Associations between Symptoms and Exercise Barriers in Breast Cancer Survivors
Abstract
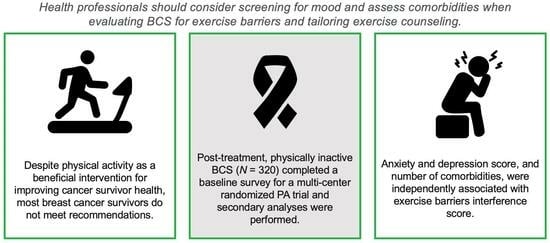
:1. Introduction
1.1. Connection to Patient Care
1.2. Study Objectives
2. Methods
2.1. Study Design
2.2. Measures
2.3. Data Analysis
3. Results
3.1. Participants
3.2. Identification of Covariates
3.3. Adjusted Correlations between Symptoms and Exercise Barriers
3.4. Linear Regression
3.5. Prevalence of Individual Barriers above the HADS Clinically Important Cut Point
4. Discussion
4.1. Strengths and Limitations
4.2. Implications
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Islami, F.; Ward, E.M.; Sung, H.; Cronin, K.A.; Tangka, F.K.L.; Sherman, R.L.; Zhao, J.; Anderson, R.N.; Henley, S.J.; Yabroff, K.R.; et al. Annual Report to the Nation on the Status of Cancer, Part 1: National Cancer Statistics. JNCI J. Natl. Cancer Inst. 2021, 113, 1648–1669. [Google Scholar] [CrossRef]
- National Cancer Institute. SEER Cancer Stat Facts: Female Breast Cancer; National Cancer Institute: Bethesda, MD, USA, 2018. [Google Scholar]
- American Cancer Society. Cancer Facts & Figures 2022; American Cancer Society: Atlanta, GA, USA, 2022. [Google Scholar]
- U.S. Department of Health and Human Services. Physical Activity Guidelines for Americans, 2nd ed.; U.S. Department of Health and Human Services: Washington, DC, USA, 2018; p. 117.
- Li, Q.; Pan, X.; Li, X.; Huang, W. Association of Physical Activity Intensity with All-Cause Mortality in Cancer Survivors: A National Prospective Cohort Study. Cancers. 2022, 14, 5760. [Google Scholar] [CrossRef]
- Pinto, B.M.; Trunzo, J.J.; Reiss, P.; Shiu, S.-Y. Exercise participation after diagnosis of breast cancer: Trends and effects on mood and quality of life. Psychooncology 2002, 11, 389–400. [Google Scholar] [CrossRef]
- Campbell, K.L.; Winters-Stone, K.M.; Wiskemann, J.; May, A.M.; Schwartz, A.L.; Courneya, K.S.; Zucker, D.S.; Matthews, C.E.; Ligibel, J.A.; Gerber, L.H.; et al. Exercise Guidelines for Cancer Survivors: Consensus statement from International Multidisciplinary Roundtable. Med. Sci. Sports Exerc. 2019, 51, 2375–2390. [Google Scholar] [CrossRef]
- Naraphong, W.; Lane, A.; Schafer, J.; Whitmer, K.; Wilson, B.R.A. Exercise intervention for fatigue-related symptoms in Thai women with breast cancer: A pilot study. Nurs. Health Sci. 2015, 17, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Blaney, J.; Lowe-Strong, A.; Rankin, J.; Campbell, A.; Allen, J.; Gracey, J. The Cancer Rehabilitation Journey: Barriers to and Facilitators of Exercise Among Patients with Cancer-Related Fatigue. Phys. Ther. 2010, 90, 1135–1147. [Google Scholar] [CrossRef]
- Ventura, E.E.; Ganz, P.A.; Bower, J.E.; Abascal, L.; Petersen, L.; Stanton, A.L.; Crespi, C.M. Barriers to physical activity and healthy eating in young breast cancer survivors: Modifiable risk factors and associations with body mass index. Breast Cancer Res. Treat. 2013, 142, 423–433. [Google Scholar] [CrossRef] [PubMed]
- Oh, P.-J.; Cho, J.-R. Changes in Fatigue, Psychological Distress, and Quality of Life After Chemotherapy in Women with Breast Cancer: A Prospective Study. Cancer Nurs. 2020, 43, E54. [Google Scholar] [CrossRef] [PubMed]
- Servaes, P.; Verhagen, C.A.H.H.V.M.; Bleijenberg, G. Relations between fatigue, neuropsychological functioning, and physical activity after treatment for breast carcinoma. Cancer 2002, 95, 2017–2026. [Google Scholar] [CrossRef]
- Ahlberg, K.; Ekman, T.; Gaston-Johansson, F.; Mock, V. Assessment and management of cancer-related fatigue in adults. Lancet 2003, 362, 640–650. [Google Scholar] [CrossRef]
- Blesch, K.S.; Paice, J.; Wickham, R.; Harte, N.; Schnoor, D.K.; Purl, S.; Rehwalt, M.; Kopp, P.L.; Manson, S.; Coveny, S.B. Correlates of fatigue in people with breast or lung cancer. Oncol. Nurs. Forum 1991, 18, 81–87. [Google Scholar] [PubMed]
- Maass, S.W.; Roorda, C.; Berendsen, A.J.; Verhaak, P.F.; de Bock, G.H. The prevalence of long-term symptoms of depression and anxiety after breast cancer treatment: A systematic review. Maturitas 2015, 82, 100–108. [Google Scholar] [CrossRef]
- Yi, J.C.; Syrjala, K.L. Anxiety and Depression in Cancer Survivors. Med. Clin. N. Am. 2017, 101, 1099–1113. [Google Scholar] [CrossRef] [PubMed]
- Otte, J.L.; Chernyak, Y.; Johns, S.A.; Jackson, L.; Ludwig, K.K.; Dodson, J.; Manchanda, S.; Bufink, E.; Draucker, C. Referral process to further evaluate poor sleep in breast cancer survivors. Cancer Med. 2022, 11, 1891–1901. [Google Scholar] [CrossRef] [PubMed]
- Koh, Y.S.; Asharani, P.V.; Devi, F.; Roystonn, K.; Wang, P.; Vaingankar, J.A.; Abdin, E.; Sum, C.F.; Lee, E.S.; Müller-Riemenschneider, F.; et al. A cross-sectional study on the perceived barriers to physical activity and their associations with domain-specific physical activity and sedentary behaviour. BMC Public Health 2022, 22, 1051. [Google Scholar] [CrossRef]
- Elshahat, S.; Treanor, C.; Donnelly, M. Factors influencing physical activity participation among people living with or beyond cancer: A systematic scoping review. Int. J. Behav. Nutr. Phys. Act. 2021, 18, 50. [Google Scholar] [CrossRef]
- Cho, D.; Park, C.L. Barriers to physical activity and healthy diet among breast cancer survivors: A multilevel perspective. Eur. J. Cancer Care 2018, 27, e12772. [Google Scholar] [CrossRef] [PubMed]
- Romero, S.A.D.; Brown, J.C.; Bauml, J.M.; Hay, J.L.; Li, Q.S.; Cohen, R.B.; Mao, J.J. Barriers to Physical Activity: A Study of Academic and Community Cancer Survivors with Pain. J. Cancer Surviv. Res. Pract. 2018, 12, 744–752. [Google Scholar] [CrossRef] [PubMed]
- Crump, C.; Sundquist, K.; Sundquist, J.; Winkleby, M.A. Exercise Is Medicine: Primary Care Counseling on Aerobic Fitness and Muscle Strengthening. J. Am. Board Fam. Med. 2019, 32, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Kirkham, A.A.; Van Patten, C.L.; Gelmon, K.A.; McKenzie, D.C.; Bonsignore, A.; Bland, K.A.; Campbell, K.L. Effectiveness of Oncologist-Referred Exercise and Healthy Eating Programming as a Part of Supportive Adjuvant Care for Early Breast Cancer. Oncologist 2018, 23, 105–115. [Google Scholar] [CrossRef]
- Rogers, L.Q.; McAuley, E.; Anton, P.M.; Courneya, K.S.; Vicari, S.; Hopkins-Price, P.; Verhulst, S.; Mocharnuk, R.; Hoelzer, K. Better exercise adherence after treatment for cancer (BEAT Cancer) study: Rationale, design, and methods. Contemp. Clin. Trials 2012, 33, 124–137. [Google Scholar] [CrossRef]
- Brown, N.I.; Pekmezi, D.W.; Oster, R.A.; Courneya, K.S.; McAuley, E.; Ehlers, D.K.; Phillips, S.M.; Anton, P.; Rogers, L.Q. Relationships between Obesity, Exercise Preferences, and Related Social Cognitive Theory Variables among Breast Cancer Survivors. Nutrients 2023, 15, 1286. [Google Scholar] [CrossRef] [PubMed]
- Groll, D.L.; To, T.; Bombardier, C.; Wright, J.G. The development of a comorbidity index with physical function as the outcome. J. Clin. Epidemiol. 2005, 58, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Rogers, L.Q.; Shah, P.; Dunnington, G.; Greive, A.; Shanmugham, A.; Dawson, B.; Courneya, K.S. Social cognitive theory and physical activity during breast cancer treatment. Oncol. Nurs. Forum 2005, 32, 807–815. [Google Scholar] [CrossRef]
- Hann, D.M.; Jacobsen, P.; Azzarello, L.M.; Martin, S.C.; Curran, S.L.; Fields, K.K.; Greenberg, H.; Lyman, G. Measurement of fatigue in cancer patients: Development and validation of the Fatigue Symptom Inventory. Qual. Life Res. Int. J. Qual. Life Asp. Treat. Care Rehabil. 1998, 7, 301–310. [Google Scholar] [CrossRef]
- Donovan, K.A.; Jacobsen, P.B.; Small, B.J.; Munster, P.N.; Andrykowski, M.A. Identifying clinically meaningful fatigue with the Fatigue Symptom Inventory. J. Pain Symptom Manag. 2008, 36, 480–487. [Google Scholar] [CrossRef] [PubMed]
- Zigmond, A.S.; Snaith, R.P. The hospital anxiety and depression scale. Acta Psychiatr. Scand. 1983, 67, 361–370. [Google Scholar] [CrossRef]
- Singer, S.; Kuhnt, S.; Götze, H.; Hauss, J.; Hinz, A.; Liebmann, A.; Krauß, O.; Lehmann, A.; Schwarz, R. Hospital anxiety and depression scale cutoff scores for cancer patients in acute care. Br. J. Cancer 2009, 100, 908–912. [Google Scholar] [CrossRef]
- Buysse, D.J.; Reynolds, C.F., III; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef]
- Aycinena, A.C.; Valdovinos, C.; Crew, K.D.; Tsai, W.Y.; Mata, J.M.; Sandoval, R.; Hershman, D.; Greenlee, H. Barriers to Recruitment and Adherence in a Randomized Controlled Diet and Exercise Weight Loss Intervention among Minority Breast Cancer Survivors. J. Immigr. Minor. Health 2017, 19, 120–129. [Google Scholar] [CrossRef]
- Cheifetz, O.; Dorsay, J.P.; Macdermid, J.C. Exercise facilitators and barriers following participation in a community-based exercise and education program for cancer survivors. J. Exerc. Rehabil. 2015, 11, 20–29. [Google Scholar] [CrossRef]
- Tsai, E.; Robertson, M.C.; Lyons, E.J.; Swartz, M.C.; Basen-Engquist, K. Physical activity and exercise self-regulation in cancer survivors: A qualitative study. Psychooncology 2018, 27, 563–568. [Google Scholar] [CrossRef]
- Omura, J.D.; Bellissimo, M.P.; Watson, K.B.; Loustalot, F.; Fulton, J.E.; Carlson, S.A. Primary care providers’ physical activity counseling and referral practices and barriers for cardiovascular disease prevention. Prev. Med. 2018, 108, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Andersen, B.L.; Lacchetti, C.; Ashing, K.; Berek, J.S.; Berman, B.S.; Bolte, S.; Dizon, D.S.; Given, B.; Nekhlyudov, L.; Pirl, W.; et al. Management of Anxiety and Depression in Adult Survivors of Cancer: ASCO Guideline Update. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2023, 41, 3426–3453. [Google Scholar] [CrossRef] [PubMed]
- Rogers, L.Q.; Vicari, S.; Trammell, R.; Hopkins-Price, P.; Fogleman, A.; Spenner, A.; Rao, K.; Courneya, K.S.; Hoelzer, K.S.; Robbs, R.; et al. Biobehavioral Factors Mediate Exercise Effects on Fatigue in Breast Cancer Survivors. Med. Sci. Sports Exerc. 2014, 46, 1077–1088. [Google Scholar] [CrossRef] [PubMed]
- Haas, B.K. Fatigue, self-efficacy, physical activity, and quality of life in women with breast cancer. Cancer Nurs. 2011, 34, 322–334. [Google Scholar] [CrossRef]
- Cheung, Y.T.; Chan, A.; Charalambous, A.; Darling, H.S.; Eng, L.; Grech, L.; Hurk, C.J.G.V.D.; Kirk, D.; Mitchell, S.A.; Poprawski, D.; et al. The use of patient-reported outcomes in routine cancer care: Preliminary insights from a multinational scoping survey of oncology practitioners. Support. Care Cancer Off. J. Multinatl. Assoc. Support. Care Cancer 2022, 30, 1427–1439. [Google Scholar] [CrossRef] [PubMed]

| Variable | Breast Cancer Survivors a |
|---|---|
| Age (years) | 55 ± 8 (21–70) |
| Ethnicity | |
| Not Hispanic/Latino | 315 (98) |
| Missing | 1 (0.3) |
| Race b | |
| White/Caucasian | 260 (81) |
| Black/African American | 48 (15) |
| Asian | 8 (3) |
| Native American/Alaska Native | 3 (1) |
| Native Hawaiian/Pacific Islander | 2 (1) |
| other | 4 (1) |
| Income | |
| <$10,000 | 14 (4) |
| $10,000 to $19,999 | 16 (5) |
| $20,000 to $34,999 | 33 (10) |
| $35,000 to $49,999 | 39 (12) |
| $50,000+ | 214 (67) |
| Missing | 4 (1) |
| Education | |
| some high school | 2 (0.6) |
| high school | 57 (17.8) |
| some college | 32 (10) |
| associate degree | 43 (13.4) |
| bachelor’s degree | 82 (25.6) |
| graduate degree or higher | 104 (32.5) |
| Mean number of comorbidities | 2.2 ± 1.8 (0–7) |
| Mean Body Mass Index (BMI) | 31.1 ± 7.34 |
| Marital status | |
| married or living with significant other | 221 (69) |
| other | 99 (31) |
| Cancer stage | |
| Ductal Carcinoma in Situ (DCIS) | 41 (13) |
| I | 125 (39) |
| II | 121 (38) |
| III | 33 (10) |
| Estrogen Receptor (self-report) | |
| Positive | 194 (61) |
| Negative | 61 (19) |
| Unsure | 62 (19) |
| Missing | 3 (1) |
| Months since cancer diagnosis | 53 ± 54 (2–276) |
| Current hormonal therapy (yes) | 160 (50) |
| Prior radiation therapy (yes) | 210 (66) |
| Prior chemotherapy (yes) | 197 (62) |
| Variable | M | SD | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|---|---|
| 1. HADS Global | 11.5 | 6.3 | – | ||||
| 2. PSQI Global | 8.2 | 4.1 | 0.46 *** | – | |||
| 3. fatigue mean interference | 3.4 | 2.1 | 0.51 *** | 0.46 *** | – | ||
| 4. fatigue mean intensity | 4.5 | 1.9 | 0.40 *** | 0.40 *** | 0.74 *** | – | |
| 5. exercise barriers | 58.3 | 12.7 | 0.23 *** | 0.19 *** | 0.17 ** | 0.17 ** | – |
| Variable | B | SE B | β |
|---|---|---|---|
| HADS Global | 0.463 | 0.112 | 0.228 ** |
| Comorbidities | 0.992 | 0.385 | 0.142 * |
| R2 | 0.088 | ||
| F | 15.286 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scott, H.; Brown, N.I.; Schleicher, E.A.; Oster, R.A.; McAuley, E.; Courneya, K.S.; Anton, P.; Ehlers, D.K.; Phillips, S.M.; Rogers, L.Q. Associations between Symptoms and Exercise Barriers in Breast Cancer Survivors. J. Clin. Med. 2023, 12, 6531. https://doi.org/10.3390/jcm12206531
Scott H, Brown NI, Schleicher EA, Oster RA, McAuley E, Courneya KS, Anton P, Ehlers DK, Phillips SM, Rogers LQ. Associations between Symptoms and Exercise Barriers in Breast Cancer Survivors. Journal of Clinical Medicine. 2023; 12(20):6531. https://doi.org/10.3390/jcm12206531
Chicago/Turabian StyleScott, Hunter, Nashira I. Brown, Erica A. Schleicher, Robert A. Oster, Edward McAuley, Kerry S. Courneya, Philip Anton, Diane K. Ehlers, Siobhan M. Phillips, and Laura Q. Rogers. 2023. "Associations between Symptoms and Exercise Barriers in Breast Cancer Survivors" Journal of Clinical Medicine 12, no. 20: 6531. https://doi.org/10.3390/jcm12206531
APA StyleScott, H., Brown, N. I., Schleicher, E. A., Oster, R. A., McAuley, E., Courneya, K. S., Anton, P., Ehlers, D. K., Phillips, S. M., & Rogers, L. Q. (2023). Associations between Symptoms and Exercise Barriers in Breast Cancer Survivors. Journal of Clinical Medicine, 12(20), 6531. https://doi.org/10.3390/jcm12206531