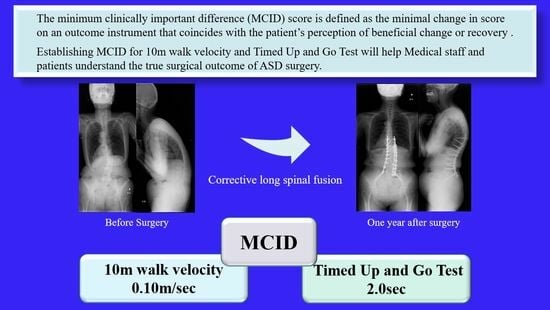
Minimal Clinically Important Differences in Gait and Balance Ability in Patients Who Underwent Corrective Long Spinal Fusion for Adult Spinal Deformity
Abstract
:1. Introduction
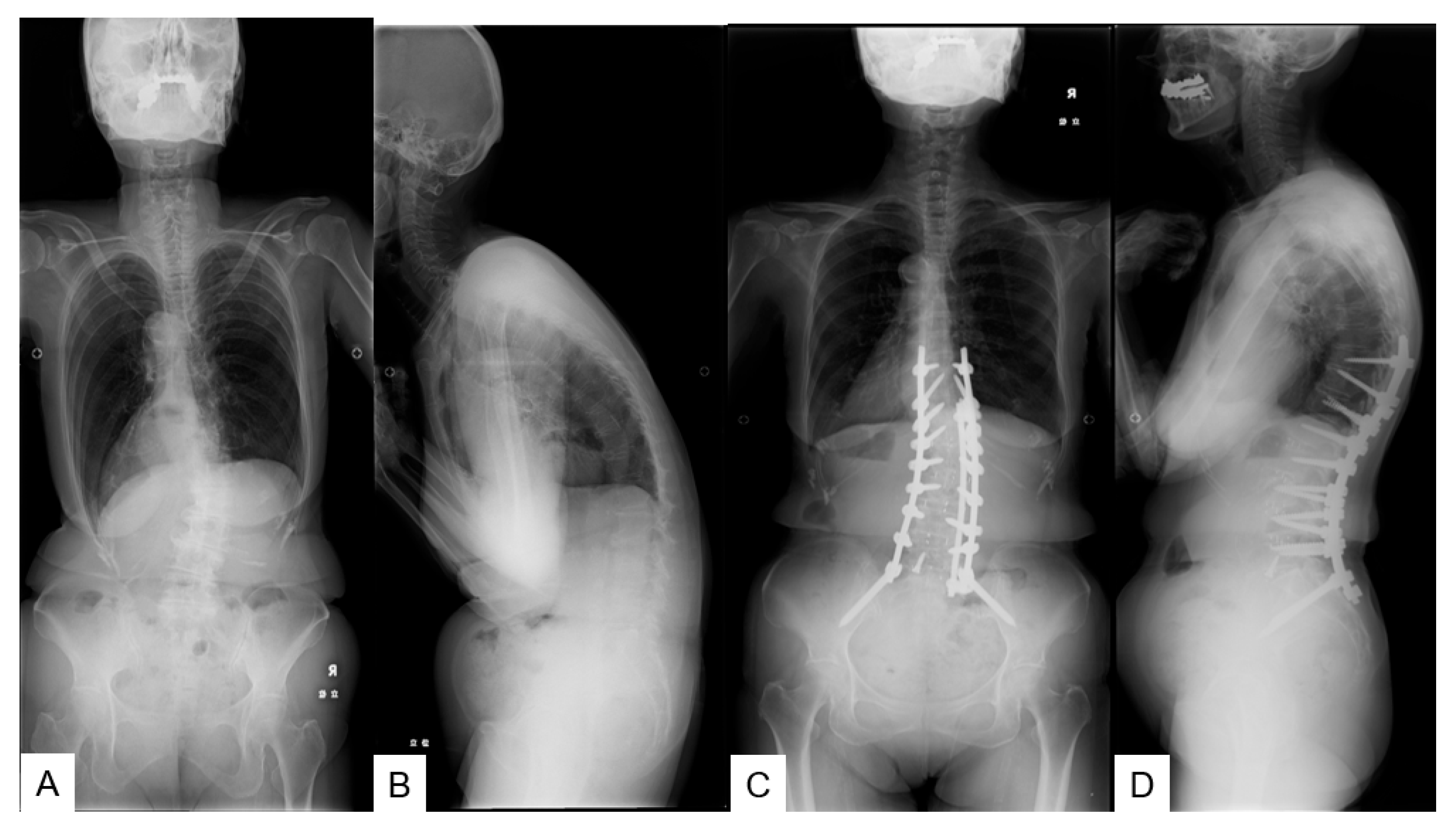
2. Materials and Methods
2.1. Patient Demographics
2.2. Outcome Assessment
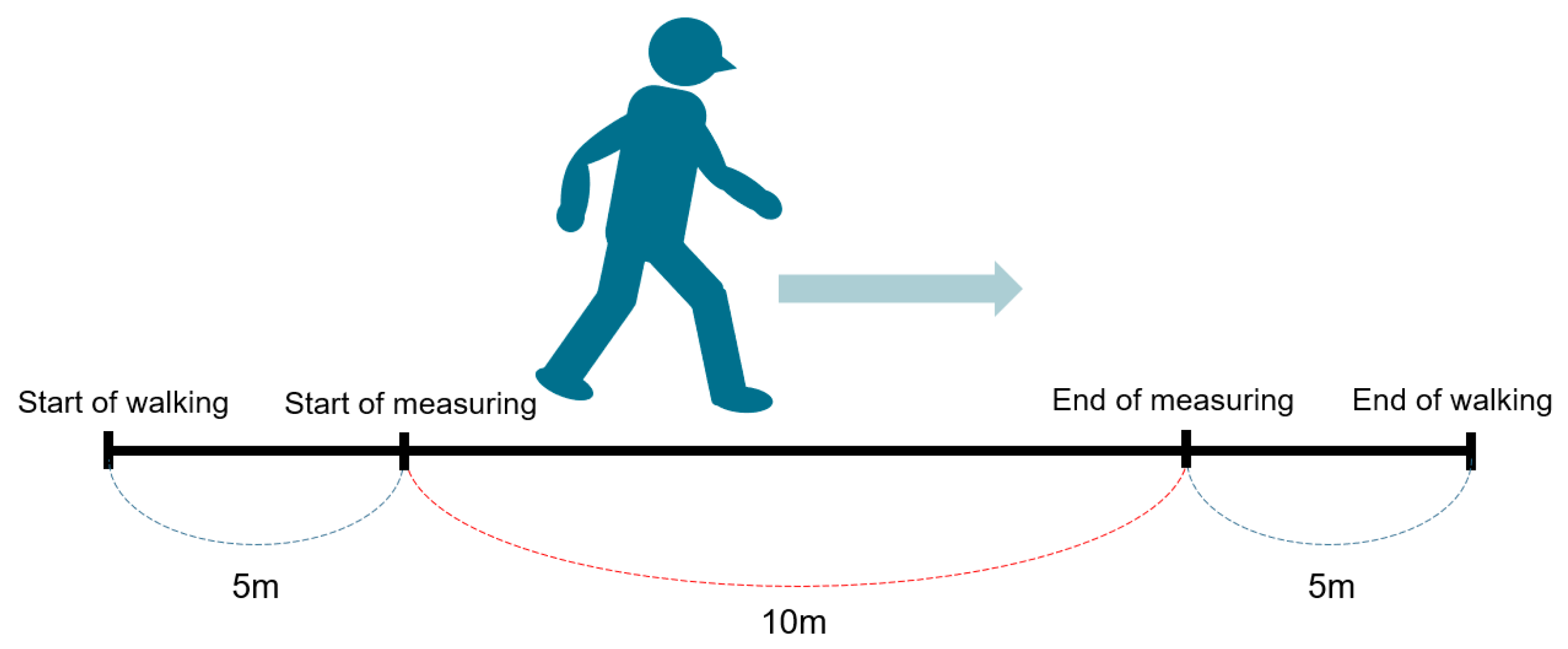
2.2.1. Ten Meter Walk Velocity
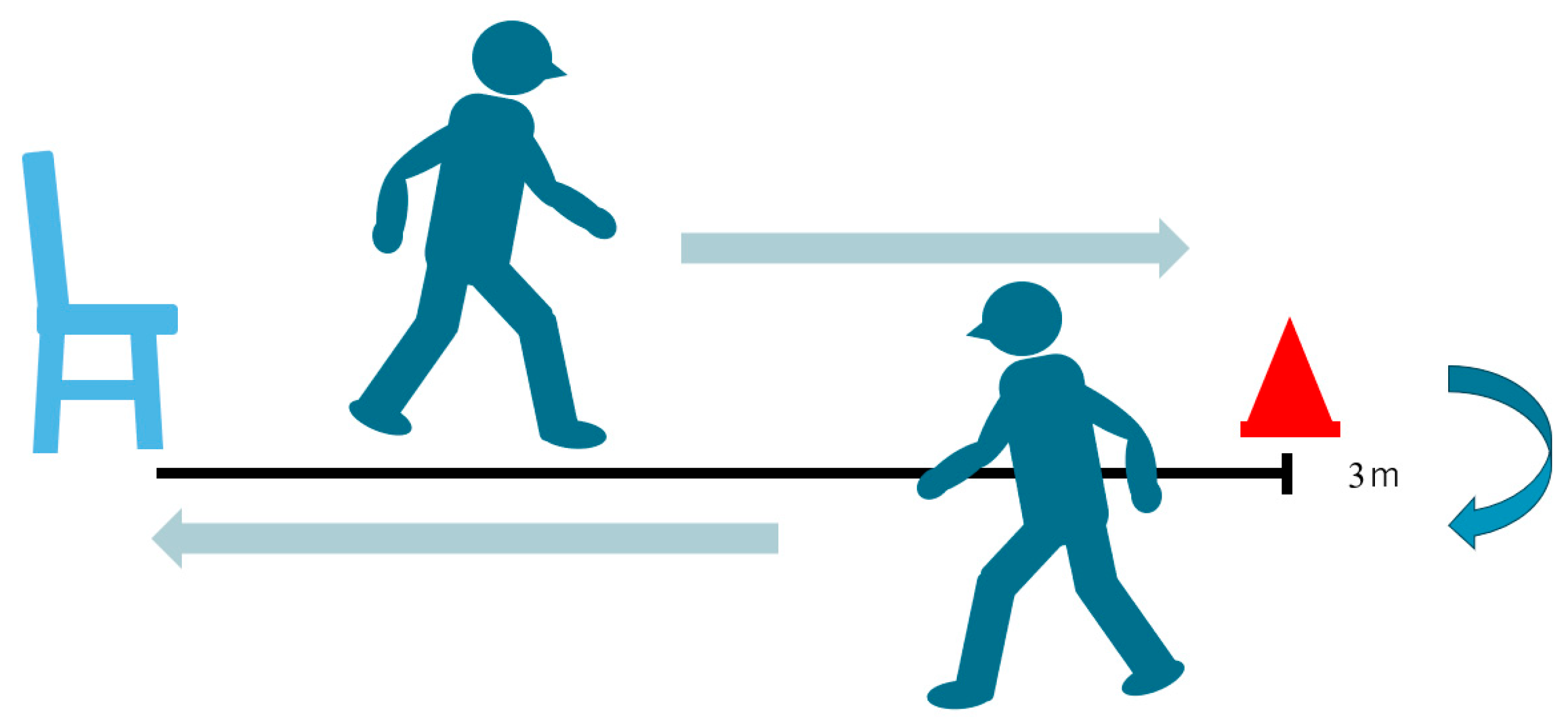
2.2.2. Timed Up-and-Go Test (TUG)
2.2.3. Patients Reported Outcomes (PRO)
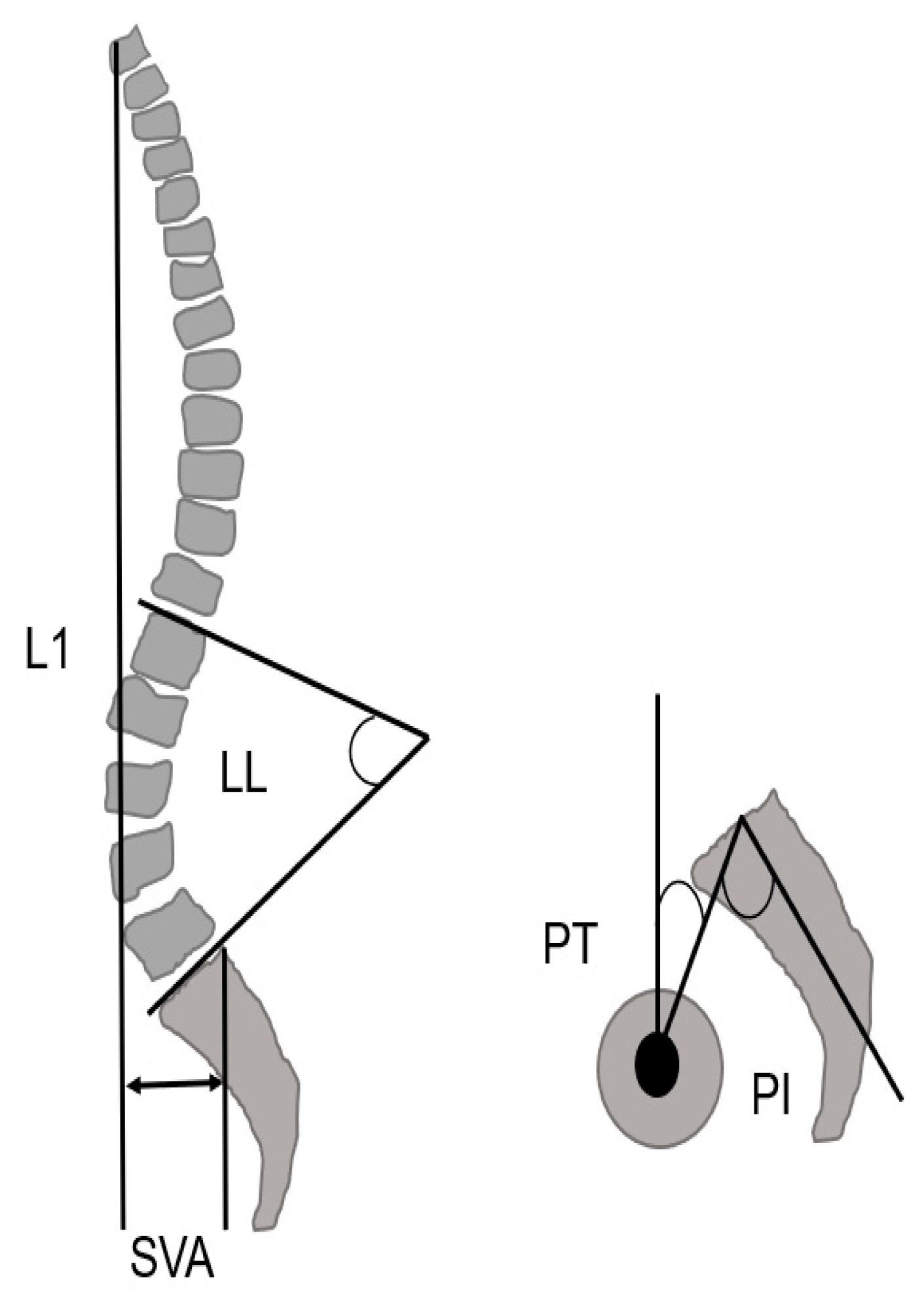
2.3. Radiographic Measurements
2.4. Statistical Analysis
3. Results
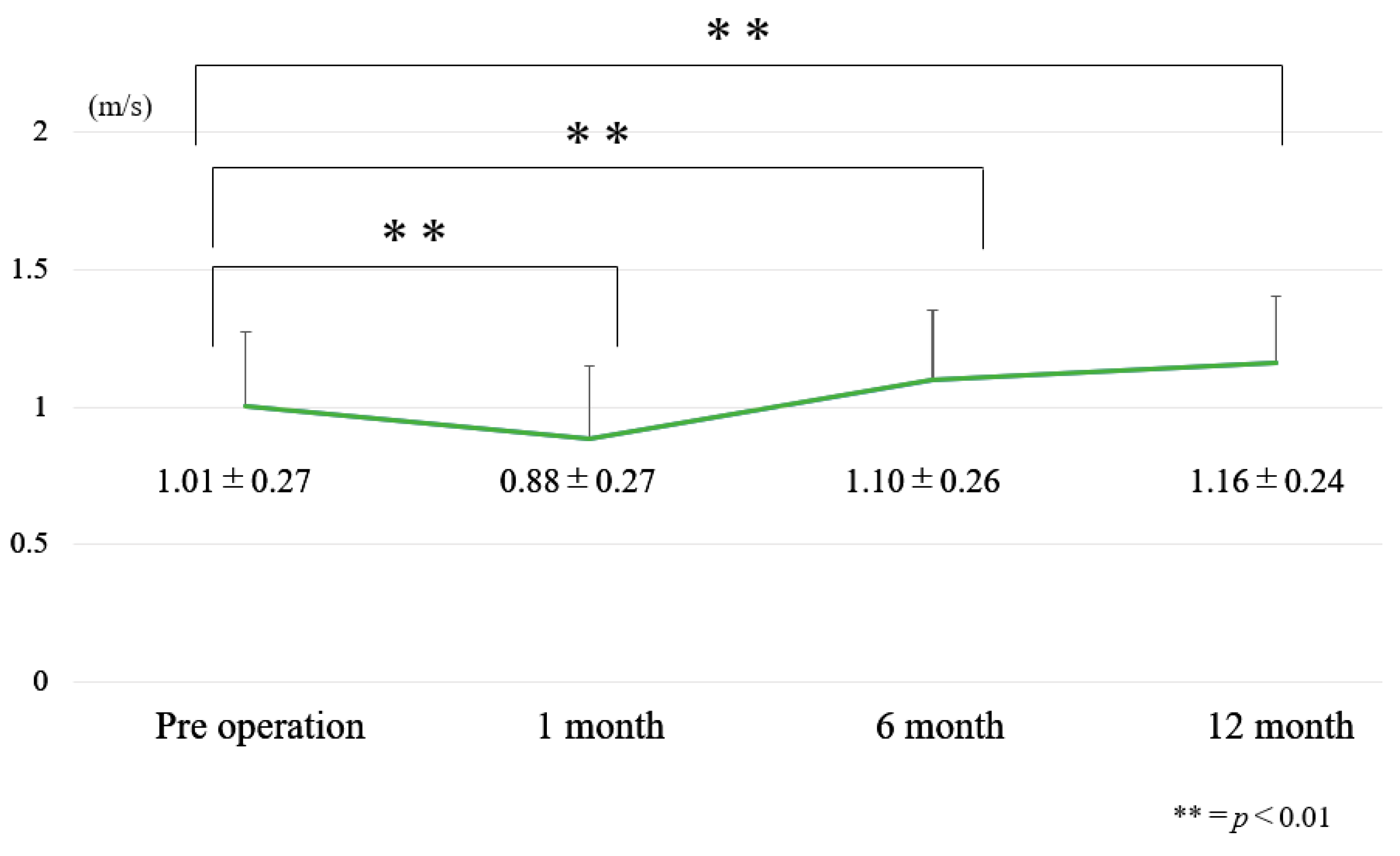
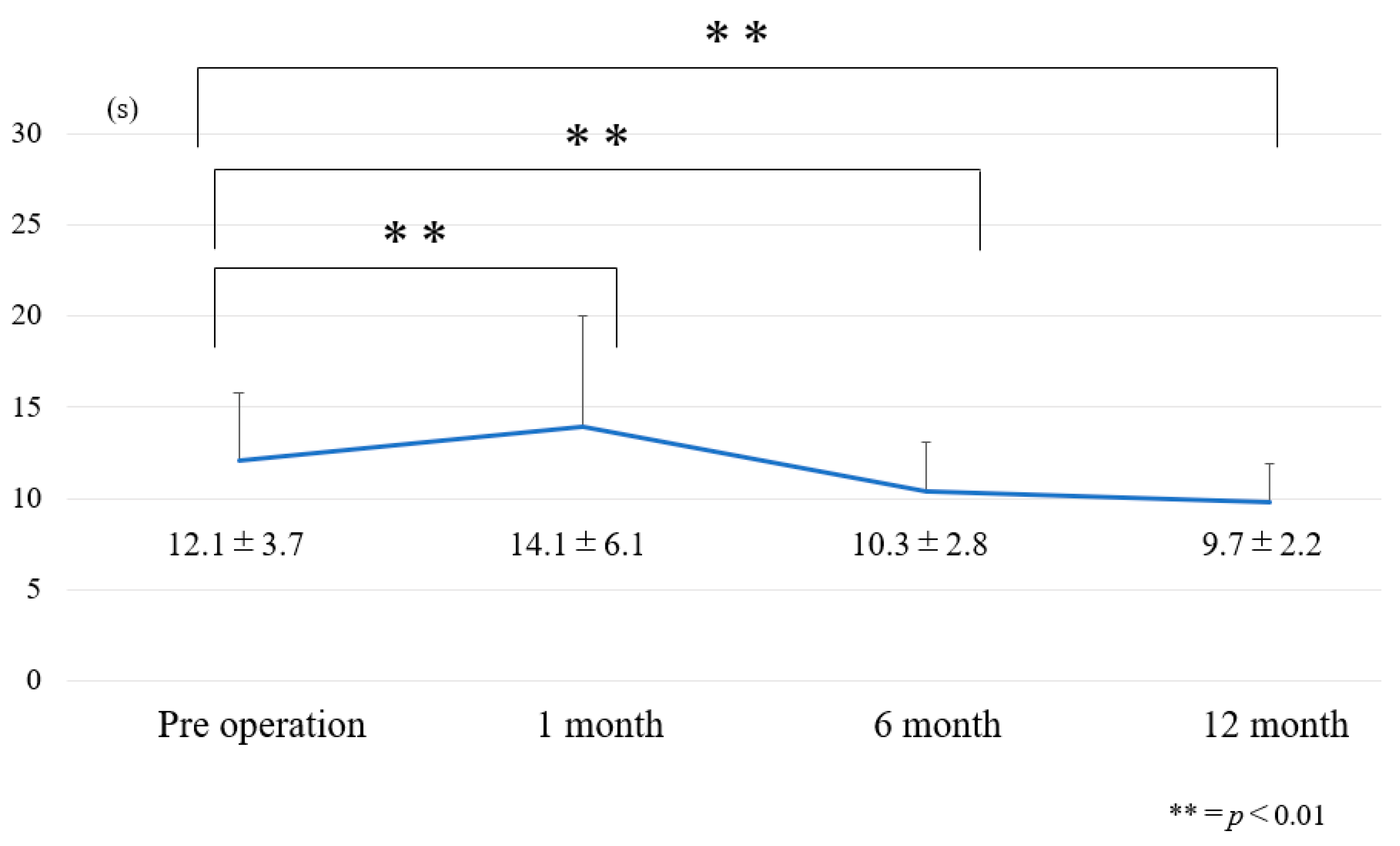
3.1. Chronological Results of 10 m Walk Velocity and TUG (Figure 6 and Figure 7)
3.2. Internal Responsiveness
3.3. External Responsiveness
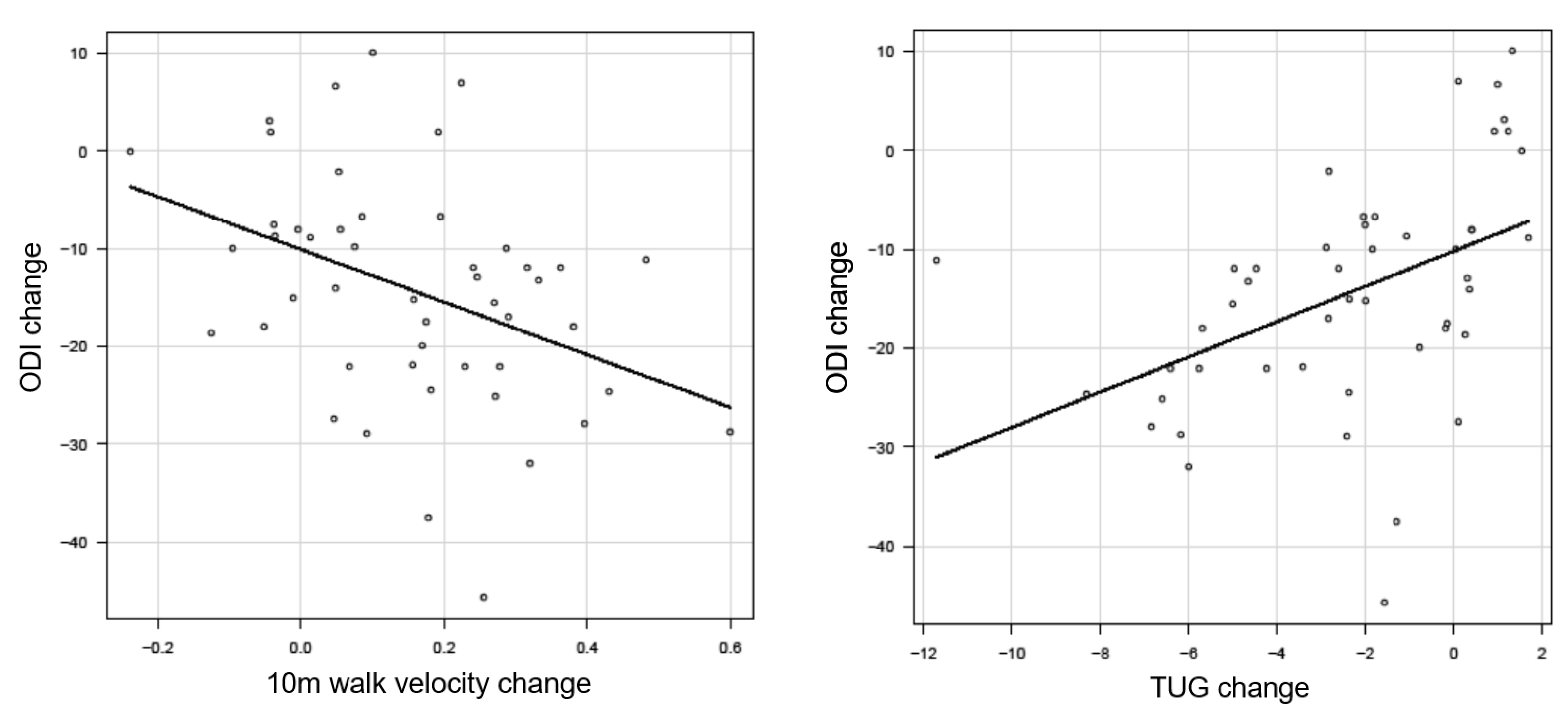
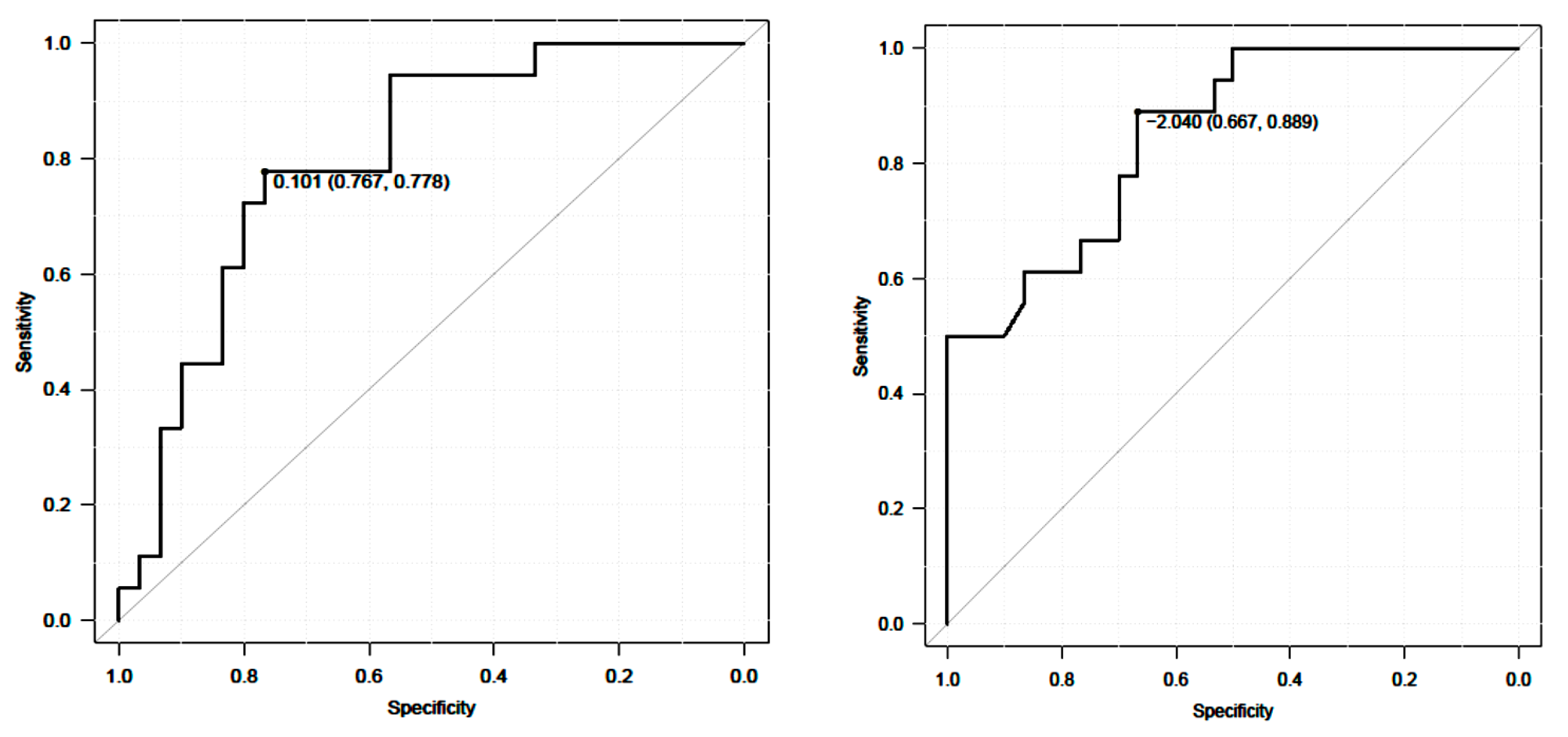
3.3.1. Anchor-Based Approach
3.3.2. Post-Operative Comparison of Responders and Non-Responders
3.3.3. Spinopelvic Parameter
4. Discussion
Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schwab, F.; Dubey, A.; Gamez, L.; El Fegoun, A.B.; Hwang, K.; Pagala, M.; Farcy, J.P. Adult scoliosis: Prevalence, SF-36, and nutritional parameters in an elderly volunteer population. Spine 2005, 30, 1082–1085. [Google Scholar] [CrossRef] [PubMed]
- Konno, S.; Kikuchi, S.; Nagaosa, Y. The relationship between intramuscular pressure of the paraspinal muscles and low back pain. Spine 1994, 9, 2186–2189. [Google Scholar] [CrossRef] [PubMed]
- Hosogane, N.; Watanabe, K.; Yagi, M.; Kaneko, S.; Toyama, Y.; Matsumoto, M. Scoliosis is Risk Factor for Gastroesophageal Reflex Disease in Adult Spinal Deformity. Clin. Spine Surg. 2017, 30, E480–E484. [Google Scholar] [CrossRef] [PubMed]
- Arima, H.; Hasegawa, T.; Yamato, Y.; Yoshida, G.; Banno, T.; Oe, S.; Mihara, Y.; Ide, K.; Watanabe, Y.; Nakai, K.; et al. Clinical Outcomes of Corrective Fusion Surgery From the Thoracic Spine to the Pelvis for Adult Spinal Deformity at 1, 2, and 5 years Postoperatively. Spine 2022, 47, 792–799. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.S.; Shaffrey, C.I.; Berven, S.; Glassman, S.; Hamill, C.; Horton, W.; Ondra, S.; Schwab, F.; Shainline, M.; Fu, K.M.; et al. Improvement of back pain with operative and nonoperative treatment in adults with scoliosis. Neurosurgery 2009, 65, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Yagi, M.; Ohne, H.; Kaneko, S.; Machida, M.; Yato, Y.; Asazuma, T. Does corrective spine surgery improve the standing balance in patients with adult spinal deformity? Spine J. 2018, 18, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, J.; Murata, S.; Horie, J.; Uematsu, A.; Hortobágyi, T.; Suzuki, S. Lumbar lordosis angle (LLA) and leg strength predict walking ability in elderly males. Arch. Gerontol. Geriatr. 2013, 56, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Li, C.Y.; Chang, C.L.; Tai, T.W. Incidence and risk factors for hip fracture in elderly patients undergoing lumbar spine surgery: A nationwide database study with 11-year follow-up. Osteoporos. Int. 2018, 29, 2717–2723. [Google Scholar] [CrossRef]
- Gautschi, O.P.; Stienen, M.N.; Corniola, M.V.; Joswig, H.; Schaller, K.; Hildebrandt, G.; Smoll, N.R. Assessment of the Minimum Clinically Important Difference in the Timed Up and Go Test After Surgery for Lumbar Degenerative Disc Disease. Neurosurgery 2017, 80, 380–385. [Google Scholar] [CrossRef]
- Sakaguchi, T.; Tanaka, M.; Suthar, H.; Fujiwara, Y.; Uotani, K.; Arataki, S.; Yamauchi, T.; Sugyo, A.; Takamatsu, K.; Yasuda, Y.; et al. Chronological evaluation of gait ability and posture balance after adult spinal deformity surgery. Appl. Sci. 2022, 12, 4285. [Google Scholar] [CrossRef]
- Sakaguchi, T.; Tanaka, M.; Sake, N.; Latka, K.; Fujiwara, Y.; Arataki, S.; Yamauchi, T.; Takamatsu, K.; Yasuda, Y.; Nakagawa, M.; et al. The Most Significant Factor Affecting Gait and Postural Balance in Patients’ Activities of Daily Living Following Corrective Surgery for Deformity of the Adult Spine. Medicina 2022, 58, 1118. [Google Scholar] [CrossRef] [PubMed]
- Jaeschke, R.; Singer, J.; Guyatt, G.H. Measurement of health status. Ascertaining the minimal clinically important difference. Control Clin. Trials 1989, 10, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Takenaka, H.; Kamiya, M.; Sugiura, H.; Nishihama, K.; Ito, A.; Suzuki, J.; Hanamura, S. Responsiveness and Minimal Clinically Important Difference of the 6-minute Walk Distance in Patients Undergoing Lumbar Spinal Canal Stenosis Surgery. Clin. Spine Surg. 2022, 35, E345–E350. [Google Scholar] [CrossRef] [PubMed]
- Roussouly, P.; Gollogly, S.; Berthonnaud, E.; Dimnet, J. Classification of the normal variation in the sagittal alignment of the lumbar spine and pelvis in the standing position. Spine 2005, 30, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Academy of Neurological Physical Therapy. Core Measure: 10 Meter Walk Test. Available online: https://www.physio-pedis.com/10_Metre_Walk_Test (accessed on 6 May 2022).
- Salbach, N.; Bayley, M.; Bayley, M.; Howe, J.-A.; Kelloway, L. iWalk Guide Online Resources 14. Quick Look-Up Sheet: Reference Values for 10mWT & 6MWT; Quick Look-Up Sheet: Reference Values for 10-metre Walk Test and 6-Minute Walk; University of Toronto: Toronto, ON, Canada, 2018. [Google Scholar]
- Wolf, S.L.; Catlin, P.A.; Gage, K.; Gurucharri, K.; Robertson, R.; Stephen, K. Establishing the reliability and validity of measurements of walking time using the Emory Functional Ambulation Profile. Phys. Ther. 1999, 79, 1122–1133. [Google Scholar] [CrossRef] [PubMed]
- Podsiadlo, D.; Richardson, S. The timed “Up & Go”: A test of basic functional mobility for frail elderly persons. J. Am. Geriatr. Soc. 1991, 39, 142–148. [Google Scholar] [PubMed]
- Lusardi, M.M.; Pellecchia, G.L.; Schulman, M. Functional Performance in Community Living Older Adults. J. Geriatr. Phys. Ther. 2003, 26, 14–22. [Google Scholar] [CrossRef]
- Shumway-Cook, A.; Brauer, S.; Woollacott, M. Predicting the probability for falls in community-dwelling older adults using the Timed Up & Go Test. Phys. Ther. 2000, 80, 896–903. [Google Scholar]
- Hofheinz, M.; Schusterschitz, C. Dual task interference in estimating the risk of falls and measuring change: A comparative, psychometric study of four measurements. Clin. Rehabil. 2010, 24, 831–842. [Google Scholar] [CrossRef]
- Suwannarat, P.; Kaewsanmung, S.; Thaweewannakij, T.; Amatachaya, S. The use of functional performance tests by primary health-care providers to determine walking ability with and without awalking device in community-dwelling elderly. Physiother. Theory Pract. 2021, 37, 64–72. [Google Scholar] [CrossRef]
- Fujiwara, A.; Kobayashi, N.; Saiki, K.; Kitagawa, T.; Tamai, K.; Saotome, K. Association of the Japanese Orthopaedic Association score with the Oswestry Disability Index, Roland-Morris Disability Questionnaire, and short-form 36. Spine 2003, 28, 1601–1607. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, G.; Hasegawa, T.; Yamato, Y.; Kobayashi, S.; Shin, O.; Banno, T.; Mihara, Y.; Arima, H.; Ushirozako, H.; Yasuda, T.; et al. Minimum Clinically Important Differences in Oswestry Disability Index Domains and Their Impact on Adult Spinal Deformity Surgery. Asian Spine J. 2019, 13, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Schwab, F.; Ungar, B.; Blondel, B.; Buchowski, J.; Coe, J.; Deinlein, D.; DeWald, C.; Mehdian, H.; Shaffrey, C.; Tribus, C.; et al. Scoliosis Research Society-Schwab adult spinal deformity classification: A validation study. Spine 2012, 37, 1077–1082. [Google Scholar] [CrossRef] [PubMed]
- Tomkins-Lane, C.C.; Battié, M.C.; Macedo, L.G. Longitudinal construct validity and responsiveness of measures of walking capacity in individuals with lumbar spinal stenosis. Spine J. 2014, 14, 1936–1943. [Google Scholar] [CrossRef] [PubMed]
- Kanda, Y. Investigation of the freely available easy-to-use software “EZR” (Easy R) for medical statistics. Bone Marrow Transpl. 2013, 48, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Arima, H.; Yamato, Y.; Hasegawa, T.; Togawa, D.; Kobayashi, S.; Yasuda, T.; Banno, T.; Oe, S.; Matsuyama, Y. Discrepancy between standing posture and sagittal balance during walking in adult spinal deformity patients. Spine 2017, 42, E25–E30. [Google Scholar] [CrossRef] [PubMed]
- Moke, L.; Severijns, P.; Schelfaut, S.; Van de Loock, K.; Hermans, L.; Molenaers, G.; Jonkers, I.; Scheys, L. Performance on Balance Evaluation Systems Test (BESTest) Impacts Health-Related Quality of Life in Adult Spinal Deformity Patients. Spine 2018, 43, 637–646. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, Y.; Miyakoshi, N.; Kasukawa, Y.; Hongo, M.; Shimada, Y. Spinal sagittal contour affecting falls: Cut-off value of the lumbar spine for falls. Gait Posture 2013, 38, 260–263. [Google Scholar] [CrossRef]
- Tinetti, M.E.; Kumar, C. The patient who falls: “It’s always a trade-off”. JAMA 2010, 303, 258–266. [Google Scholar] [CrossRef]
- Kwon, S.; Perera, S.; Pahor, M.; Katula, J.A.; King, A.C.; Groessl, E.J.; Studenski, S.A. What is a meaningful change in physical performance? Findings from a clinical trial in older adults (the LIFE-P study). J. Nutr. Health Aging 2009, 13, 538–544. [Google Scholar] [CrossRef]
- Kelly, M.P.; Lurie, J.D.; Yanik, E.L.; Shaffrey, C.I.; Baldus, C.R.; Boachie-Adjei, O.; Buchowski, J.M.; Carreon, L.Y.; Crawford, C.H., 3rd; Edwards, C., 2nd; et al. Operative Versus Nonoperative Treatment for Adult Symptomatic Lumbar Scoliosis. J. Bone Joint Surg. Am. 2019, 101, 338–352. [Google Scholar] [CrossRef] [PubMed]
- Jakobsson, M.; Brisby, H.; Gutke, A.; Lundberg, M.; Smeets, R. One-minute stair climbing, 50-foot walk, and timed up-and-go were responsive measures for patients with chronic low back pain undergoing lumbar fusion surgery. BMC Musculoskelet. Disord. 2019, 20, 137. [Google Scholar] [CrossRef]
- Fors, M.; Enthoven, P.; Abbott, A.; Öberg, B. Effects of pre-surgery physiotherapy on walking ability and lower extremity strength in patients with degenerative lumbar spine disorder: Secondary outcomes of the PREPARE randomised controlled trial. BMC Musculoskelet. Disord. 2019, 20, 468. [Google Scholar] [CrossRef]
- Granacher, U.; Lacroix, A.; Muehlbauer, T.; Roettger, K.; Gollhofer, A. Effects of core instability strength training on trunk muscle strength, spinal mobility, dynamic balance and functional mobility in older adults. Gerontology 2013, 59, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Kondo, R.; Yamato, Y.; Nagafusa, T.; Mizushima, T.; Hasegawa, T.; Kobayashi, S.; Togawa, D.; Oe, S.; Kurosu, K.; Matsuyama, Y. Effect of corrective long spinal fusion to the ilium on physical function in patients with adult spinal deformity. Eur. Spine J. 2017, 26, 2138–2145. [Google Scholar] [CrossRef] [PubMed]
- Ilves, O.E.; Neva, M.H.; Häkkinen, K.; Dekker, J.; Kraemer, W.J.; Tarnanen, S.; Kyrölä, K.; Ylinen, J.; Piitulainen, K.; Järvenpää, S.; et al. Trunk Muscle Strength After Lumbar Spine Fusion: A 12-Month Follow-up. Neurospine 2019, 16, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Coronado, R.A.; Master, H.; White, D.K.; Pennings, J.S.; Bird, M.L.; Devin, C.J.; Buchowski, M.S.; Mathis, S.L.; McGirt, M.J.; Cheng, J.S.; et al. Early postoperative physical activity and function: A descriptive case series study of 53 patients after lumbar spine surgery. BMC Musculoskelet. Disord. 2020, 21, 783. [Google Scholar] [CrossRef] [PubMed]
- Snavely, J.E.; Weiner, J.A.; Johnson, D.J.; Hsu, W.K.; Patel, A.A. Preoperative PROMIS Scores Predict Postoperative Outcomes in Lumbar Spine Surgery Patients. Spine 2021, 46, 1139–1146. [Google Scholar] [CrossRef]
- Leyton-Mange, A.; Truumees, E.; Bozic, K.J.; Singh, D.; Liu, T.C.; Stokes, J.K.; Mahometa, M.J.; Geck, M.J. Preoperative patient-reported outcome score thresholds predict the likelihood of reaching MCID with surgical correction of adult spinal deformity. Spine Deform. 2021, 9, 207–219. [Google Scholar] [CrossRef]
| n = 48 | |
|---|---|
| Gender (Men:Women) | 4:44 |
| Age (mean ± S.D.) (year) | 71.0 ± 7.4 |
| Height (mean ± S.D.) (cm) | 151.0 ± 7.7 |
| Body weight (mean ± S.D.) (kg) | 52.8 ± 9.9 |
| Body mass index (mean ± S.D.) (kg/m2) | 23.2 ± 3.7 |
| Comorbidity | 13 (27.1%) |
| Heart disease | 5 (10.4%) |
| Diabetes mellitus | 4 (8.3%) |
| Depression | 4 (8.3%) |
| n = 48 | |
|---|---|
| Operation time (min) | |
| 1st stage (OLIF) | 200.2 ± 47.8 |
| 2nd stage (posterior corrective fusion) | 280.5 ± 53.5 |
| Bleeding (mL) | |
| Blood loss at OLIF surgery (mL) | 437.4 ± 317.1 |
| Blood loss at posterior surgery (mL) | 824.3 ± 385.3 |
| Type of Posterior fusion (OPEN/MIS) | 30/18 |
| Upper instrumented vertebra (UIV) | T6: 2, T9: 2, T10: 44 |
| 10 m Walk Velocity | TUG | ODI | |
|---|---|---|---|
| Pre-operative | 1.0 ± 0.3 | 12.1 ± 3.7 | 38.6 ± 12.8 |
| Post-operative 1 year | 1.2 ± 0.3 | 9.7 ± 2.2 | 24.2 ± 15.9 |
| Amount of change | 0.2 ± 0.2 | −2.3 ± 3 | −14.4 ± 11.6 |
| p value | <0.01 | <0.01 | <0.01 |
| Effect size | 0.63 | 0.78 | 1.0 |
| 10 m Walk Velocity | Change ODI (n = 30) |
|---|---|
| Anchor-Based Approach | |
| Average change 10 m walk velocity | 0.22 ± 0.2 |
| Change difference 10 m walk velocity | 0.18 |
| ROC analysis | |
| AUC (95% CI) | 0.8 |
| Cutoff value | 0.10 |
| Sensitivity | 0.78 |
| Specificity | 0.77 |
| Timed Up and Go Test | Change ODI (n = 30) |
|---|---|
| Anchor-Based Approach | |
| Average change 10 m walk velocity | −3.6 ± 2.9 |
| Change difference 10 m walk velocity | −3.3 |
| ROC analysis | |
| AUC (95% CI) | 0.85 |
| Cutoff value | −2.04 |
| Sensitivity | 0.89 |
| Specificity | 0.67 |
| Responders (n = 30) | Non Responders (n = 18) | p Value | |
|---|---|---|---|
| Age at surgery (year) | 70.9 ± 8.1 | 72.6 ± 6.4 | 0.53 |
| Sex (male/female) | 4/26 | 0/18 | 0.17 |
| Body Mass Index (kg/m2) | 22.9 ± 3.4 | 23.5 ± 4.3 | 0.94 |
| 10 m walk velocity (m/s) | 0.98 ± 0.3 | 1.04 ± 0.2 | 0.25 |
| TUG (s) | 12.9 ± 4.3 | 10.6 ± 1.7 | 0.12 |
| Oswestry disability index (%) | 39.2 ± 12.8 | 37.8 ± 12.4 | 0.59 |
| Sagittal vertical axis (mm) | 108 ± 46.1 | 106.4 ± 44.7 | 0.72 |
| Lumbar lordosis (degree) | 14.1 ± 17.8 | 7.7 ± 0.3 | 0.17 |
| Pelvic tilt (degree) | 34.4 ± 9.9 | 34.2 ± 7.2 | 0.96 |
| Pelvic incidence (degree) | 53 ± 7.9 | 54.1 ± 7.2 | 0.71 |
| PI-LL (degree) | 41.5 ± 19.1 | 43.4 ± 11.9 | 0.69 |
| Preoperation | Postoperation | p Value | |
|---|---|---|---|
| Sagittal vertical axis (mm) | 107.4 ± 46.1 | 38.3 ± 32.3 | <0.001 |
| Lumbar lordosis (degree) | 11.6 ± 15.8 | 45.7 ± 9.6 | <0.001 |
| Pelvic tilt (degree) | 34.3 ± 9.1 | 19.6 ± 9.8 | <0.001 |
| Pelvic incidence (degree) | 52.3 ± 6.8 | 52.1 ± 7.2 | 0.19 |
| PI-LL (degree) | 41.7 ± 16.6 | 7.71 ± 12.3 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sakaguchi, T.; Meena, U.; Tanaka, M.; Xiang, H.; Fujiwara, Y.; Arataki, S.; Taoka, T.; Takamatsu, K.; Yasuda, Y.; Nakagawa, M.; et al. Minimal Clinically Important Differences in Gait and Balance Ability in Patients Who Underwent Corrective Long Spinal Fusion for Adult Spinal Deformity. J. Clin. Med. 2023, 12, 6500. https://doi.org/10.3390/jcm12206500
Sakaguchi T, Meena U, Tanaka M, Xiang H, Fujiwara Y, Arataki S, Taoka T, Takamatsu K, Yasuda Y, Nakagawa M, et al. Minimal Clinically Important Differences in Gait and Balance Ability in Patients Who Underwent Corrective Long Spinal Fusion for Adult Spinal Deformity. Journal of Clinical Medicine. 2023; 12(20):6500. https://doi.org/10.3390/jcm12206500
Chicago/Turabian StyleSakaguchi, Tomoyoshi, Umesh Meena, Masato Tanaka, Hongfei Xiang, Yoshihiro Fujiwara, Shinya Arataki, Takuya Taoka, Kazuhiko Takamatsu, Yosuke Yasuda, Masami Nakagawa, and et al. 2023. "Minimal Clinically Important Differences in Gait and Balance Ability in Patients Who Underwent Corrective Long Spinal Fusion for Adult Spinal Deformity" Journal of Clinical Medicine 12, no. 20: 6500. https://doi.org/10.3390/jcm12206500