Influence of Snoring on the Incidence of Metabolic Syndrome: A Community-Based Prospective Cohort Study in Rural Northeast China
Abstract
1. Introduction
2. Methods
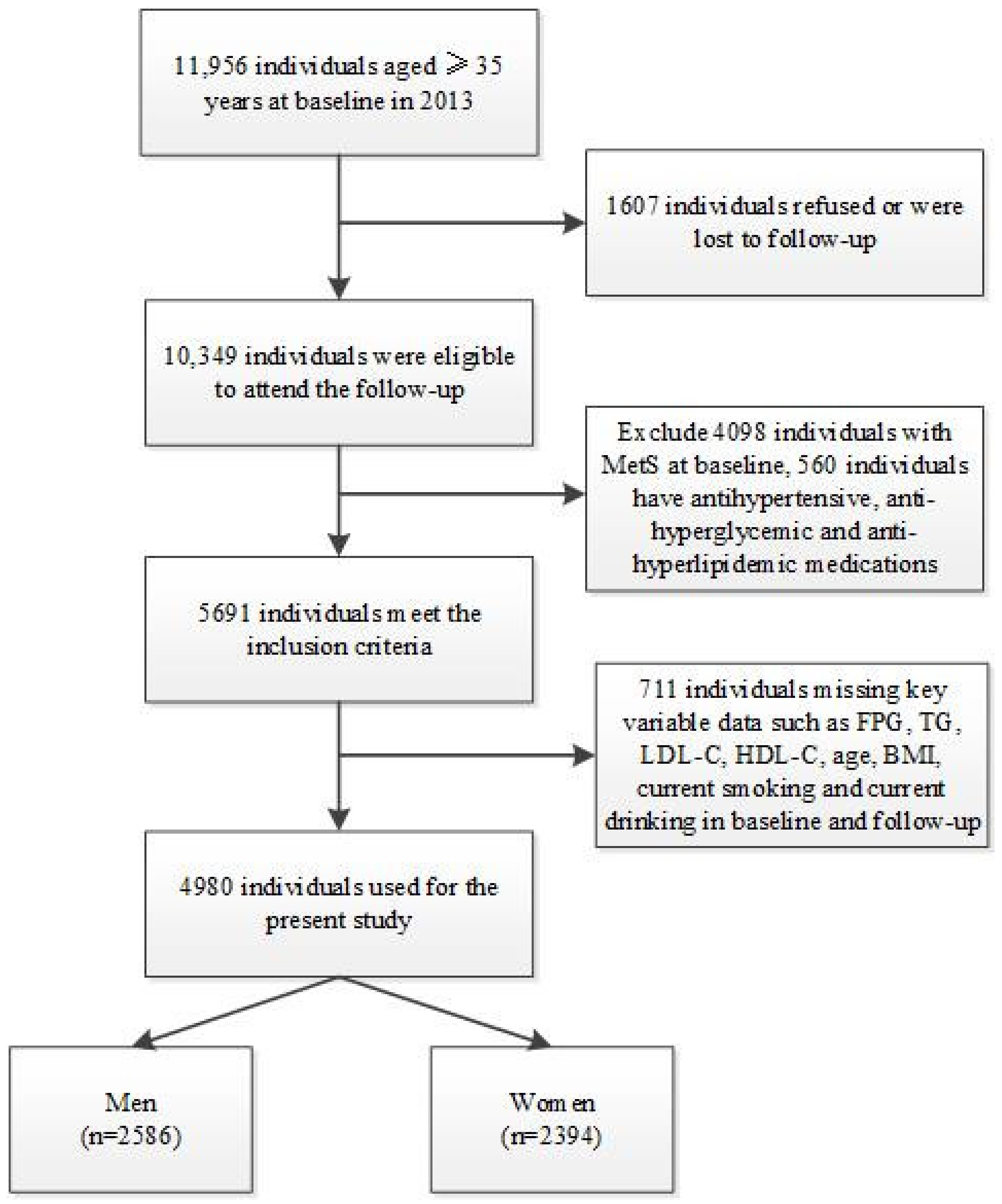
2.1. Study Design and Data Source
2.2. Study Variables
2.3. Statistical Analysis
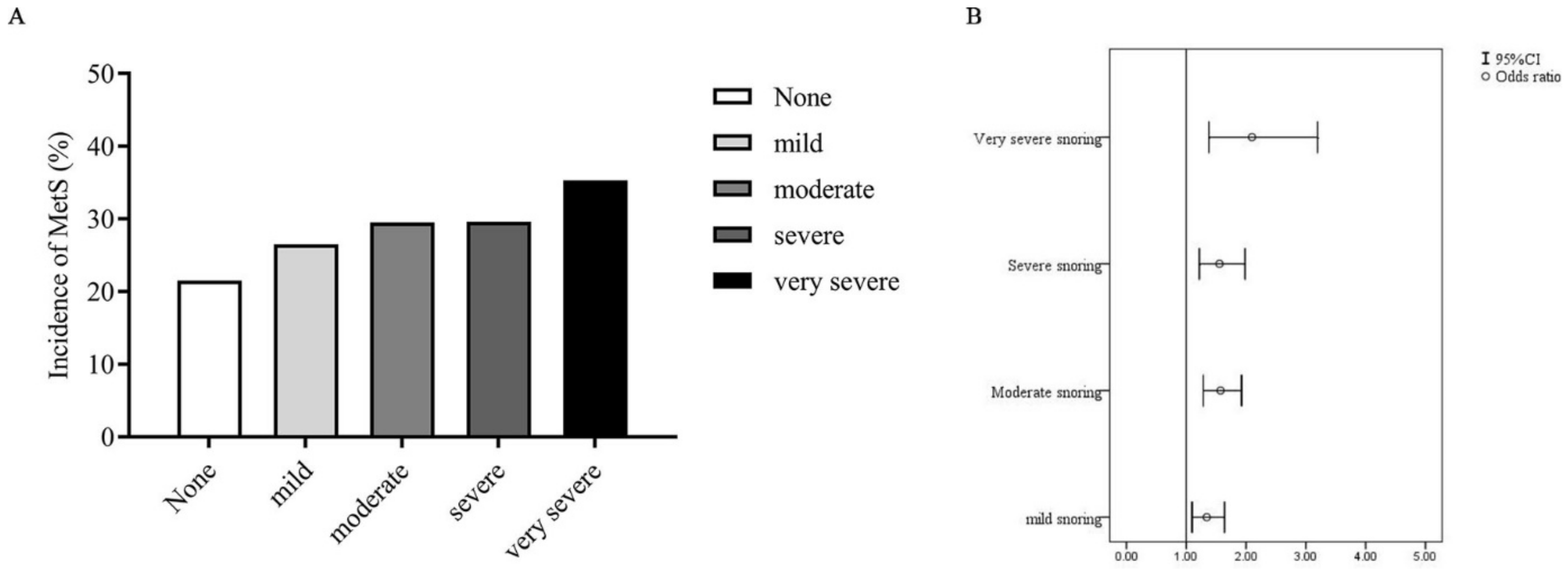
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, X.; Li, X.D.; Feng, G.S.; Xu, Z.F.; Du, J.N.; Wang, G.X.; Ma, J.; Hu, P.J.; Yan, X.Y.; Zhang, J.; et al. The prevalence of snoring and its related family factors of children from 3 to 14 years old in Beijing. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi Chin. J. Otorhinolaryngol. Head Neck Surg. 2019, 54, 902–906. [Google Scholar]
- Wali, S.O.; Abaalkhail, B.A. Prevalence and predictors of habitual snoring in a sample of Saudi middle-aged adults. Saudi Med. J. 2015, 36, 920–927. [Google Scholar] [CrossRef] [PubMed]
- Ward, S.A.; Pase, M.P. Advances in pathophysiology and neuroimaging: Implications for sleep and dementia. Respirology 2020, 25, 580–592. [Google Scholar] [CrossRef] [PubMed]
- Collop, N.A. Obstructive sleep apnea syndromes. Semin. Respir. Crit. Care Med. 2005, 26, 13–24. [Google Scholar] [CrossRef]
- Yunus, F.M.; Khan, S.; Mitra, D.K.; Mistry, S.K.; Afsana, K.; Rahman, M. Relationship of sleep pattern and snoring with chronic disease: Findings from a nationwide population-based survey. Sleep Health 2018, 4, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Gao, Q.; Li, L.; Shen, Y.; Lu, Q.; Huang, J.; Sun, C.; Wang, H.; Qiao, N.; Wang, C.; et al. Additive interaction of snoring and body mass index on the prevalence of metabolic syndrome among Chinese coal mine employees: A cross-sectional study. BMC Endocr. Disord. 2019, 19, 28. [Google Scholar] [CrossRef]
- Huang, J.; Qi, J.; Lin, Q.; Li, S.; Chen, G.; Ding, H.; Zhao, J. Snoring and components of metabolic syndrome in Southeastern Chinese adults: A community-based study. Clin. Respir. J. 2018, 12, 966–973. [Google Scholar] [CrossRef]
- Alberti, K.G.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.C.; James, W.P.; Loria, C.M.; Smith, S.C., Jr. Harmonizing the metabolic syndrome: A joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009, 120, 1640–1645. [Google Scholar]
- Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z. The metabolic syndrome. Lancet 2005, 365, 1415–1428. [Google Scholar] [CrossRef]
- Zhou, X.; Guan, H.; Zheng, L.; Li, Z.; Guo, X.; Yang, H.; Yu, S.; Sun, G.; Li, W.; Hu, W.; et al. Prevalence and awareness of diabetes mellitus among a rural population in China: Results from Liaoning Province. Diabet. Med. J. Br. Diabet. Assoc. 2015, 32, 332–342. [Google Scholar] [CrossRef]
- Sun, Y.; Mu, J.; Wang, D.W.; Ouyang, N.; Xing, L.; Guo, X.; Zhao, C.; Ren, G.; Ye, N.; Zhou, Y.; et al. A village doctor-led multifaceted intervention for blood pressure control in rural China: An open, cluster randomised trial. Lancet 2022, 399, 1964–1975. [Google Scholar] [CrossRef]
- Li, Z.; Guo, X.; Zheng, L.; Sun, Z.; Yang, H.; Sun, G.; Yu, S.; Li, W.; Zou, L.; Wang, J.; et al. Prehypertension in rural northeastern China: Results from the northeast China rural cardiovascular health study. J. Clin. Hypertens. 2014, 16, 664–670. [Google Scholar] [CrossRef]
- Yu, S.; Yang, H.; Guo, X.; Zheng, L.; Sun, Y. Metabolic syndrome and depressive symptoms among rural Northeast general population in China. BMC Public Health 2017, 17, 43. [Google Scholar] [CrossRef][Green Version]
- Chobanian, A.V.; Bakris, G.L.; Black, H.R.; Cushman, W.C.; Green, L.A.; Izzo, J.L., Jr.; Jones, D.W.; Materson, B.J.; Oparil, S.; Wright, J.T., Jr.; et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: The JNC 7 report. Jama 2003, 289, 2560–2572. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F., 3rd; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Shin, M.H.; Kweon, S.S.; Choi, B.Y.; Kim, M.K.; Chun, B.Y.; Shin, D.H.; Lee, Y.H. Self-reported snoring and metabolic syndrome: The Korean Multi-Rural Communities Cohort Study. Sleep Breath. Schlaf Atm. 2014, 18, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.E.; Shin, S.; Lee, H.W.; Lim, J.; Lee, J.K.; Kang, D. Frequency of Loud Snoring and Metabolic Syndrome among Korean Adults: Results from the Health Examinees (HEXA) Study. Int. J. Environ. Res. Public Health 2017, 14, 1294. [Google Scholar] [CrossRef]
- Zou, J.; Song, F.; Xu, H.; Fu, Y.; Xia, Y.; Qian, Y.; Zou, J.; Liu, S.; Fang, F.; Meng, L.; et al. The Relationship between Simple Snoring and Metabolic Syndrome: A Cross-Sectional Study. J. Diabetes Res. 2019, 2019, 9578391. [Google Scholar] [CrossRef]
- Thomas, G.N.; Jiang, C.Q.; Lao, X.Q.; McGhee, S.M.; Zhang, W.S.; Schooling, C.M.; Adab, P.; Lam, T.H.; Cheng, K.K. Snoring and vascular risk factors and disease in a low-risk Chinese population: The Guangzhou Biobank Cohort Study. Sleep 2006, 29, 896–900. [Google Scholar] [CrossRef][Green Version]
- Cho, N.; Joo, S.; Kim, J.; Abbott, R.D.; Kim, J.; Kimm, K.; Shin, C. Relation of habitual snoring with components of metabolic syndrome in Korean adults. Diabetes Res. Clin. Pract. 2006, 71, 256–263. [Google Scholar] [CrossRef]
- Goto, R.; Tanigawa, T.; Maruyama, K.; Tomooka, K.; Eguchi, E.; Osawa, H.; Saito, I. Associations of snoring frequency with blood pressure among the lean Japanese population: The Toon Health Study. J. Hum. Hypertens. 2020, 34, 271–277. [Google Scholar] [CrossRef]
- Hoffstein, V. Blood pressure, snoring, obesity, and nocturnal hypoxaemia. Lancet 1994, 344, 643–645. [Google Scholar] [CrossRef] [PubMed]
- Hoffstein, V.; Mateika, J. Evening-to-morning blood pressure variations in snoring patients with and without obstructive sleep apnea. Chest 1992, 101, 379–384. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, T.; Nakano, H.; Yoshihara, K.; Sudo, N. The Relationship between Snoring Sound Intensity and Morning Blood Pressure in Workers. J. Clin. Sleep Med. JCSM 2016, 12, 1601–1606. [Google Scholar] [CrossRef] [PubMed]
- Al-Delaimy, W.K.; Manson, J.E.; Willett, W.C.; Stampfer, M.J.; Hu, F.B. Snoring as a risk factor for type II diabetes mellitus: A prospective study. Am. J. Epidemiol. 2002, 155, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Pan, A.; Yu, Z.; Li, H.; Shi, A.; Yu, D.; Zhang, G.; Zong, G.; Liu, Y.; Lin, X. Snoring, inflammatory markers, adipokines and metabolic syndrome in apparently healthy Chinese. PLoS ONE 2011, 6, e27515. [Google Scholar] [CrossRef]
- Fava, C.; Montagnana, M.; Favaloro, E.J.; Guidi, G.C.; Lippi, G. Obstructive sleep apnea syndrome and cardiovascular diseases. Semin. Thromb. Hemost. 2011, 37, 280–297. [Google Scholar] [CrossRef]
- Wang, T.; Lu, J.; Wang, W.; Mu, Y.; Zhao, J.; Liu, C.; Chen, L.; Shi, L.; Li, Q.; Yang, T.; et al. Sleep duration and snoring associate with hypertension and glycaemic control in patients with diabetes. Diabet. Med. J. Br. Diabet. Assoc. 2015, 32, 1001–1007. [Google Scholar] [CrossRef]
| Men | Women | |||
|---|---|---|---|---|
| Non-Snorers | Snorers | Non-Snorers | Snorers | |
| Participants, n | 1550 | 1036 | 1782 | 612 |
| Incidence of MetS | 305 (19.7) | 271 (26.2) | 413 (23.2) | 205 (33.5) |
| Age, years | 54.51 ± 11.03 | 53.88 ± 9.78 | 50.12 ± 9.61 | 53.21 ±8.97 |
| Height, (cm) | 165.75 ± 6.47 | 166.13 ± 6.45 | 155.82 ± 6.08 | 155.46 ± 6.44 |
| Weight, kg | 64.21 ± 8.60 | 66.76 ± 9.16 | 56.38 ± 8.67 | 58.65 ± 9.84 |
| BMI a (kg/m2) | 23.37 ± 2.94 | 24.19 ± 3.13 | 23.20 ± 3.23 | 24.25 ± 3.67 |
| Waist circumference (cm) | 79.51 ± 7.71 | 81.27 ± 8.15 | 75.68 ± 8.04 | 78.59 ± 9.08 |
| SBP b (mmHg) | 139.60 ± 21.97 | 140.36 ± 21.63 | 131.93 ± 20.83 | 135.80 ± 22.36 |
| DBP c (mmHg) | 81. 01 ± 10.87 | 82.28 ± 11.36 | 77.42 ± 10.46 | 78.91 ± 10.88 |
| FPG f (mmol/L) | 5.61 ± 1.14 | 5.65 ± 1.25 | 5.37 ± 0.90 | 5.39 ± 0.82 |
| TC g (mmol/L) | 5.07 ± 0.98 | 5.12 ± 0.94 | 5.07 ± 1.00 | 5.28 ± 1.05 |
| TG h (mmol/L) | 1.18 ± 0.99 | 1.17 ± 0.69 | 1.08 ± 0.58 | 1.15 ± 0.50 |
| HDL-C d (mmol/L) | 1.52 ± 0.42 | 1.50 ± 0.43 | 1.54 ± 0.34 | 1.54 ± 0.34 |
| LDL-C e (mmol/L) | 2.80 ± 0.74 | 2.85 ± 0.74 | 2.79 ± 0.76 | 2.95 ± 0.83 |
| eGFR (mL/min/1.73 m2) | 95.61 ± 12.75 | 96.18 ± 14.82 | 95.59 ± 14.82 | 93.69 ± 14.73 |
| HbA1c | 5.22 ± 0.85 | 5.31 ± 0.90 | 5.16 ± 0.64 | 5.32 ± 0.59 |
| Sleep time (hours) | 7.38 ± 1.62 | 7.47 ± 1.56 | 7.12 ± 1.69 | 7.10 ± 1.75 |
| Current smoker (%) | 58.6 | 60.5 | 14.8 | 21.1 |
| Current drinker (%) | 43.5 | 51.4 | 2.6 | 3.9 |
| Regular exercise (%) | 19.2 | 18.8 | 17.3 | 19.6 |
| Men | Women | |||||
|---|---|---|---|---|---|---|
| Non-Snorers | Snorers | p Values | Non-Snorers | Snorers | p Values | |
| Participants (n) | 1550 | 1036 | 1782 | 612 | ||
| Incidence of MetS (%) | 19.7 | 26.2 | <0.001 | 23.2 | 33.5 | <0.001 |
| △Weight (kg) | 0.09 ± 0.01 | 0.12 ± 0.01 | 0.893 | 0.33 ± 0.12 | 0.61 ± 0.22 | 0.281 |
| △BMI a (kg/m2) | 0.85 ± 0.07 | 0.92 ± 0.09 | 0.524 | −0.38 ± 0.07 | −0.34 ± 0.11 | 0.764 |
| △Waist circumference (cm) | 3.96 ± 0.18 | 4.08 ± 0.22 | 0.526 | 3.82 ± 0.18 | 4.35 ± 0.27 | 0.122 |
| △SBP b (mmHg) | −3.68 ± 0.46 | −2.04 ± 0.56 | 0.024 | −4.58 ± 0.42 | −5.07 ± 0.72 | 0.555 |
| △DBP c (mmHg) | −0.45 ± 0.14 | 0.51 ± 0.19 | 0.012 | −1.11 ± 0.21 | −0.06 ± 0.35 | 0.763 |
| △FBG f (mmol/L) | 0.07 ± 0.03 | 0.09 ± 0.03 | 0.672 | −0.06 ± 0.02 | 0.06 ± 0.04 | 0.003 |
| △TC g (mmol/L) | −0.29 ± 0.02 | −0.33 ± 0.03 | 0.336 | −0.29 ± 0.02 | −0.28 ± 0.03 | 0.895 |
| △TG h (mmol/L) | 0.24 ± 0.03 | 0.32 ± 0.04 | 0.135 | 0.27 ± 0.02 | 0.31 ± 0.03 | 0.306 |
| △HDL-C d (mmol/L) | −0.08 ± 0.01 | −0.10 ± 0.01 | 0.148 | −0.08 ± 0.02 | −0.08 ± 0.03 | 0.624 |
| △LDL-C e (mmol/L) | 0.19 ± 0.02 | 0.17 ± 0.02 | 0.501 | 0.22 ± 0.02 | 0.25 ± 0.03 | 0.456 |
| △eGFR (mL/min/1.73 m2) | −3.24 ± 0.27 | −2.62 ± 0.40 | 0.176 | 0.04 ± 0.01 | 0.21 ± 0.02 | 0.776 |
| △Current smoker (%) | −2.4 | −5.0 | <0.001 | −1.0 | −7.3 | <0.001 |
| △Current drinker (%) | 2.4 | 2.8 | 0.476 | 1.4 | 2.0 | 0.126 |
| Non-Snorers | Snorers | p Value | ||
|---|---|---|---|---|
| Total | MetS | 718(21.5) | 476(28.9) | <0.001 |
| Abdominal obesity | 1155(34.7) | 702(42.6) | <0.001 | |
| Hypertension | 1621(48.6) | 948(57.5) | <0.001 | |
| High TG | 764(22.9) | 450(27.3) | <0.001 | |
| Low HDL-C | 780(23.4) | 347(21.1) | 0.033 | |
| Hyperglycaemia | 957(28.7) | 579(35.1) | <0.001 | |
| Men | MetS | 305(19.7) | 271(26.2) | <0.001 |
| Abdominal obesity | 343(22.1) | 322(31.1) | <0.001 | |
| Hypertension | 919(59.3) | 657(63.4) | 0.019 | |
| High TG | 379(24.5) | 281(27.1) | 0.070 | |
| Low HDL-C | 200(12.9) | 134(12.9) | 0.513 | |
| Hyperglycaemia | 583(37.6) | 412(39.8) | 0.144 | |
| Women | MetS | 413(23.2) | 205(33.5) | <0.001 |
| Abdominal obesity | 812(45.6) | 380(62.1) | <0.001 | |
| Hypertension | 702(39.4) | 291(47.5) | <0.001 | |
| High TG | 385(21.6) | 169(27.6) | 0.002 | |
| Low HDL-C | 580(32.5) | 213(34.8) | 0.165 | |
| Hyperglycaemia | 374(21.0) | 167(27.3) | 0.001 |
| ORs (95% CIs) | ||
|---|---|---|
| Crude | Multivariate | |
| Snoring (ref: no) | 1.48 (1.29–1.69) | 1.51 (1.32–1.74) |
| Men (ref: women) | 0.82 (0.72–0.94) | 0.68 (0.58–0.80) |
| Age (1-year increase) | 1.02 (1.01–1.02) | 1.02 (1.01–1.03) |
| eGFR (1 mL/min/1.73 m2 increase) | 0.99 (0.98–0.99) | 0.99 (0.99–1.00) |
| Sleep duration (1 h increase) | 1.01 (0.97–1.05) | 1.03 (0.99–1.07) |
| Exercise (ref: no) | 1.17 (0.99–1.38) | 1.06 (0.89–1.26) |
| Current smoker (ref: never smoked or a former smoker) | 0.91 (0.80–1.04) | 0.94 (0.81–1.10) |
| Current drinker (ref: never drank or a former drinker) | 1.01 (0.87–1.17) | 1.20 (1.00–1.44) |
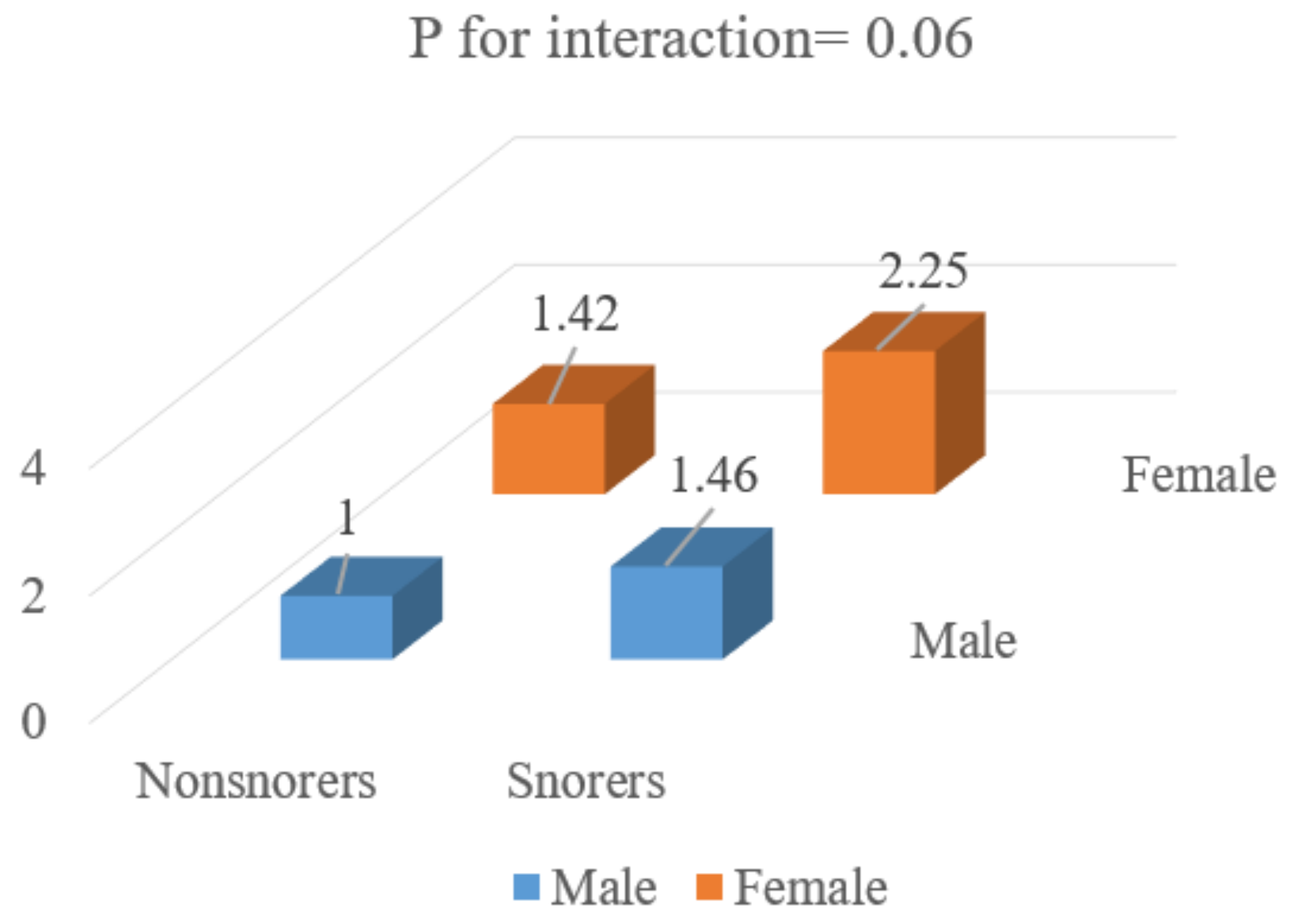
| OR (95% CI) | ||||
|---|---|---|---|---|
| Men | Women | |||
| Crude | Multivariate | Crude | Multivariate | |
| Snoring (ref: no) | 1.45 | 1.43 | 1.67 | 1.51 |
| (1.20–1.74) | (1.19–1.73) | (1.37–2.04) | (1.23–1.85) | |
| Age (1-year increase) | 1.00 | 0.99 | 1.04 | 1.04 |
| (0.99–1.01) | (0.99–1.01) | (1.03–1.05) | (1.03–1.05) | |
| eGFR (1 mL/min/1.73 m2 increase) | 0.99 | 0.99 | 0.99 | 1.00 |
| (0.98–0.99) | (0.98–1.00) | (0.98–0.99) | (0.99–1.01) | |
| Sleep duration (1 h increase) | 1.01 | 1.02 | 1.01 | 1.06 |
| (0.96–1.07) | (0.96–1.08) | (0.96–1.07) | (1.00–1.12) | |
| Exercise (ref: no) | 1.04 | 1.01 | 1.33 | 1.16 |
| (0.82–1.31) | (0.79–1.29) | (1.06–1.68) | (0.91–1.47) | |
| Current smoker (ref: never smoked or a former smoker) | 0.85 | 0.81 | 1.29 | 1.07 |
| (0.70–1.02) | (0.67–0.99) | (1.02–1.64) | (0.83–1.37) | |
| Current drinker (ref: never drank or a former drinker) | 1.20 | 1.26 | 1.05 | 0.86 |
| (0.99–1.44) | (1.03–1.53) | (0.62–1.79) | (0.49–1.51) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, S.; Guo, X.; Li, G.; Yang, H.; Sun, Y. Influence of Snoring on the Incidence of Metabolic Syndrome: A Community-Based Prospective Cohort Study in Rural Northeast China. J. Clin. Med. 2023, 12, 447. https://doi.org/10.3390/jcm12020447
Yu S, Guo X, Li G, Yang H, Sun Y. Influence of Snoring on the Incidence of Metabolic Syndrome: A Community-Based Prospective Cohort Study in Rural Northeast China. Journal of Clinical Medicine. 2023; 12(2):447. https://doi.org/10.3390/jcm12020447
Chicago/Turabian StyleYu, Shasha, Xiaofan Guo, Guangxiao Li, Hongmei Yang, and Yingxian Sun. 2023. "Influence of Snoring on the Incidence of Metabolic Syndrome: A Community-Based Prospective Cohort Study in Rural Northeast China" Journal of Clinical Medicine 12, no. 2: 447. https://doi.org/10.3390/jcm12020447
APA StyleYu, S., Guo, X., Li, G., Yang, H., & Sun, Y. (2023). Influence of Snoring on the Incidence of Metabolic Syndrome: A Community-Based Prospective Cohort Study in Rural Northeast China. Journal of Clinical Medicine, 12(2), 447. https://doi.org/10.3390/jcm12020447






