Evaluation of the Properties of 3D-Printed Ti Alloy Plates: In Vivo and In Vitro Comparative Experimental Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of the Ti Plates Specimens
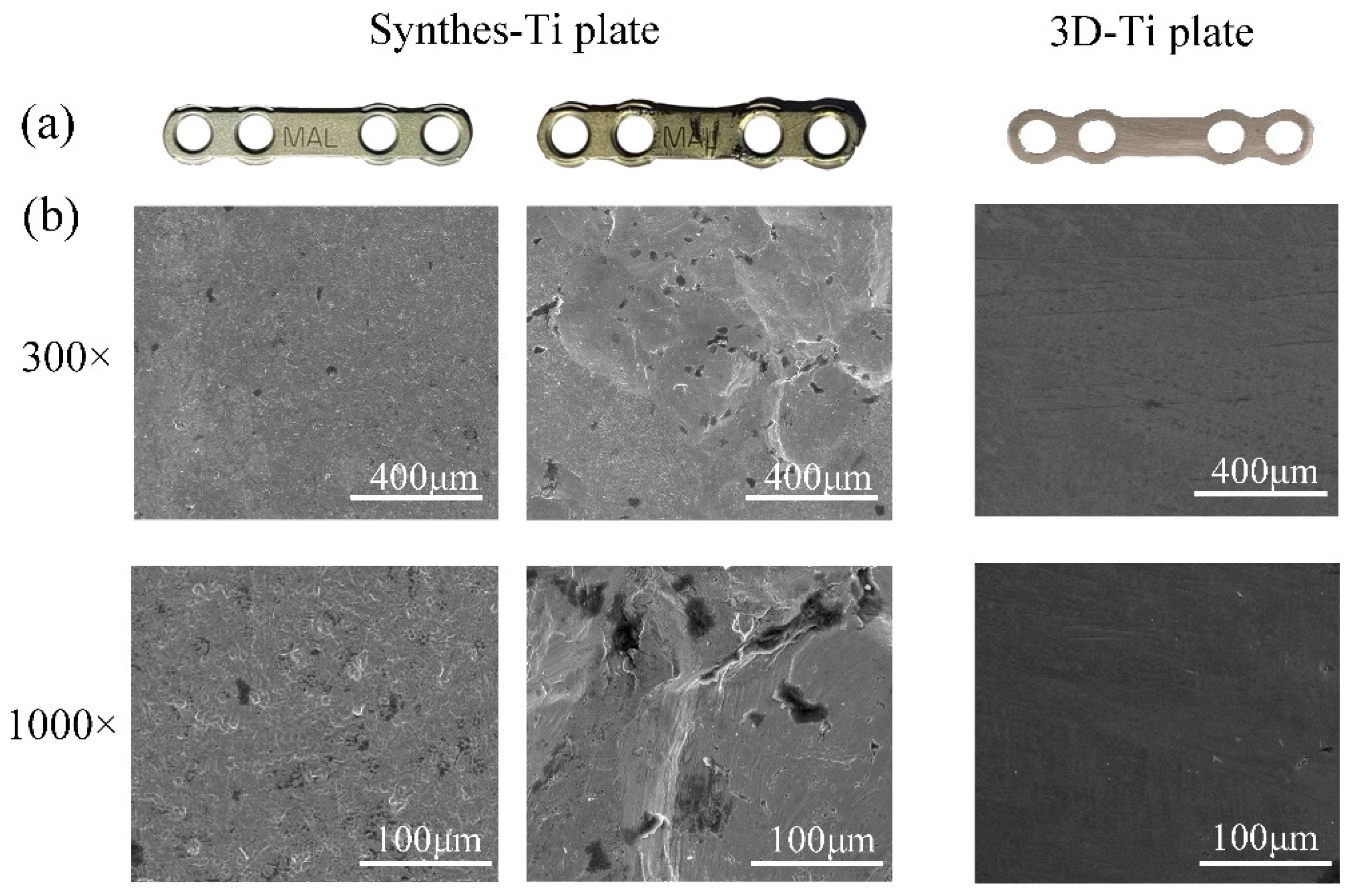
2.2. Surface Morphology
2.3. Mechanical Properties
2.4. In Vitro Cell Behavior
2.4.1. Cell Adhesion
2.4.2. Cell Proliferation
2.5. Animal Study
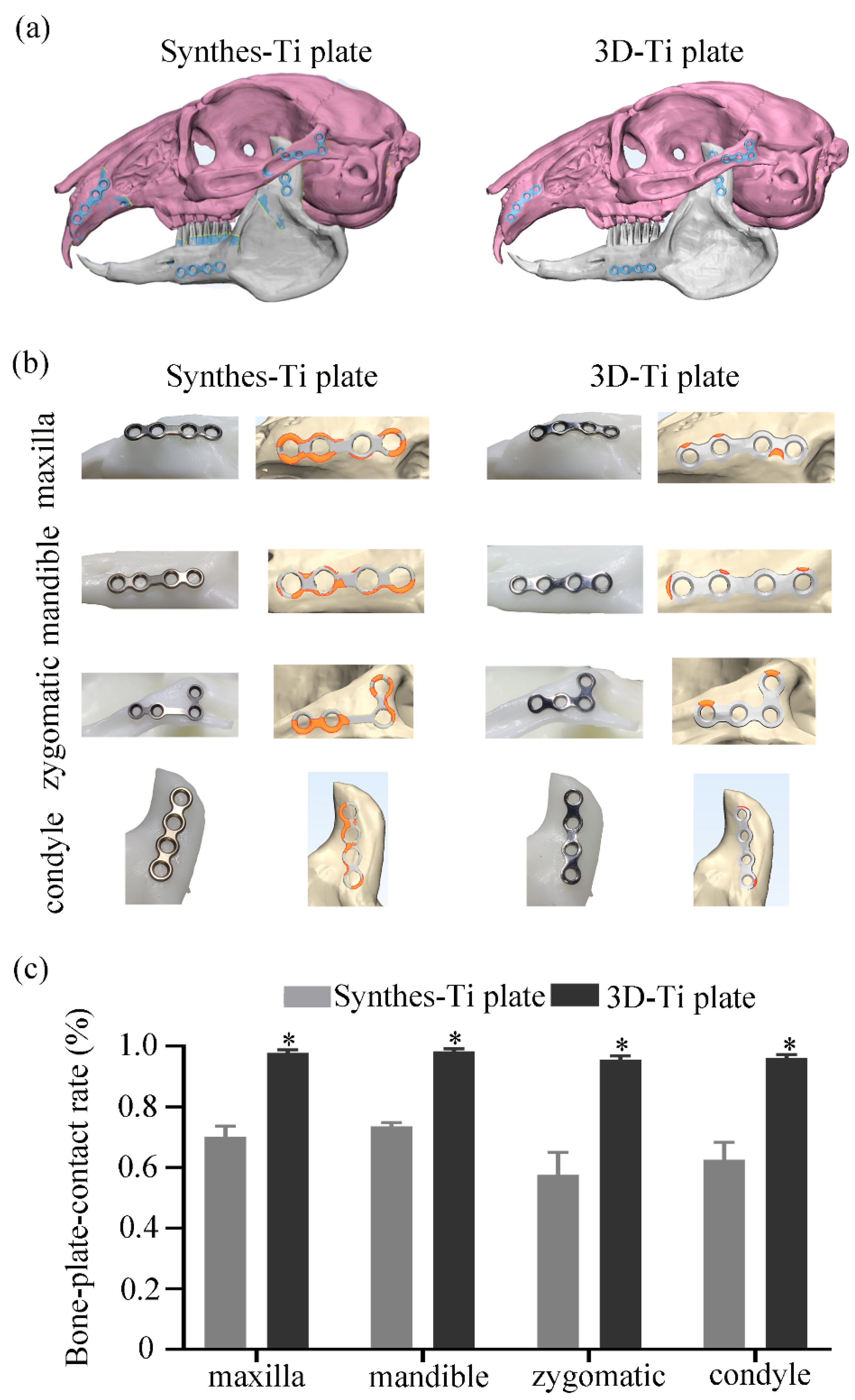
2.5.1. Accuracy Evaluation of the Ti Plate Shaping
2.5.2. Animal Model and Surgery
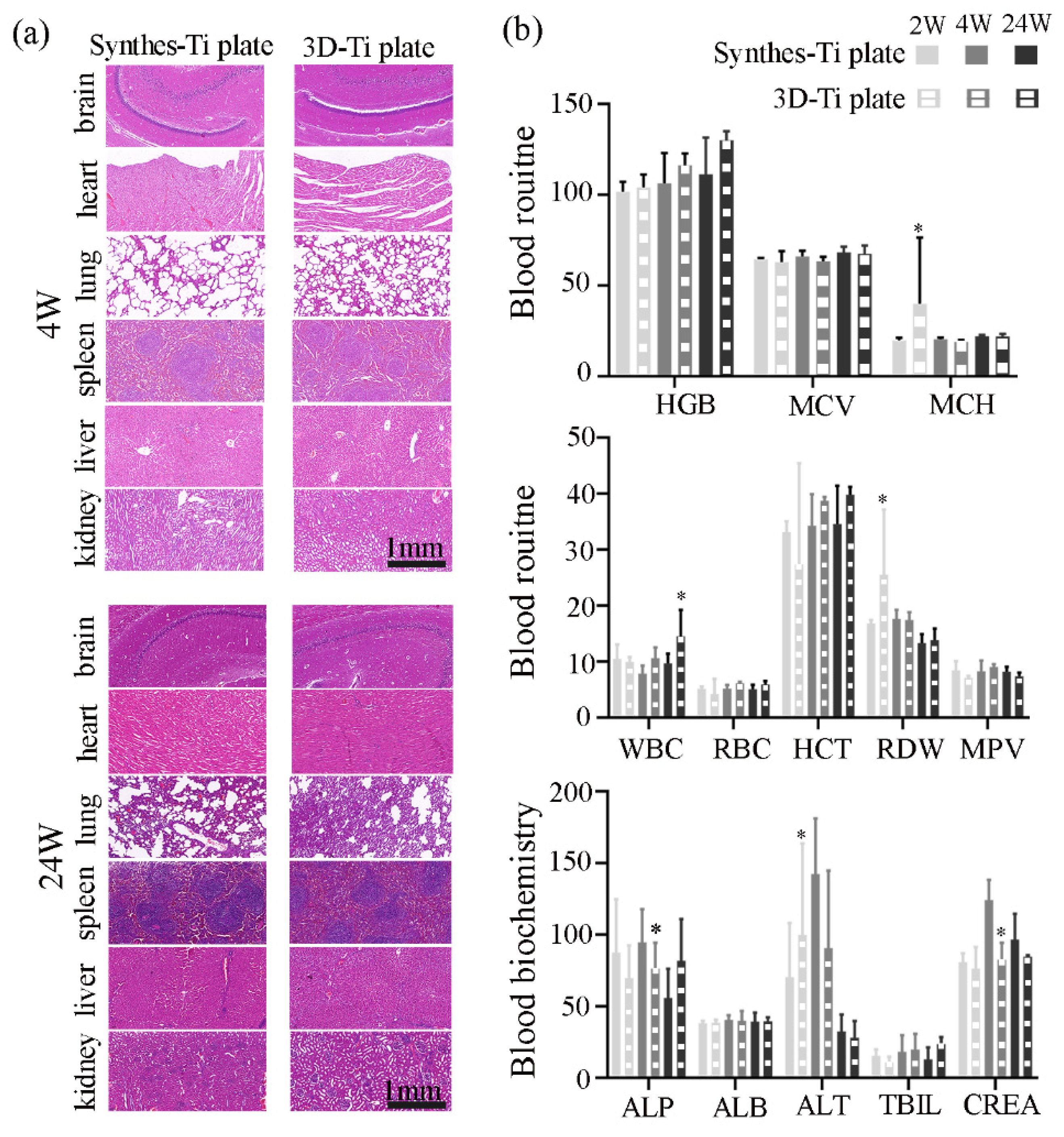
2.5.3. Biosafety Assessment
2.5.4. Evaluation Effectiveness of the Ti Plates in the Fracture Fixation
2.5.5. Evaluation of the Mechanical Stability of the Ti Plates
2.6. Statistics
3. Results
3.1. The Mechanical Properties and Surface Morphology of the Ti Plates
3.2. In Vitro Cell Behavior
3.2.1. Cell Adhesion
3.2.2. Cell Proliferation
3.3. In Vivo Animal Study
3.3.1. Accuracy of the Ti Plate Shaping
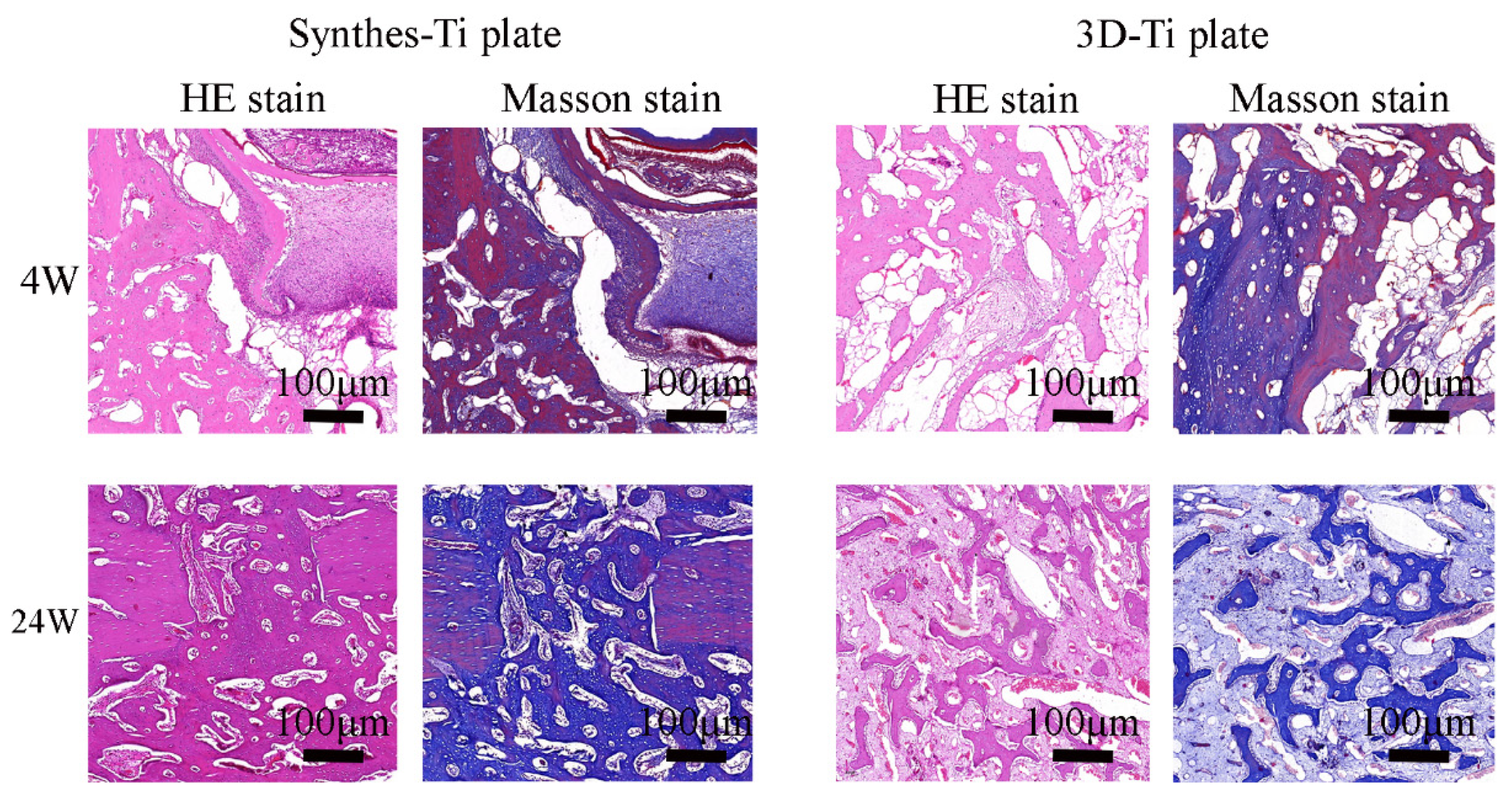
3.3.2. Biosafety of the Ti Plates
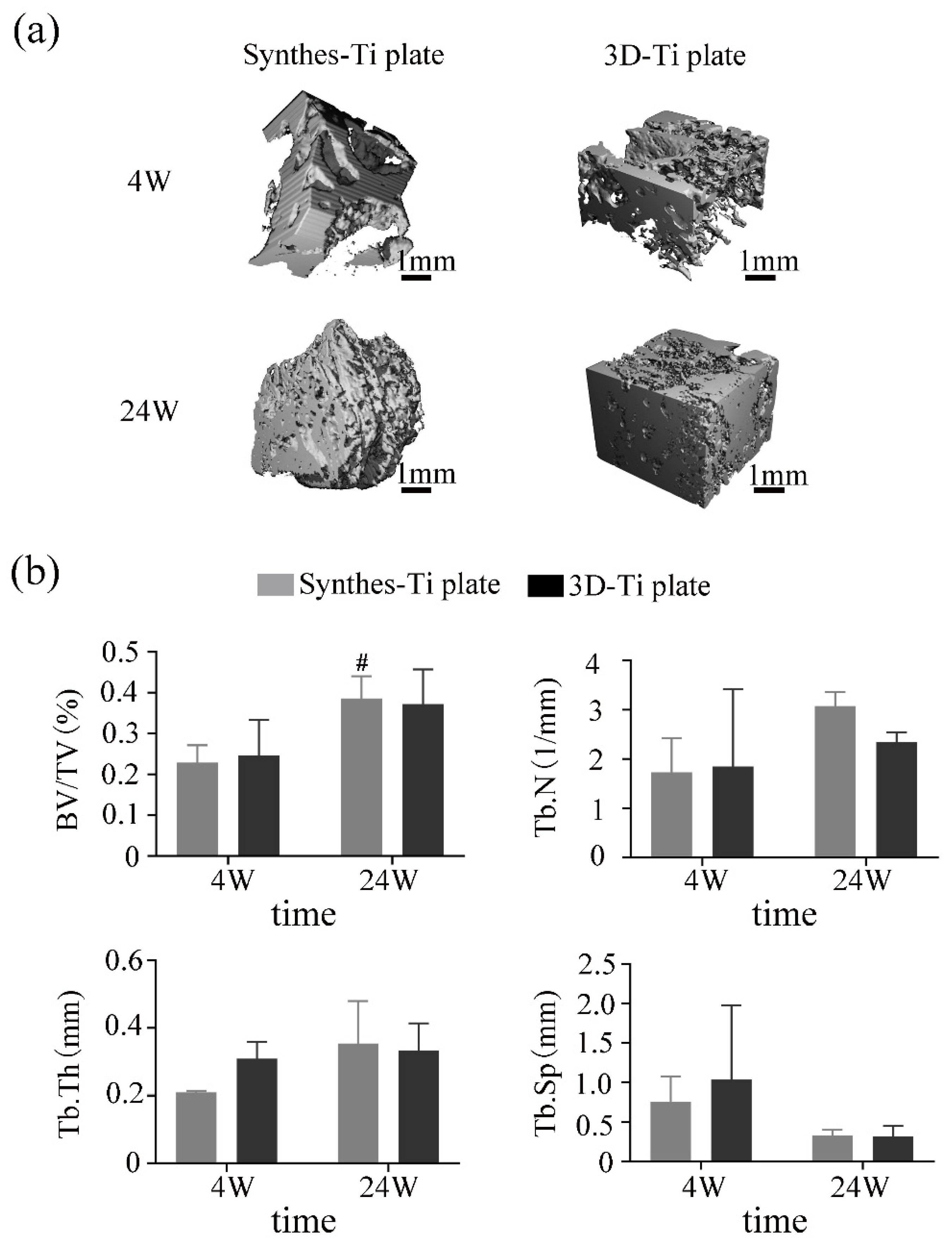
3.3.3. The Effectiveness of the Ti Plates in the Fracture Fixation
3.3.4. Mechanical Stability of 3D-Ti Plate
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kirby, B.; Kenkel, J.M.; Zhang, A.Y.; Amirlak, B.; Suszynski, T.M. Three-dimensional (3D) synthetic printing for the manufacture of non-biodegradable models, tools and implants used in surgery: A review of current methods. J. Med. Eng. Technol. 2021, 45, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Xue, X.; Yu, J.; Jiang, T.; Li, X.; Lin, K.; Zhang, L.; Wang, X. The performance tests of three-dimensional printing titanium alloy craniomaxillofacial bone plate: A preliminary preclinical study. J. Dent. Sci. 2021, 206, 82–98. [Google Scholar] [CrossRef]
- Liu, P.-c.; Yang, Y.-j.; Liu, R.; Shu, H.-x.; Gong, J.-p.; Yang, Y.; Sun, Q.; Wu, X.; Cai, M. A study on the mechanical characteristics of the EBM-printed Ti-6Al-4V LCP plates in vitro. J. Orthop. Surg. 2014, 9, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feller, L.; Chandran, R.; Khammissa, R.A.; Meyerov, R.; Jadwat, Y.; Bouckaert, M.; Schechter, I.; Lemmer, J. Osseointegration: Biological events in relation to characteristics of the implant surface. S. Afr. Dent. J. 2014, 69, 112–117. [Google Scholar]
- Martola, M.; Lindqvist, C.; Hänninen, H.; Al-Sukhun, J. Fracture of titanium plates used for mandibular reconstruction following ablative tumor surgery. J. Biomed. Mater. Res. Part B Appl. Biomater. Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2007, 80, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Loukota, R.; Shelton, J. Mechanical analysis of maxillofacial miniplates. Br. J. Oral Maxillofac. Surg. 1995, 33, 174–179. [Google Scholar] [CrossRef]
- Liu, L.-N.; Li, J.-Y.; Li, K.-H.; Wu, Q.-H.; Liu, Y.; Luo, E. Deformation Assessment of the Manually Pre-Bent Titanium Miniplates in Orthognathic Surgery with Finite Element Analysis. J. Craniofacial Surg. 2021, 32, 883–887. [Google Scholar] [CrossRef]
- Jardini, A.L.; Larosa, M.A.; Maciel Filho, R.; de Carvalho Zavaglia, C.A.; Bernardes, L.F.; Lambert, C.S.; Calderoni, D.R.; Kharmandayan, P. Cranial reconstruction: 3D biomodel and custom-built implant created using additive manufacturing. J. Cranio-Maxillofac. Surg. 2014, 42, 1877–1884. [Google Scholar] [CrossRef]
- Philippe, B. Guided maxillofacial surgery: Simulation and surgery aided by stereolithographic guides and custom-made miniplates. Rev. Stomatol. Chir Maxillofac. Chir Orale 2013, 114, 228–246. [Google Scholar] [CrossRef]
- Wang, C.-F.; Yu, Y.; Bai, W.; Han, J.-M.; Zhang, W.-B.; Peng, X. Mechanical properties of three-dimensionally printed titanium plates used in jaw reconstruction: Preliminary study. Int. J. Oral Maxillofac. Surg. 2022, 51, 754–761. [Google Scholar] [CrossRef]
- Lauria, A.; de Medeiros, R.C.; Rodrigues, D.C.; Sato, F.R.L.; Moreira, R.W.F. Evaluation of cyclic and linear mechanical resistance of prebent and manually-bent plates used for maxillary advancement in orthognathic surgery. Br. J. Oral Maxillofac. Surg. 2016, 54, 987–991. [Google Scholar] [CrossRef] [PubMed]
- Jazayeri, H.E.; Khavanin, N.; Yu, J.W.; Lopez, J.; Shamliyan, T.; Peacock, Z.S.; Dorafshar, A.H. Fixation Points in the Treatment of Traumatic Zygomaticomaxillary Complex Fractures: A Systematic Review and Meta-Analysis. J. Oral Maxillofac. Surg. 2019, 77, 2064–2073. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, H.; Lai, Q.; Xue, R.; Zhu, K.; Deng, Y. Finite element analysis of 3D-printed personalized titanium plates for mandibular angle fracture. Comput. Methods Biomech. Biomed. Eng. 2022, 26, 78–89. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, T. An overview of the corrosion aspect of dental implants (titanium and its alloys). Indian J. Dent. Res. 2009, 20, 91. [Google Scholar] [CrossRef]
- Lin, X.; Xiao, X.; Wang, Y.; Gu, C.; Wang, C.; Chen, J.; Liu, H.; Luo, J.; Li, T.; Wang, D. Biocompatibility of bespoke 3D-printed titanium alloy plates for treating acetabular fractures. BioMed Res. Int. 2018, 2018, 2053486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ng, S.L.; Das, S.; Ting, Y.-P.; Wong, R.C.W.; Chanchareonsook, N. Benefits and biosafety of use of 3d-printing technology for titanium biomedical implants: A pilot study in the rabbit model. Int. J. Mol. Sci. 2021, 22, 8480. [Google Scholar] [CrossRef]
- Wei, H.-p.; Wang, X.-d. Advances in titanium bone implants made by rapid prototyping technology. Chin. J. Tissue Eng. Res. 2017, 21, 3583. [Google Scholar]
- Ponader, S.; Vairaktaris, E.; Heinl, P.; Wilmowsky, C.v.; Rottmair, A.; Körner, C.; Singer, R.F.; Holst, S.; Schlegel, K.A.; Neukam, F.W. Effects of topographical surface modifications of electron beam melted Ti-6Al-4V titanium on human fetal osteoblasts. J. Biomed. Mater. Res. Part A: Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2008, 84, 1111–1119. [Google Scholar] [CrossRef]
- Gómez-Barrachina, R.; Montiel-Company, J.; García-Sanz, V.; Almerich-Silla, J.; Paredes-Gallardo, V.; Bellot-Arcís, C. Titanium plate removal in orthognathic surgery: Prevalence, causes and risk factors. A systematic literature review and meta-analysis. Int. J. Oral Maxillofac. Surg. 2020, 49, 770–778. [Google Scholar] [CrossRef]
- Mazurek-Popczyk, J.; Palka, L.; Arkusz, K.; Dalewski, B.; Baldy-Chudzik, K. Personalized, 3D-printed fracture fixation plates versus commonly used orthopedic implant materials-biomaterials characteristics and bacterial biofilm formation. Injury 2022, 53, 938–946. [Google Scholar] [CrossRef]
- Ellis, E., 3rd. Rigid skeletal fixation of fractures. J. Oral Maxillofac. Surg. 1993, 51, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Katakura, A.; Shibahara, T.; Noma, H.; Yoshinari, M. Material analysis of AO plate fracture cases. J. Oral Maxillofac. Surg. 2004, 62, 348–352. [Google Scholar] [CrossRef] [PubMed]
- Sidambe, A.T. Biocompatibility of advanced manufactured titanium implants—A review. Materials 2014, 7, 8168–8188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ni, J.; Ling, H.; Zhang, S.; Wang, Z.; Peng, Z.; Benyshek, C.; Zan, R.; Miri, A.; Li, Z.; Zhang, X. Three-dimensional printing of metals for biomedical applications. Mater. Today Bio. 2019, 3, 100024. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.-F.; Choi, W.S.; Leung, Y.Y.; Curtin, J.P.; Du, R.; Zhang, C.-Y.; Chen, X.-S.; Su, Y.-X. Three-dimensional printing of patient-specific surgical plates in head and neck reconstruction: A prospective pilot study. Oral Oncol. 2018, 78, 31–36. [Google Scholar] [CrossRef]
- Yang, W.-f.; Zhang, C.-y.; Choi, W.; Zhu, W.-Y.; Li, D.; Chen, X.-S.; Du, R.; Su, Y.-X. A novel ‘surgeon-dominated’approach to the design of 3D-printed patient-specific surgical plates in mandibular reconstruction: A proof-of-concept study. Int. J. Oral Maxillofac. Surg. 2020, 49, 13–21. [Google Scholar] [CrossRef]
- Yang, W.-f.; Choi, W.S.; Wong, M.C.-M.; Powcharoen, W.; Zhu, W.-y.; Tsoi, J.K.-H.; Chow, M.; Kwok, K.-W.; Su, Y.-x. Three-dimensionally printed patient-specific surgical plates increase accuracy of oncologic head and neck reconstruction versus conventional surgical plates: A comparative study. Ann. Surg. Oncol. 2021, 28, 363–375. [Google Scholar] [CrossRef]
- Van den Bempt, M.; Liebregts, J.; Maal, T.; Berge, S.; Xi, T. Toward a higher accuracy in orthognathic surgery by using intraoperative computer navigation, 3D surgical guides, and/or customized osteosynthesis plates: A systematic review. J. Cranio Maxillofac. Surg. 2018, 46, 2108–2119. [Google Scholar] [CrossRef]
- Figueiredo, C.; Paranhos, L.; da Silva, R.; Herval, Á.; Blumenberg, C.; Zanetta-Barbosa, D. Accuracy of orthognathic surgery with customized titanium plates–Systematic review. J. Stomatol. Oral Maxillofac. Surg. 2021, 122, 88–97. [Google Scholar] [CrossRef]
- Mazzoni, S.; Bianchi, A.; Schiariti, G.; Badiali, G.; Marchetti, C. Computer-aided design and computer-aided manufacturing cutting guides and customized titanium plates are useful in upper maxilla waferless repositioning. J. Oral Maxillofac. Surg. 2015, 73, 701–707. [Google Scholar] [CrossRef]
- Heufelder, M.; Wilde, F.; Pietzka, S.; Mascha, F.; Winter, K.; Schramm, A.; Rana, M. Clinical accuracy of waferless maxillary positioning using customized surgical guides and patient specific osteosynthesis in bimaxillary orthognathic surgery. J. Cranio Maxillofac. Surg. 2017, 45, 1578–1585. [Google Scholar] [CrossRef] [PubMed]
- Badiali, G.; Roncari, A.; Bianchi, A.; Taddei, F.; Marchetti, C.; Schileo, E. Navigation in orthognathic surgery: 3D accuracy. Facial Plast. Surg. 2015, 31, 463–473. [Google Scholar] [PubMed]
- Rios, O.; Lerhe, B.; Chamorey, E.; Savoldelli, C. Accuracy of Segmented Le Fort I Osteotomy with Virtual Planning in Orthognathic Surgery Using Patient-Specific Implants: A Case Series. J. Clin. Med. 2022, 11, 5495. [Google Scholar] [CrossRef] [PubMed]


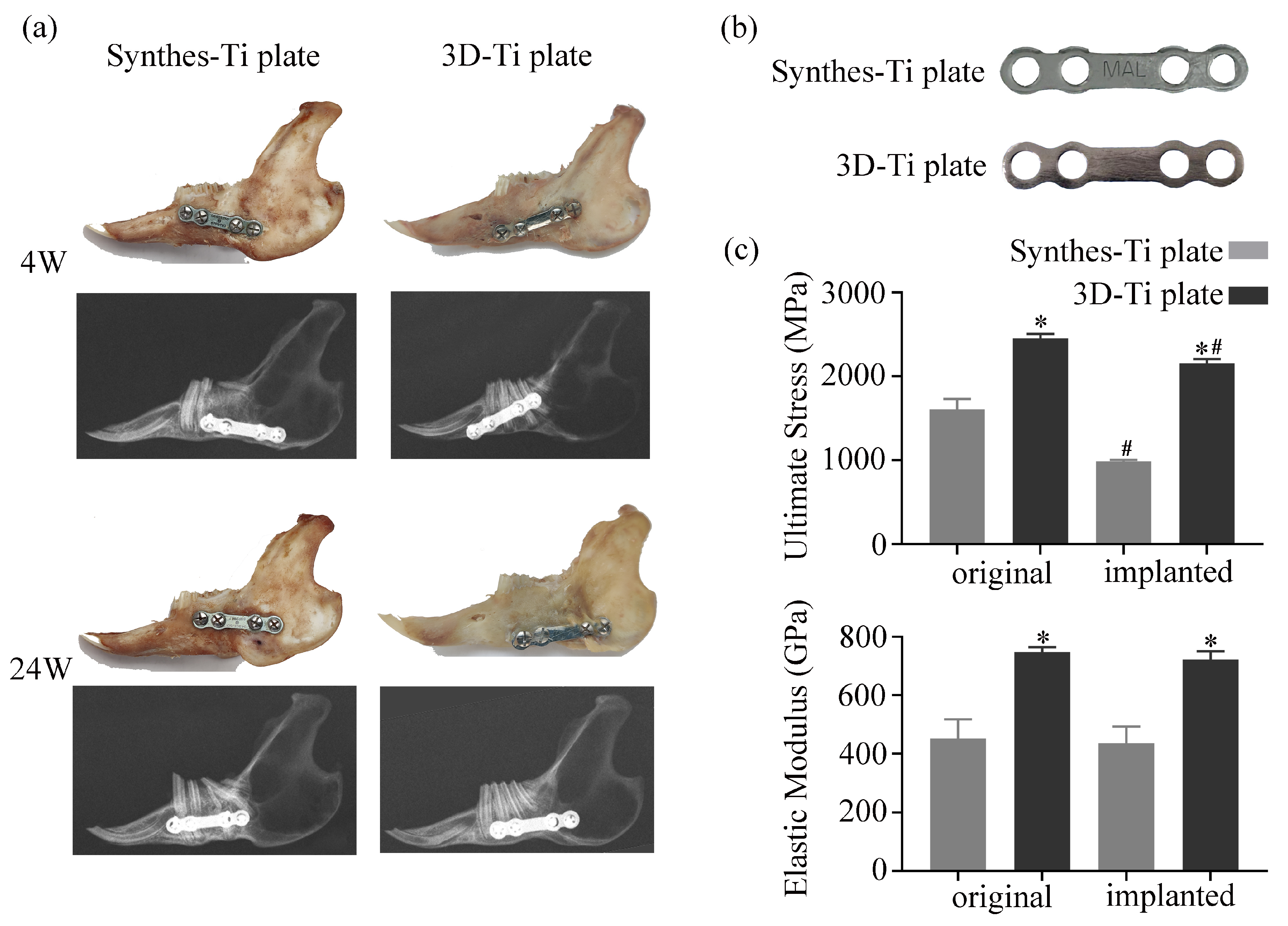
| Hardness (HV10) | Ultimate Stress (MPa) | Elasticity Modulus (GPa) | |
|---|---|---|---|
| Synthes-Ti plate | 156.04 ± 1.37 | 1670.77 ± 52.81 | 451.75 ± 66.139 |
| 3D-Ti plate | 370.78 ± 1.25 | 2153.13 ± 62.15 | 747.81 ± 16.813 |
| p | <0.0001 | <0.0001 | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Q.; Telha, W.; Wu, Y.; Abotaleb, B.; Jiang, N.; Zhu, S. Evaluation of the Properties of 3D-Printed Ti Alloy Plates: In Vivo and In Vitro Comparative Experimental Study. J. Clin. Med. 2023, 12, 444. https://doi.org/10.3390/jcm12020444
Wang Q, Telha W, Wu Y, Abotaleb B, Jiang N, Zhu S. Evaluation of the Properties of 3D-Printed Ti Alloy Plates: In Vivo and In Vitro Comparative Experimental Study. Journal of Clinical Medicine. 2023; 12(2):444. https://doi.org/10.3390/jcm12020444
Chicago/Turabian StyleWang, Qi, Wael Telha, Yange Wu, Bassam Abotaleb, Nan Jiang, and Songsong Zhu. 2023. "Evaluation of the Properties of 3D-Printed Ti Alloy Plates: In Vivo and In Vitro Comparative Experimental Study" Journal of Clinical Medicine 12, no. 2: 444. https://doi.org/10.3390/jcm12020444
APA StyleWang, Q., Telha, W., Wu, Y., Abotaleb, B., Jiang, N., & Zhu, S. (2023). Evaluation of the Properties of 3D-Printed Ti Alloy Plates: In Vivo and In Vitro Comparative Experimental Study. Journal of Clinical Medicine, 12(2), 444. https://doi.org/10.3390/jcm12020444








