; Jorge, R.; Park, L.; Abdi, S.; Cho, R.Y. Home-based self-administered transcranial direct current stimulation in older adults with knee osteoarthritis pain: An open-label study. J. Clin. Neurosci. 2019, 66, 61–65. [Google Scholar] [CrossRef]
- Ahn, H.; Zhong, C.; Miao, H.; Chaoul, A.; Park, L.; Yen, I.H.; Vila, M.A.; Sorkpor, S.; Abdi, S. Efficacy of combining home-based transcranial direct current stimulation with mindfulness-based meditation for pain in older adults with knee osteoarthritis: A randomized controlled pilot study. J. Clin. Neurosci. 2019, 70, 140–145. [Google Scholar] [CrossRef]
- Luedtke, K.; Rushton, A.; Wright, C.; Geiss, B.; Juergens, T.P.; May, A. Transcranial direct current stimulation for the reduction of clinical and experimentally induced pain: A systematic review and meta-analysis. Clin. J. Pain 2012, 28, 452–461. [Google Scholar] [CrossRef]
- O’Connell, N.E.; Wand, B.M.; Marston, L.; Spencer, S.; Desouza, L.H. Non-invasive brain stimulation techniques for chronic pain. Cochrane Database Syst. Rev. 2014, CD008208. [Google Scholar] [CrossRef] [Green Version]
- Vaseghi, B.; Zoghi, M.; Jaberzadeh, S. Does anodal transcranial direct current stimulation modulate sensory perception and pain? A meta-analysis study. Clin. Neurophysiol. 2014, 125, 1847–1858. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, J.-P.; Nizard, J.; Keravel, Y.; Lefaucheur, J.-P. Invasive brain stimulation for the treatment of neuropathic pain. Nat. Rev. Neurol. 2011, 7, 699–709. [Google Scholar] [CrossRef] [PubMed]
- Peyron, R.; Faillenot, I.; Mertens, P.; Laurent, B.; Garcia-Larrea, L. Motor cortex stimulation in neuropathic pain. Correlations between analgesic effect and hemodynamic changes in the brain. A PET study. Neuroimage 2007, 34, 310–321. [Google Scholar] [CrossRef] [PubMed]
- Lefaucheur, J.-P. A comprehensive database of published tDCS clinical trials (2005–2016). Neurophysiol. Clin. 2016, 46, 319–398. [Google Scholar] [CrossRef]
- Majdi, A.; van Boekholdt, L.; Sadigh-Eteghad, S.; Mc Laughlin, M. A systematic review and meta-analysis of transcranial direct-current stimulation effects on cognitive function in patients with Alzheimer’s disease. Mol. Psychiatry 2022, 27, 2000–2009. [Google Scholar] [CrossRef]
- Martorella, G.; Mathis, K.; Miao, H.; Wang, D.; Park, L.; Ahn, H. Self-administered Transcranial Direct Current Stimulation for Pain in Older Adults with Knee Osteoarthritis: A Randomized Controlled Trial. Brain Stimul. 2022, 15, 902–909. [Google Scholar] [CrossRef]
- Martorella, G.; Mathis, K.; Miao, H.; Wang, D.; Park, L.; Ahn, H. Efficacy of Home-Based Transcranial Direct Current Stimulation on Experimental Pain Sensitivity in Older Adults with Knee Osteoarthritis: A Randomized, Sham-Controlled Clinical Trial. J. Clin. Med. 2022, 11, 5209. [Google Scholar] [CrossRef]
- Charvet, L.E.; Kasschau, M.; Datta, A.; Knotkova, H.; Stevens, M.; Alonzo, A.; Loo, C.; Krull, K.R.; Bikson, M. Remotely-supervised transcranial direct current stimulation (tDCS) for clinical trials: Guidelines for technology and protocols. Front. Syst. Neurosci. 2015, 9, 26. [Google Scholar] [CrossRef] [Green Version]
- Thibaut, A.; O’Brien, A.T.; Fregni, F. Strategies for replacing non-invasive brain stimulation sessions: Recommendations for designing neurostimulation clinical trials. Expert Rev. Med. Devices 2017, 14, 633–649. [Google Scholar] [CrossRef]
- Probst, J.C.; Laditka, S.B.; Wang, J.-Y.; Johnson, A.O. Effects of residence and race on burden of travel for care: Cross sectional analysis of the 2001 US National Household Travel Survey. BMC Health Serv. Res. 2007, 7, 40. [Google Scholar] [CrossRef] [Green Version]
- Witham, M.; McMurdo, M.E.T. How to Get Older People Included in Clinical Studies. Drugs Aging 2007, 24, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Cammisuli, D.M.; Cignoni, F.; Ceravolo, R.; Bonuccelli, U.; Castelnuovo, G. Transcranial Direct Current Stimulation (tDCS) as a Useful Rehabilitation Strategy to Improve Cognition in Patients With Alzheimer’s Disease and Parkinson’s Disease: An Updated Systematic Review of Randomized Controlled Trials. Front. Neurol. 2022, 12. [Google Scholar] [CrossRef] [PubMed]
- André, S.; Heinrich, S.; Kayser, F.; Menzler, K.; Kesselring, J.; Khader, P.H.; Lefaucheur, J.-P.; Mylius, V. At-home tDCS of the left dorsolateral prefrontal cortex improves visual short-term memory in mild vascular dementia. J. Neurol. Sci. 2016, 369, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Im, J.J.; Jeong, H.; Bikson, M.; Woods, A.J.; Unal, G.; Oh, J.K.; Na, S.; Park, J.-S.; Knotkova, H.; Song, I.-U.; et al. Effects of 6-month at-home transcranial direct current stimulation on cognition and cerebral glucose metabolism in Alzheimer’s disease. Brain Stimul. 2019, 12, 1222–1228. [Google Scholar] [CrossRef]
- Castillo-Saavedra, L.; Gebodh, N.; Bikson, M.; Diaz-Cruz, C.; Brandao, R.; Coutinho, L.; Truong, D.; Datta, A.; Shani-Hershkovich, R.; Weiss, M.; et al. Clinically Effective Treatment of Fibromyalgia Pain With High-Definition Transcranial Direct Current Stimulation: Phase II Open-Label Dose Optimization. J. Pain 2015, 17, 14–26. [Google Scholar] [CrossRef] [Green Version]
- Morris, J.C. The Clinical Dementia Rating (CDR): Current version and scoring rules. Neurology 1993, 43, 2412–2414. [Google Scholar] [CrossRef]
- Knotkova, H.; Riggs, A.; Berisha, D.; Borges, H.; Bernstein, H.; Patel, V.; Truong, D.Q.; Unal, G.; Arce, D.; Datta, A.; et al. Automatic M1-SO Montage Headgear for Transcranial Direct Current Stimulation (TDCS) Suitable for Home and High-Throughput In-Clinic Applications. Neuromodulation Technol. Neural Interface 2018, 22, 904–910. [Google Scholar] [CrossRef]
- Fregni, F.; Gimenes, R.; Valle, A.C.; Ferreira, M.J.L.; Rocha, R.R.; Natalle, L.; Bravo, R.; Rigonatti, S.P.; Freedman, S.D.; Nitsche, M.A.; et al. A randomized, sham-controlled, proof of principle study of transcranial direct current stimulation for the treatment of pain in fibromyalgia. Arthritis Rheum. 2006, 54, 3988–3998. [Google Scholar] [CrossRef]
- Gandiga, P.C.; Hummel, F.C.; Cohen, L.G. Transcranial DC stimulation (tDCS): A tool for double-blind sham-controlled clinical studies in brain stimulation. Clin. Neurophysiol. 2006, 117, 845–850. [Google Scholar] [CrossRef]
- Dworkin, R.H.; Turk, D.C.; McDermott, M.P.; Peirce-Sandner, S.; Burke, L.B.; Cowan, P.; Farrar, J.T.; Hertz, S.; Raja, S.N.; Rappaport, B.A.; et al. Interpreting the clinical importance of group differences in chronic pain clinical trials: IMMPACT recommendations. Pain 2009, 146, 238–244. [Google Scholar] [CrossRef]
- Husebo, B.S. Mobilization-Observation-Behaviour-Intensity-Dementia-2 Pain Scale (MOBID-2). J. Physiother. 2017, 63, 261. [Google Scholar] [CrossRef] [PubMed]
- Husebo, B.; Ostelo, R.; Strand, L. The MOBID -2 pain scale: Reliability and responsiveness to pain in patients with dementia. Eur. J. Pain 2014, 18, 1419–1430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cohen-Mansfield, J.; Marx, M.S.; Rosenthal, A.S. A Description of Agitation in a Nursing Home. J. Gerontol. 1989, 44, M77–M84. [Google Scholar] [CrossRef] [PubMed]
- Koss, E.; Weiner, M.; Ernesto, C.; Cohen-Mansfield, J.; Ferris, S.H.; Grundman, M.; Schafer, K.; Sano, M.; Thal, L.J.; Thomas, R.; et al. Assessing patterns of agitation in Alzheimer’s disease patients with the Cohen-Mansfield Agitation Inventory. The Alzheimer’s Disease Cooperative Study. Alzheimer Dis. Assoc. Disord. 1997, 11 (Suppl. 2), S45–S50. [Google Scholar] [CrossRef]
- Cohen-Mansfield, J.; Libin, A. Assessment of agitation in elderly patients with dementia: Correlations between informant rating and direct observation. Int. J. Geriatr. Psychiatry 2004, 19, 881–891. [Google Scholar] [CrossRef]
- Cohen-Mansfield, J.; Libin, A. Verbal and physical non-aggressive agitated behaviors in elderly persons with dementia: Robustness of syndromes. J. Psychiatr. Res. 2005, 39, 325–332. [Google Scholar] [CrossRef]
- Cummings, J.L.; Mega, M.; Gray, K.; Rosenberg-Thompson, S.; Carusi, D.A.; Gornbein, J. The Neuropsychiatric Inventory: Comprehensive assessment of psychopathology in dementia. Neurology 1994, 44, 2308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cummings, J.L. The Neuropsychiatric Inventory: Assessing psychopathology in dementia patients. Neurology 1997, 48 (Suppl. 6), 10S–16S. [Google Scholar] [CrossRef] [Green Version]
- Cummings, J. The Neuropsychiatric Inventory: Development and Applications. J. Geriatr. Psychiatry Neurol. 2020, 33, 73–84. [Google Scholar] [CrossRef]
- Moher, D.; Hopewell, S.; Schulz, K.F.; Montori, V.; Gotzsche, P.C.; Devereaux, P.J.; Elbourne, D.; Egger, M.; Altman, D.G. CONSORT 2010 explanation and elaboration: Updated guidelines for reporting parallel group randomised trials. BMJ 2010, 340, c869. [Google Scholar]
- Gillick, B.T.; Feyma, T.; Menk, J.; Usset, M.; Vaith, A.; Wood, T.J.; Worthington, R.; Krach, L. Safety and Feasibility of Transcranial Direct Current Stimulation in Pediatric Hemiparesis: Randomized Controlled Preliminary Study. Phys. Ther. 2015, 95, 337–349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cha, Y.-H.; Urbano, D.; Pariseau, N. Randomized Single Blind Sham Controlled Trial of Adjunctive Home-Based tDCS after rTMS for Mal De Debarquement Syndrome: Safety, Efficacy, and Participant Satisfaction Assessment. Brain Stimul. 2016, 9, 537–544. [Google Scholar] [CrossRef]
- Larsen, D.L.; Attkisson, C.C.; Hargreaves, W.A.; Nguyen, T.D. Assessment of client/patient satisfaction: Development of a general scale. Eval. Progr. Plan. 1979, 2, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.D.; Attkisson, C.; Stegner, B.L. Assessment of patient satisfaction: Development and refinement of a Service Evaluation Questionnaire. Eval. Progr. Plan. 1983, 6, 299–313. [Google Scholar] [CrossRef]
- Bikson, M.; Grossman, P.; Thomas, C.; Zannou, A.L.; Jiang, J.; Adnan, T.; Mourdoukoutas, A.P.; Kronberg, G.; Truong, D.; Boggio, P.; et al. Safety of Transcranial Direct Current Stimulation: Evidence Based Update 2016. Brain Stimul. 2016, 9, 641–661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farrar, J.T.; Young, J.P., Jr.; LaMoreaux, L.; Werth, J.L.; Poole, R.M. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain 2001, 94, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Saxena, V.; Pal, A. Role of Transcranial Direct Current Stimulation in the Management of Alzheimer’s Disease: A Meta-analysis of Effects, Adherence and Adverse Effects. Clin. Psychopharmacol. Neurosci. 2021, 19, 589–599. [Google Scholar] [CrossRef]
- Riggs, A.; Patel, V.; Paneri, B.; Portenoy, R.K.; Bikson, M.; Knotkova, H. At-Home Transcranial Direct Current Stimulation (tDCS) With Telehealth Support for Symptom Control in Chronically-Ill Patients With Multiple Symptoms. Front. Behav. Neurosci. 2018, 12. [Google Scholar] [CrossRef] [PubMed]
- Montero-Hernandez, S.; Martorella, G.; Miao, H.; Pollonini, L.; Ahn, H. Inter- and Intra-Region Functional Connectivity in Nonpharmacological Pain Management. In Proceedings of the Society of Functional Near-Infrared Spectroscopy 2022 Conference, Boston, MA, USA, 9–12 October 2022. [Google Scholar]
- Pollonini, L.; Miao, H.; Ahn, H. Longitudinal effect of transcranial direct current stimulation on knee osteoarthritis patients measured by functional infrared spectroscopy: A pilot study. Neurophotonics 2020, 7, 025004. [Google Scholar] [CrossRef]
- Pollonini, L.; Montero-Hernandez, S.; Park, L.; Miao, H.; Mathis, K.; Ahn, H. Functional Near-Infrared Spectroscopy to Assess Central Pain Responses in a Nonpharmacologic Treatment Trial of Osteoarthritis. J. Neuroimaging 2020, 30, 808–814. [Google Scholar] [CrossRef]
- Keeser, D.; Meindl, T.; Bor, J.; Palm, U.; Pogarell, O.; Mulert, C.; Brunelin, J.; Möller, H.-J.; Reiser, M.; Padberg, F. Prefrontal Transcranial Direct Current Stimulation Changes Connectivity of Resting-State Networks during fMRI. J. Neurosci. 2011, 31, 15284–15293. [Google Scholar] [CrossRef]
- Naegel, S.; Biermann, J.; Theysohn, N.; Kleinschnitz, C.; Diener, H.-C.; Katsarava, Z.; Obermann, M.; Holle, D. Polarity-specific modulation of pain processing by transcranial direct current stimulation—A blinded longitudinal fMRI study. J. Headache Pain 2018, 19, 99. [Google Scholar] [CrossRef] [PubMed]
- Cummiford, C.M.; Nascimento, T.D.; Foerster, B.R.; Clauw, D.J.; Zubieta, J.-K.; Harris, R.E.; DaSilva, A.F. Changes in resting state functional connectivity after repetitive transcranial direct current stimulation applied to motor cortex in fibromyalgia patients. Arthritis Res. Ther. 2016, 18, 40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Da Silva, J.T.; Letzen, J.E.; Haythornthwaite, J.A.; Finan, P.H.; Campbell, C.M.; Seminowicz, D. Do chronic pain and comorbidities affect brain function in sickle cell patients? A systematic review of neuroimaging and treatment approaches. Pain 2019, 160, 1933–1945. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Fisher, D.W.; Yu, T.; Dong, H. The link between chronic pain and Alzheimer’s disease. J. Neuroinflamm. 2019, 16, 204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Husebo, B.S.; Corbett, A. Dementia: Pain management in dementia--the value of proxy measures. Nat. Rev. Neurol. 2014, 10, 313–314. [Google Scholar] [CrossRef]
- Amspoker, A.B.; Snow, A.L.; Renn, B.; Block, P.; Pickens, S.; Morgan, R.O.; Kunik, M.E. Patient Versus Informal Caregiver Proxy Reports of Pain Interference in Persons With Dementia. J. Appl. Gerontol. 2020, 40, 414–422. [Google Scholar] [CrossRef]
- Tavares, D.R.B.; Okazaki, J.E.F.; Santana, M.V.D.A.; Pinto, A.C.P.N.; Tutiya, K.K.; Gazoni, F.M.; Pinto, C.B.; Santos, F.C.; Fregni, F.; Trevisani, V.F.M. Motor cortex transcranial direct current stimulation effects on knee osteoarthritis pain in elderly subjects with dysfunctional descending pain inhibitory system: A randomized controlled trial. Brain Stimul. 2021, 14, 477–487. [Google Scholar] [CrossRef]
- Loo, C.K.; Husain, M.M.; McDonald, W.M.; Aaronson, S.; O’Reardon, J.P.; Alonzo, A.; Weickert, C.S.; Martin, D.M.; McClintock, S.M.; Mohan, A.; et al. International randomized-controlled trial of transcranial Direct Current Stimulation in depression. Brain Stimul. 2018, 11, 125–133. [Google Scholar] [CrossRef]
- Pacheco-Barrios, K.; Cardenas-Rojas, A.; Thibaut, A.; Costa, B.; Ferreira, I.; Caumo, W.; Fregni, F. Methods and strategies of tDCS for the treatment of pain: Current status and future directions. Expert Rev. Med. Devices 2020, 17, 879–898. [Google Scholar] [CrossRef]


| Sham tDCS (N = 20) | Active tDCS (N = 20) | p Value | |
|---|---|---|---|
| Age, years, M (SD) | 71.90 (9.20) | 74.15 (6.32) | 0.37 |
| Gender, n (%) | 0.72 | ||
| Male | 5 (25.0%) | 6 (30.0%) | |
| Female | 15 (75.0%) | 14 (70.0%) | |
| Race, n (%) | 0.55 | ||
| Asian | 1 (5.0%) | 1 (5.0%) | |
| Black African | 0 (0.0%) | 1 (5.0%) | |
| White | 19 (95.0%) | 17 (85.0%) | |
| Hispanic or Latino | 0 (0.0%) | 1 (5.0%) | |
| BMI, kg/m2, M (SD) | 26.92 (5.35) | 26.73 (5.35) | 0.91 |
| Marital Status | 0.64 | ||
| Married | 11 (55.0%) | 12 (60.0%) | |
| Widowed | 3 (15.0%) | 2 (10.0%) | |
| Divorced | 4 (20.0%) | 4 (20.0%) | |
| Separated | 1 (5.0%) | 0 (0.0%) | |
| Never married | 0 (0.0%) | 1 (5.0%) | |
| Living with partner | 0 (0.0%) | 1 (5.0%) | |
| Refused | 1 (5.0%) | 0 (0.0%) | |
| Education, n (%) | 0.73 | ||
| Some school but did not complete high school | 1 (5.0%) | 0 (0.0%) | |
| High school degree | 4 (20.0%) | 5 (25.0%) | |
| Two-year college degree | 3 (15.0%) | 2 (10.0%) | |
| Four-year college degree | 3 (15.0%) | 6 (30.0%) | |
| Master’s degree | 4 (20.0%) | 4 (20.0%) | |
| Doctoral degree | 5 (25.0%) | 3 (15.0%) | |
| NRS, M (SD) | 37.95 (30.83) | 45.75 (27.54) | 0.27 |
| MOBID-2, M (SD) | 3.05 (1.93) | 4.50 (2.84) | 0.07 |
| CMAI, M (SD) | 36.80 (10.87) | 37.80 (9.06) | 0.51 |
| NPI, M (SD) | 9.25 (12.17) | 12.85 (15.65) | 0.42 |
| Variable | Sham tDCS (N = 20) | Active tDCS (N = 20) | Effect Size (d) | p-Value |
|---|---|---|---|---|
| NRS changes between 5-day and baseline | −3.30 (14.81) | −13.55 (14.90) | 0.69 | 0.02 |
| NRS changes between 3-month after intervention and baseline | −8.30 (27.59) | −18.7 (27.48) | 0.39 | 0.23 |
| MOBID changes between 5-day and baseline | −0.40 (1.67) | −2.70 (2.39) | 1.12 | <0.01 |
| MOBID changes between 3-month after intervention and baseline | −0.30 (1.81) | −2.55 (3.46) | 0.82 | 0.06 |
| CMAI changes between 5-day and baseline | −2.95 (5.24) | −2.35 (5.99) | 0.11 | 0.95 |
| CMAI changes between 3-month after intervention and baseline | −3.90 (7.16) | −5.30 (9.80) | 0.16 | 0.53 |
| NPI changes between 5-day and baseline | −2.30 (11.59) | −2.9 (15.08) | 0.10 | 0.57 |
| NPI changes between 3-month after intervention and baseline | −1.00 (5.88) | −7.05 (11.96) | 0.05 | 0.09 |
| Variable | Estimate | Standard Error | 95% Confidence Intervals | T Value | p Value | |
|---|---|---|---|---|---|---|
| Intercept | 37.95 | 5.74 | 26.84 | 49.06 | 6.61 | 0.50 |
| Day 5 | −3.30 | 4.88 | −12.67 | 6.07 | −0.68 | 0.50 |
| Month1 | −3.30 | 4.88 | −12.67 | 6.07 | −0.68 | 0.31 |
| Month2 | −4.95 | 4.88 | −14.32 | 4.42 | −1.01 | 0.09 |
| Month3 | −8.30 | 4.88 | −12.67 | 6.07 | −1.70 | 0.34 |
| Active tDCS | 7.80 | 8.12 | −17.67 | 1.07 | 0.96 | 0.34 |
| Day 5: Active tDCS | −10.25 | 6.90 | −23.50 | 3.00 | −1.49 | 0.14 |
| Month1: Active tDCS | −14.80 | 6.90 | −28.05 | −1.54 | −2.15 | 0.03 |
| Month2: Active tDCS | −16.15 | 6.90 | −29.40 | −2.89 | −2.34 | 0.02 |
| Month3: Active tDCS | −10.40 | 6.90 | −23.65 | 2.85 | −1.51 | 0.13 |
| Variable | Estimate | Standard Error | 95% Confidence Intervals | T Value | p Value | |
|---|---|---|---|---|---|---|
| Intercept | 37.95 | 5.74 | 26.83 | 49.06 | 6.61 | <0.01 |
| Day 5 | −3.30 | 4.88 | −12.67 | 6.07 | −0.68 | 0.50 |
| Month1 | −3.30 | 4.88 | −12.67 | 6.07 | −0.68 | 0.50 |
| Month2 | −4.95 | 4.88 | −14.32 | 4.21 | −1.02 | 0.31 |
| Month3 | −8.30 | 4.88 | −17.67 | 1.07 | −1.70 | 0.09 |
| Active tDCS | 7.80 | 8.12 | −7.91 | 23.51 | 0.96 | 0.34 |
| Day 5: Active tDCS | −10.25 | 6.90 | −23.50 | 3.00 | −1.49 | 0.14 |
| Month1: Active tDCS | −14.80 | 6.90 | −28.05 | −1.55 | −2.15 | 0.03 |
| Month2: Active tDCS | −16.15 | 6.90 | −29.40 | −2.89 | −2.34 | 0.02 |
| Month3: Active tDCS | −10.4 | 6.90 | −23.65 | 2.85 | −1.51 | 0.13 |
| Items of the tDCS Experience Questionnaire | Mean (SD) |
|---|---|
| 1. It was easy to prepare the device and accessories (0–10) | 8.98 (2.36) |
| 2. The device was unnecessarily complex (0–10) | 0.80 (2.26) |
| 3. The device was easy to use (0–10) | 9.45 (1.80) |
| 4. I felt the video conferences with a technical person were helpful (0–10) | 9.27 (1.94) |
| 5. I would imagine that most people would learn to use this device quickly (0–10) | 9.60 (0.98) |
| 6. The device was cumbersome to use (0–10) | 1.25 (2.68) |
| 7. I felt confident using the device (0–10) | 9.35 (1.85) |
| 8. I needed to learn a lot of things before I could get going with this device (0–10) | 2.20 (3.62) |
| 9. The effectiveness of the treatment increased over the course of treatment (0–10) | 6.90 (3.48) |
| 10. Overall, I felt that transcranial electrical stimulation treatment benefited me (0–10) | 6.80 (3.44) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martorella, G.; Miao, H.; Wang, D.; Park, L.; Mathis, K.; Park, J.; Sheffler, J.; Granville, L.; Teixeira, A.L.; Schulz, P.E.; et al. Feasibility, Acceptability, and Efficacy of Home-Based Transcranial Direct Current Stimulation on Pain in Older Adults with Alzheimer’s Disease and Related Dementias: A Randomized Sham-Controlled Pilot Clinical Trial. J. Clin. Med. 2023, 12, 401. https://doi.org/10.3390/jcm12020401
Martorella G, Miao H, Wang D, Park L, Mathis K, Park J, Sheffler J, Granville L, Teixeira AL, Schulz PE, et al. Feasibility, Acceptability, and Efficacy of Home-Based Transcranial Direct Current Stimulation on Pain in Older Adults with Alzheimer’s Disease and Related Dementias: A Randomized Sham-Controlled Pilot Clinical Trial. Journal of Clinical Medicine. 2023; 12(2):401. https://doi.org/10.3390/jcm12020401
Chicago/Turabian StyleMartorella, Geraldine, Hongyu Miao, Duo Wang, Lindsey Park, Kenneth Mathis, JuYoung Park, Julia Sheffler, Lisa Granville, Antonio L. Teixeira, Paul E. Schulz, and et al. 2023. "Feasibility, Acceptability, and Efficacy of Home-Based Transcranial Direct Current Stimulation on Pain in Older Adults with Alzheimer’s Disease and Related Dementias: A Randomized Sham-Controlled Pilot Clinical Trial" Journal of Clinical Medicine 12, no. 2: 401. https://doi.org/10.3390/jcm12020401






