Evolving Clinical–Translational Investigations of Cerebroprotection in Ischemic Stroke
Abstract
1. Introduction
2. Clot Lysis
3. Mechanical Recanalization
4. Physiologic Manipulation
5. Oxidative Stress
6. Excitotoxicity
- (1)
- Induction of NMDA-Receptor 2B (NR2B) receptors to raise [Ca++]i, thereby activating neuronal nitric oxide synthase (nNOS) and NADPH oxidase 2 (NOX2). This activation generates ROS and (RNS). These toxic byproducts are associated with Zinc (Zn++) overload following Ca++ influx;
- (2)
- Amplification of the Ca++ influx signal by Ca++/Zn++ activation of other channels and feed-forward Glut release due to astrocytic swelling and ROS;
- (3)
- Expression of multiple pathways mediating cell-death activated by Ca++/Zn++ overload, potassium (K+) efflux, hydrogen (H+) influx and oxidative stress to promote calpain-mediated death with severe necrosis in the core and enhancement of oxidative damage if reperfusion does occur;
- (4)
- Promotion of inflammation and subsequent leukocyte infiltration.
7. Inflammation
7.1. Intercellular Adhesion Molecule (ICAM)-1 Blockade
7.2. Very Late Antigen (VLA)-4 Blockade
7.3. C-C Chemokine Receptor 5 (CCR-5) Blockade
7.4. Interleukin-1 (IL-1) Blockade
8. Stem Cell Therapy
Putative Reasons for Stem Cell Failure
9. Pleiotropic Drugs
10. Non-Pharmacologic Approaches
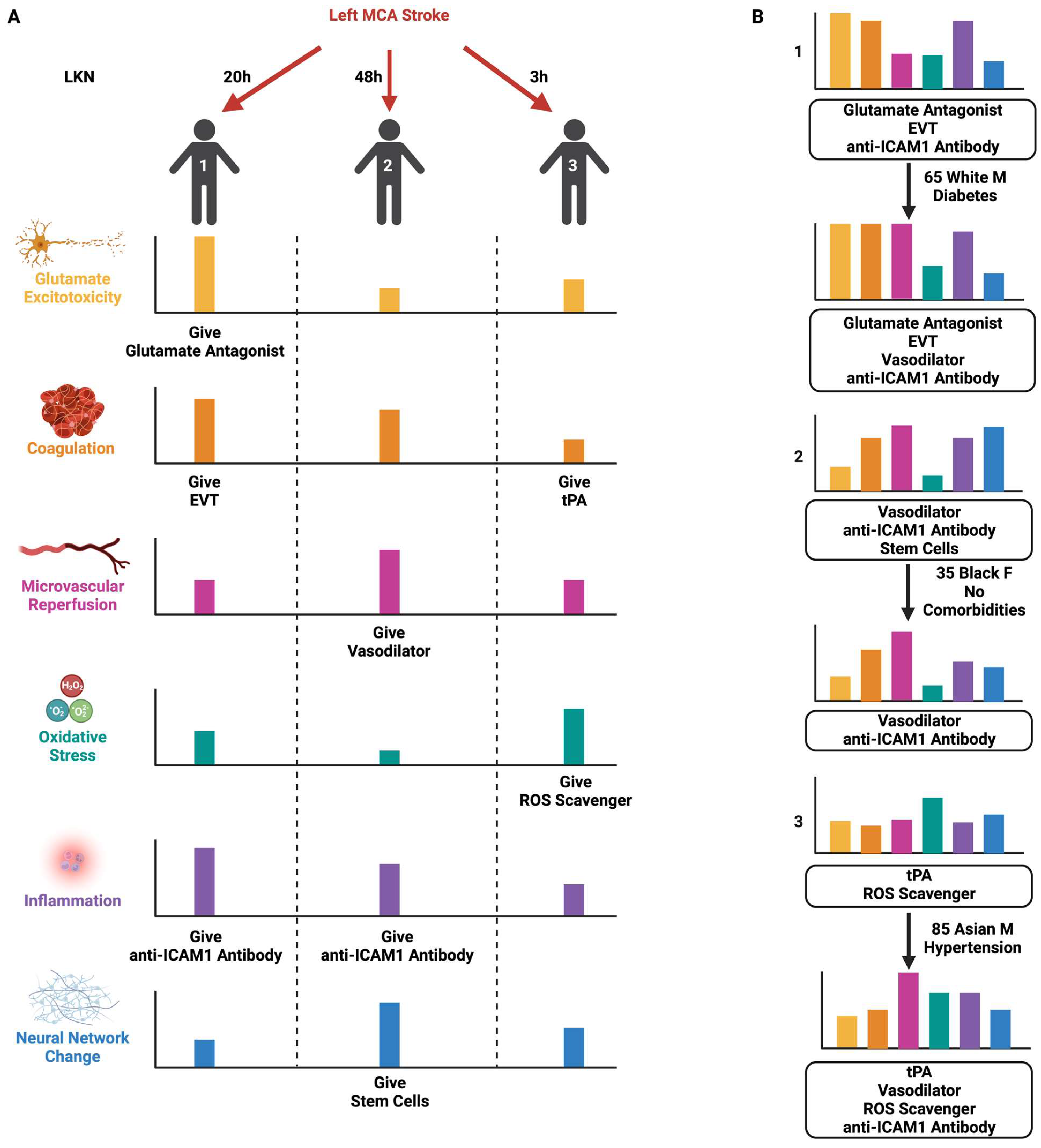
11. Bridging the Translational–Clinical Gap with SPAN
12. Discussion
13. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sacco, R.L.; Kasner, S.E.; Broderick, J.P.; Caplan, L.R.; Connors, J.J.; Culebras, A.; Elkind, M.S.V.; Grorge, M.G.; Hamdan, A.D.; Higashida, R.T.; et al. An updated definition of stroke for the 21st century: A statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2013, 44, 2064–2089. [Google Scholar] [CrossRef]
- Feigin, V.L.; Stark, B.A.; Johnson, C.O.; Roth, G.A.; Bisignano, C.; Abady, G.G.; Abbasifard, M.; Abbasi-Kangevari, M.; Abd-Allah, F.; Abedi, V.; et al. Global, regional, and national burden of stroke and its risk factors, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 2021, 20, 795–820. [Google Scholar] [CrossRef]
- Tissue plasminogen activator for acute ischemic stroke. N. Engl. J. Med. 1995, 333, 1581–1587. [CrossRef]
- Goyal, M.; Menon, B.K.; van Zwam, W.H.; Dippel, D.W.; Mitchell, P.J.; Demchuk, A.M.; Dávalos, A.; Majoie, C.B.; van der Lugt, A.; de Miquel, M.A.; et al. Endovascular thrombectomy after large-vessel ischaemic stroke: A meta-analysis of individual patient data from five randomised trials. Lancet 2016, 387, 1723–1731. [Google Scholar] [CrossRef] [PubMed]
- Atchley, T.J.; Estevez-Ordonez, D.; Laskay, N.M.B.; Tabibian, B.E.; Harrigan, M.R. Endovascular Thrombectomy for the Treatment of Large Ischemic Stroke: A Systematic Review and Meta-Analysis of Randomized Control Trials. Neurosurgery 2023. [Google Scholar] [CrossRef]
- Emberson, J.; Lees, K.R.; Lyden, P.; Blackwell, L.; Albers, G.; Bluhmki, E.; Brott, T.; Cohen, G.; Davis, S.; Donnan, G.; et al. Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: A meta-analysis of individual patient data from randomised trials. Lancet 2014, 384, 1929–1935. [Google Scholar] [CrossRef] [PubMed]
- Ng, F.C.; Churilov, L.; Yassi, N.; Kleinig, T.J.; Thijs, V.; Wu, T.; Shah, D.; Dewey, H.; Sharma, G.; Desmond, P.; et al. Prevalence and Significance of Impaired Microvascular Tissue Reperfusion Despite Macrovascular Angiographic Reperfusion (No-Reflow). Neurology 2022, 98, e790–e801. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; He, Y.; Yan, S.; Chen, L.; Zhang, R.; Xu, J.; Hu, H.; Liebeskind, D.S.; Lou, M. Reperfusion Injury Is Associated With Poor Outcome in Patients With Recanalization After Thrombectomy. Stroke 2023, 54, 96–104. [Google Scholar] [CrossRef]
- Kloner, R.A.; King, K.S.; Harrington, M.G. No-reflow phenomenon in the heart and brain. Am. J. Physiol. Heart Circ. Physiol. 2018, 315, H550–H562. [Google Scholar] [CrossRef]
- Baron, J.C. The core/penumbra model: Implications for acute stroke treatment and patient selection in 2021. Eur. J. Neurol. 2021, 28, 2794–2803. [Google Scholar] [CrossRef]
- Enzmann, G.; Kargaran, S.; Engelhardt, B. Ischemia-reperfusion injury in stroke: Impact of the brain barriers and brain immune privilege on neutrophil function. Ther. Adv. Neurol. Disord. 2018, 11, 1756286418794184. [Google Scholar] [CrossRef]
- Moskowitz, M.A.; Lo, E.H.; Iadecola, C. The science of stroke: Mechanisms in search of treatments. Neuron 2010, 67, 181–198. [Google Scholar] [CrossRef]
- Iadecola, C.; Buckwalter, M.S.; Anrather, J. Immune responses to stroke: Mechanisms, modulation, and therapeutic potential. J. Clin. Investig. 2020, 130, 2777–2788. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.T.; Goyal, M.; Levy, E.I.; Liebeskind, D.; Jahan, R.; Pereira, V.M.; Gralla, J.; Bonafe, A.; Saver, J.L. Onset to reperfusion time as a determinant of outcomes across a wide range of ASPECTS in endovascular thrombectomy: Pooled analysis of the SWIFT, SWIFT PRIME, and STAR studies. J. Neurointerv. Surg. 2020, 12, 240–245. [Google Scholar] [CrossRef]
- Li, M.; Tang, H.; Li, Z.; Tang, W. Emerging Treatment Strategies for Cerebral Ischemia-Reperfusion Injury. Neuroscience 2022, 507, 112–124. [Google Scholar] [CrossRef] [PubMed]
- Lyden, P.D. Cerebroprotection for Acute Ischemic Stroke: Looking Ahead. Stroke 2021, 52, 3033–3044. [Google Scholar] [CrossRef] [PubMed]
- Stroke Preclinical Assessment Network (SPAN) to Support Translational Studies For Acute Cerebroprotection-Interventions (U01 Clinical Trial Not Allowed [Internet]). Available online: https://grants.nih.gov/grants/guide/rfa-files/RFA-NS-22-066.html (accessed on 16 May 2022).
- Ginsberg, M.D. Neuroprotection for ischemic stroke: Past, present and future. Neuropharmacology 2008, 55, 363–389. [Google Scholar] [CrossRef] [PubMed]
- Powers, W.J.; Rabinstein, A.A.; Ackerson, T.; Adeoye, O.M.; Bambakidis, N.C.; Becker, K.; Biller, J.; Brown, M.; Demaerschalk, B.M.; Hoh, B.; et al. Guidelines for the Early Management of Patients With Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke 2019, 50, e344–e418. [Google Scholar] [CrossRef]
- Kleindorfer, D.O.; Towfighi, A.; Chaturvedi, S.; Cockroft, K.M.; Gutierrez, J.; Lombardi-Hill, D.; Kamel, H.; Kernan, W.N.; Kittner, S.J.; Leira, E.C.; et al. 2021 Guideline for the Prevention of Stroke in Patients With Stroke and Transient Ischemic Attack: A Guideline From the American Heart Association/American Stroke Association. Stroke 2021, 52, e364–e467. [Google Scholar] [CrossRef]
- Macrae, I.M. Preclinical stroke research--advantages and disadvantages of the most common rodent models of focal ischaemia. Br. J. Pharmacol. 2011, 164, 1062–1078. [Google Scholar] [CrossRef]
- Sommer, C.J. Ischemic stroke: Experimental models and reality. Acta Neuropathol. 2017, 133, 245–261. [Google Scholar] [CrossRef]
- Jolugbo, P.; Ariëns, R.A.S. Thrombus Composition and Efficacy of Thrombolysis and Thrombectomy in Acute Ischemic Stroke. Stroke 2021, 52, 1131–1142. [Google Scholar] [CrossRef]
- Hacke, W.; Kaste, M.; Bluhmki, E.; Brozman, M.; Dávalos, A.; Guidetti, D.; Larrue, V.; Lees, K.R.; Medeghri, Z.; Machnig, T.; et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N. Engl. J. Med. 2008, 359, 1317–1329. [Google Scholar] [CrossRef]
- Thelwell, C.; Longstaff, C. The regulation by fibrinogen and fibrin of tissue plasminogen activator kinetics and inhibition by plasminogen activator inhibitor 1. J. Thromb. Haemost. 2007, 5, 804–811. [Google Scholar] [CrossRef]
- Gravanis, I.; Tsirka, S.E. Tissue-type plasminogen activator as a therapeutic target in stroke. Expert Opin. Ther. Targets 2008, 12, 159–170. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhang, C.; An, J.; Strickland, D.K.; Yepes, M. The low-density lipoprotein receptor-related protein 1 mediates tissue-type plasminogen activator-induced microglial activation in the ischemic brain. Am. J. Pathol. 2009, 174, 586–594. [Google Scholar] [CrossRef] [PubMed]
- Tanswell, P.; Modi, N.; Combs, D.; Danays, T. Pharmacokinetics and pharmacodynamics of tenecteplase in fibrinolytic therapy of acute myocardial infarction. Clin. Pharmacokinet. 2002, 41, 1229–1245. [Google Scholar] [CrossRef] [PubMed]
- Campbell, B.C.V.; Mitchell, P.J.; Churilov, L.; Yassi, N.; Kleinig, T.J.; Dowling, R.J.; Yan, B.; Bush, S.J.; Dewey, H.M.; Thijs, V.; et al. Tenecteplase versus Alteplase before Thrombectomy for Ischemic Stroke. N. Engl. J. Med. 2018, 378, 1573–1582. [Google Scholar] [CrossRef]
- Zhong, C.S.; Beharry, J.; Salazar, D.; Smith, K.; Withington, S.; Campbell, B.C.V.; Wilson, D.; Le Heron, C.; Mason, D.; Duncan, R.; et al. Routine Use of Tenecteplase for Thrombolysis in Acute Ischemic Stroke. Stroke 2021, 52, 1087–1090. [Google Scholar] [CrossRef]
- Wang, Y.; Li, S.; Pan, Y.; Li, H.; Parsons, M.W.; Campbell, B.C.V.; Schwamm, L.H.; Fisher, M.; Che, F.; Dai, H.; et al. Tenecteplase versus alteplase in acute ischaemic cerebrovascular events (TRACE-2): A phase 3, multicentre, open-label, randomised controlled, non-inferiority trial. Lancet 2023, 401, 645–654. [Google Scholar] [CrossRef]
- Checkouri, T.; Gerschenfeld, G.; Seners, P.; Yger, M.; Ben Hassen, W.; Chausson, N.; Olindo, S.; Caroff, J.; Marnat, G.; Clarençon, F.; et al. Early Recanalization Among Patients Undergoing Bridging Therapy With Tenecteplase or Alteplase. Stroke 2023, 54, 2491–2499. [Google Scholar] [CrossRef] [PubMed]
- Murata, Y.; Rosell, A.; Scannevin, R.H.; Rhodes, K.J.; Wang, X.; Lo, E.H. Extension of the thrombolytic time window with minocycline in experimental stroke. Stroke 2008, 39, 3372–3377. [Google Scholar] [CrossRef] [PubMed]
- Ishiguro, M.; Kawasaki, K.; Suzuki, Y.; Ishizuka, F.; Mishiro, K.; Egashira, Y.; Ikegaki, I.; Tsuruma, K.; Shimazawa, M.; Yoshimura, S.; et al. A Rho kinase (ROCK) inhibitor, fasudil, prevents matrix metalloproteinase-9-related hemorrhagic transformation in mice treated with tissue plasminogen activator. Neuroscience 2012, 220, 302–312. [Google Scholar] [CrossRef] [PubMed]
- Kamalian, S.; Morais, L.T.; Pomerantz, S.R.; Aceves, M.; Sit, S.P.; Bose, A.; Hirsch, J.A.; Lev, M.H.; Yoo, A.J. Clot length distribution and predictors in anterior circulation stroke: Implications for intra-arterial therapy. Stroke 2013, 44, 3553–3556. [Google Scholar] [CrossRef]
- Jovin, T.G.; Chamorro, A.; Cobo, E.; de Miquel, M.A.; Molina, C.A.; Rovira, A.; San Román, L.; Serena, J.; Abilleira, S.; Ribó, M.; et al. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N. Engl. J. Med. 2015, 372, 2296–2306. [Google Scholar] [CrossRef]
- Goyal, M.; Demchuk, A.M.; Menon, B.K.; Eesa, M.; Rempel, J.L.; Thornton, J.; Roy, D.; Jovin, T.G.; Willinsky, R.A.; Sapkota, B.L.; et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N. Engl. J. Med. 2015, 372, 1019–1030. [Google Scholar] [CrossRef]
- Saver, J.L.; Goyal, M.; Bonafe, A.; Diener, H.C.; Levy, E.I.; Pereira, V.M.; Albers, G.W.; Cognard, C.; Cohen, D.J.; Hacke, W.; et al. Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N. Engl. J. Med. 2015, 372, 2285–2295. [Google Scholar] [CrossRef]
- Campbell, B.C.; Mitchell, P.J.; Kleinig, T.J.; Dewey, H.M.; Churilov, L.; Yassi, N.; Yan, B.; Dowling, R.J.; Parsons, M.W.; Oxley, T.J.; et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N. Engl. J. Med. 2015, 372, 1009–1018. [Google Scholar] [CrossRef]
- Nogueira, R.G.; Jadhav, A.P.; Haussen, D.C.; Bonafe, A.; Budzik, R.F.; Bhuva, P.; Yavagal, D.R.; Ribo, M.; Cognard, C.; Hanel, R.A.; et al. Thrombectomy 6 to 24 Hours after Stroke with a Mismatch between Deficit and Infarct. N. Engl. J. Med. 2018, 378, 11–21. [Google Scholar] [CrossRef]
- Albers, G.W.; Marks, M.P.; Kemp, S.; Christensen, S.; Tsai, J.P.; Ortega-Gutierrez, S.; McTaggart, R.A.; Torbey, M.T.; Kim-Tenser, M.; Leslie-Mazwi, T.; et al. Thrombectomy for Stroke at 6 to 16 Hours with Selection by Perfusion Imaging. N. Engl. J. Med. 2018, 378, 708–718. [Google Scholar] [CrossRef]
- Huo, X.; Ma, G.; Tong, X.; Zhang, X.; Pan, Y.; Nguyen, T.N.; Yuan, G.; Han, H.; Chen, W.; Wei, M.; et al. Trial of Endovascular Therapy for Acute Ischemic Stroke with Large Infarct. N. Engl. J. Med. 2023, 388, 1272–1283. [Google Scholar] [CrossRef]
- Sarraj, A.; Hassan, A.E.; Abraham, M.G.; Ortega-Gutierrez, S.; Kasner, S.E.; Hussain, M.S.; Chen, M.; Blackburn, S.; Sitton, C.W.; Churilov, L.; et al. Trial of Endovascular Thrombectomy for Large Ischemic Strokes. N. Engl. J. Med. 2023, 388, 1259–1271. [Google Scholar] [CrossRef]
- Olthuis, S.G.H.; Pirson, F.A.V.; Pinckaers, F.M.E.; Hinsenveld, W.H.; Nieboer, D.; Ceulemans, A.; Knapen, R.R.M.M.; Robbe, M.M.Q.; Berkhemer, O.A.; van Walderveen, M.A.A.; et al. Endovascular treatment versus no endovascular treatment after 6–24 h in patients with ischaemic stroke and collateral flow on CT angiography (MR CLEAN-LATE) in the Netherlands: A multicentre, open-label, blinded-endpoint, randomised, controlled, phase 3 trial. Lancet 2023, 401, 1371–1380. [Google Scholar] [CrossRef]
- Renú, A.; Millán, M.; San Román, L.; Blasco, J.; Martí-Fàbregas, J.; Terceño, M.; Amaro, S.; Serena, J.; Urra, X.; Laredo, C.; et al. Effect of Intra-arterial Alteplase vs Placebo Following Successful Thrombectomy on Functional Outcomes in Patients With Large Vessel Occlusion Acute Ischemic Stroke: The CHOICE Randomized Clinical Trial. JAMA 2022, 327, 826–835. [Google Scholar] [CrossRef] [PubMed]
- Fischer, U.; Kaesmacher, J.; Strbian, D.; Eker, O.; Cognard, C.; Plattner, P.S.; Bütikofer, L.; Mordasini, P.; Deppeler, S.; Pereira, V.M.; et al. Thrombectomy alone versus intravenous alteplase plus thrombectomy in patients with stroke: An open-label, blinded-outcome, randomised non-inferiority trial. Lancet 2022, 400, 104–115. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Matsumaru, Y.; Takeuchi, M.; Morimoto, M.; Kanazawa, R.; Takayama, Y.; Kamiya, Y.; Shigeta, K.; Okubo, S.; Hayakawa, M.; et al. Effect of Mechanical Thrombectomy Without vs With Intravenous Thrombolysis on Functional Outcome Among Patients With Acute Ischemic Stroke: The SKIP Randomized Clinical Trial. JAMA 2021, 325, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Zhang, Y.; Zhang, L.; Zhang, Y.; Treurniet, K.M.; Chen, W.; Peng, Y.; Han, H.; Wang, J.; Wang, S.; et al. Endovascular Thrombectomy with or without Intravenous Alteplase in Acute Stroke. N. Engl. J. Med. 2020, 382, 1981–1993. [Google Scholar] [CrossRef] [PubMed]
- Zi, W.; Qiu, Z.; Li, F.; Sang, H.; Wu, D.; Luo, W.; Liu, S.; Yuan, J.; Song, J.; Shi, Z.; et al. Effect of Endovascular Treatment Alone vs Intravenous Alteplase Plus Endovascular Treatment on Functional Independence in Patients With Acute Ischemic Stroke: The DEVT Randomized Clinical Trial. JAMA 2021, 325, 234–243. [Google Scholar] [CrossRef]
- Faizy, T.D.; Broocks, G.; Heit, J.J.; Kniep, H.; Flottmann, F.; Meyer, L.; Sporns, P.; Hanning, U.; Kaesmacher, J.; Deb-Chatterji, M.; et al. Association Between Intravenous Thrombolysis and Clinical Outcomes Among Patients With Ischemic Stroke and Unsuccessful Mechanical Reperfusion. JAMA Netw. Open 2023, 6, e2310213. [Google Scholar] [CrossRef]
- Schwarz, S.; Georgiadis, D.; Aschoff, A.; Schwab, S. Effects of body position on intracranial pressure and cerebral perfusion in patients with large hemispheric stroke. Stroke 2002, 33, 497–501. [Google Scholar] [CrossRef]
- Schwarz, S.; Georgiadis, D.; Aschoff, A.; Schwab, S. Effects of induced hypertension on intracranial pressure and flow velocities of the middle cerebral arteries in patients with large hemispheric stroke. Stroke 2002, 33, 998–1004. [Google Scholar] [CrossRef] [PubMed]
- Leonardi-Bee, J.; Bath, P.M.; Phillips, S.J.; Sandercock, P.A. Blood pressure and clinical outcomes in the International Stroke Trial. Stroke 2002, 33, 1315–1320. [Google Scholar] [CrossRef]
- Bang, O.Y.; Chung, J.W.; Kim, S.K.; Kim, S.J.; Lee, M.J.; Hwang, J.; Seo, W.K.; Ha, Y.S.; Sung, S.M.; Kim, E.G.; et al. Therapeutic-induced hypertension in patients with noncardioembolic acute stroke. Neurology 2019, 93, e1955–e1963. [Google Scholar] [CrossRef]
- He, J.; Zhang, Y.; Xu, T.; Zhao, Q.; Wang, D.; Chen, C.S.; Tong, W.; Liu, C.; Xu, T.; Ju, Z.; et al. Effects of immediate blood pressure reduction on death and major disability in patients with acute ischemic stroke: The CATIS randomized clinical trial. JAMA 2014, 311, 479–489. [Google Scholar] [CrossRef]
- Mistry, E.A.; Mayer, S.A.; Khatri, P. Blood Pressure Management after Mechanical Thrombectomy for Acute Ischemic Stroke: A Survey of the StrokeNet Sites. J. Stroke Cerebrovasc. Dis. 2018, 27, 2474–2478. [Google Scholar] [CrossRef]
- Mistry, E.A.; Sucharew, H.; Mistry, A.M.; Mehta, T.; Arora, N.; Starosciak, A.K.; De Los Rios La Rosa, F.; Siegler, J.E., 3rd; Barnhill, N.R.; Patel, K.; et al. Blood Pressure after Endovascular Therapy for Ischemic Stroke (BEST): A Multicenter Prospective Cohort Study. Stroke 2019, 50, 3449–3455. [Google Scholar] [CrossRef]
- Singhal, A.; Partners SPOTRIAS Investigators. A phase IIB clinical trial of normobaric oxygen therapy (NBO) in acute ischemic stroke (AIS) (S02.001). Neurology 2013, 80 (Suppl. S7), S02.001. [Google Scholar]
- Rusyniak, D.E.; Kirk, M.A.; May, J.D.; Kao, L.W.; Brizendine, E.J.; Welch, J.L.; Cordell, W.H.; Alonso, R.J. Hyperbaric oxygen therapy in acute ischemic stroke: Results of the Hyperbaric Oxygen in Acute Ischemic Stroke Trial Pilot Study. Stroke 2003, 34, 571–574. [Google Scholar] [CrossRef]
- Poli, S.; Mbroh, J.; Baron, J.C.; Singhal, A.B.; Strbian, D.; Molina, C.; Lemmens, R.; Turc, G.; Mikulik, R.; Michel, P.; et al. Penumbral Rescue by normobaric O = O administration in patients with ischemic stroke and target mismatch proFile (PROOF): Study protocol of a phase IIb trial. Int. J. Stroke 2023, 17474930231185275. [Google Scholar] [CrossRef]
- Roffe, C.; Nevatte, T.; Sim, J.; Bishop, J.; Ives, N.; Ferdinand, P.; Gray, R. Effect of Routine Low-Dose Oxygen Supplementation on Death and Disability in Adults With Acute Stroke: The Stroke Oxygen Study Randomized Clinical Trial. JAMA 2017, 318, 1125–1135. [Google Scholar] [CrossRef]
- Lyden, P.D.; Krieger, D.; Yenari, M.; Dietrich, W.D. Therapeutic hypothermia for acute stroke. Int. J. Stroke 2006, 1, 9–19. [Google Scholar] [CrossRef]
- You, J.S.; Kim, J.Y.; Yenari, M.A. Therapeutic hypothermia for stroke: Unique challenges at the bedside. Front. Neurol. 2022, 13, 951586. [Google Scholar] [CrossRef] [PubMed]
- Geurts, M.; Petersson, J.; Brizzi, M.; Olsson-Hau, S.; Luijckx, G.J.; Algra, A.; Dippel, D.W.; Kappelle, L.J.; van der Worp, H.B. COOLIST (Cooling for Ischemic Stroke Trial): A Multicenter, Open, Randomized, Phase II, Clinical Trial. Stroke 2017, 48, 219–221. [Google Scholar] [CrossRef] [PubMed]
- De Georgia, M.A.; Krieger, D.W.; Abou-Chebl, A.; Devlin, T.G.; Jauss, M.; Davis, S.M.; Koroshetz, W.J.; Rordorf, G.; Warach, S. Cooling for Acute Ischemic Brain Damage (COOL AID): A feasibility trial of endovascular cooling. Neurology 2004, 63, 312–317. [Google Scholar] [CrossRef]
- Lyden, P.; Hemmen, T.; Grotta, J.; Rapp, K.; Ernstrom, K.; Rzesiewicz, T.; Parker, S.; Concha, M.; Hussain, S.; Agarwal, S.; et al. Results of the ICTuS 2 Trial (Intravascular Cooling in the Treatment of Stroke 2). Stroke 2016, 47, 2888–2895. [Google Scholar] [CrossRef] [PubMed]
- Lehner, C.; Gehwolf, R.; Tempfer, H.; Krizbai, I.; Hennig, B.; Bauer, H.C.; Bauer, H. Oxidative stress and blood-brain barrier dysfunction under particular consideration of matrix metalloproteinases. Antioxid. Redox Signal 2011, 15, 1305–1323. [Google Scholar] [CrossRef]
- Simpson, D.S.A.; Oliver, P.L. ROS Generation in Microglia: Understanding Oxidative Stress and Inflammation in Neurodegenerative Disease. Antioxidants 2020, 9, 743. [Google Scholar] [CrossRef]
- Chen, Y.; Qin, C.; Huang, J.; Tang, X.; Liu, C.; Huang, K.; Xu, J.; Guo, G.; Tong, A.; Zhou, L. The role of astrocytes in oxidative stress of central nervous system: A mixed blessing. Cell Prolif. 2020, 53, e12781. [Google Scholar] [CrossRef]
- Carbone, F.; Teixeira, P.C.; Braunersreuther, V.; Mach, F.; Vuilleumier, N.; Montecucco, F. Pathophysiology and Treatments of Oxidative Injury in Ischemic Stroke: Focus on the Phagocytic NADPH Oxidase 2. Antioxid. Redox Signal 2015, 23, 460–489. [Google Scholar] [CrossRef]
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef] [PubMed]
- Griendling, K.K.; Camargo, L.L.; Rios, F.J.; Alves-Lopes, R.; Montezano, A.C.; Touyz, R.M. Oxidative Stress and Hypertension. Circ. Res. 2021, 128, 993–1020. [Google Scholar] [CrossRef]
- Iino, M.; Takamatsu, T.; Mizukami, Y.; Bando, S.; Kato, Y.; Nakagawa, H.; Kowashi, Y.; Kato, H. Silent period and initial occlusal sliding time in patients with premature contacts. Nihon Shishubyo Gakkai Kaishi 1989, 31, 1130–1137. [Google Scholar] [CrossRef]
- Feng, S.; Yang, M.; Liu, S.; He, Y.; Deng, S.; Gong, Y. Oxidative stress as a bridge between age and stroke: A narrative review. J. Intensive Med. 2023, in press. [Google Scholar] [CrossRef]
- Forman, H.J.; Zhang, H. Targeting oxidative stress in disease: Promise and limitations of antioxidant therapy. Nat. Rev. Drug Discov. 2021, 20, 689–709. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Cheng, M.; Maples, K.R.; Ma, J.Y.; Buchan, A.M. NXY-059, a novel free radical trapping compound, reduces cortical infarction after permanent focal cerebral ischemia in the rat. Brain Res. 2001, 909, 46–50. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, S.; Tsuchidate, R.; Smith, M.L.; Maples, K.R.; Siesjö, B.K. Neuroprotective effects of a novel nitrone, NXY-059, after transient focal cerebral ischemia in the rat. J. Cereb. Blood Flow Metab. 1999, 19, 778–787. [Google Scholar] [CrossRef]
- Marshall, J.W.; Duffin, K.J.; Green, A.R.; Ridley, R.M. NXY-059, a free radical--trapping agent, substantially lessens the functional disability resulting from cerebral ischemia in a primate species. Stroke 2001, 32, 190–198. [Google Scholar] [CrossRef]
- Lees, K.R.; Barer, D.; Ford, G.A.; Hacke, W.; Kostulas, V.; Sharma, A.K.; Odergren, T.; SA-NXY-0004 Investigators. Tolerability of NXY-059 at higher target concentrations in patients with acute stroke. Stroke 2003, 34, 482–487. [Google Scholar] [CrossRef]
- Lees, K.R.; Zivin, J.A.; Ashwood, T.; Davalos, A.; Davis, S.M.; Diener, H.C.; Grotta, J.; Lyden, P.; Shuaib, A.; Hårdemark, H.G.; et al. NXY-059 for acute ischemic stroke. N. Engl. J. Med. 2006, 354, 588–600. [Google Scholar] [CrossRef]
- Muir, K.W.; Weir, C.J.; Murray, G.D.; Povey, C.; Lees, K.R. Comparison of neurological scales and scoring systems for acute stroke prognosis. Stroke 1996, 27, 1817–1820. [Google Scholar] [CrossRef]
- Shuaib, A.; Lees, K.R.; Lyden, P.; Grotta, J.; Davalos, A.; Davis, S.M.; Diener, H.C.; Ashwood, T.; Wasiewski, W.W.; Emeribe, U.; et al. NXY-059 for the treatment of acute ischemic stroke. N. Engl. J. Med. 2007, 357, 562–571. [Google Scholar] [CrossRef]
- Diener, H.C.; Lees, K.R.; Lyden, P.; Grotta, J.; Davalos, A.; Davis, S.M.; Shuaib, A.; Ashwood, T.; Wasiewski, W.; Alderfer, V.; et al. NXY-059 for the treatment of acute stroke: Pooled analysis of the SAINT I and II Trials. Stroke 2008, 39, 1751–1758. [Google Scholar] [CrossRef]
- Minnerup, J.; Sutherland, B.A.; Buchan, A.M.; Kleinschnitz, C. Neuroprotection for stroke: Current status and future perspectives. Int. J. Mol. Sci. 2012, 13, 11753–11772. [Google Scholar] [CrossRef]
- Bath, P.M.; Gray, L.J.; Bath, A.J.; Buchan, A.; Miyata, T.; Green, A.R. Effects of NXY-059 in experimental stroke: An individual animal meta-analysis. Br. J. Pharmacol. 2009, 157, 1157–1171. [Google Scholar] [CrossRef] [PubMed]
- Fisher, M.; Lees, K.; Papadakis, M.; Buchan, A.M. NXY-059: Brain or vessel protection. Stroke 2006, 37, 2189–2190. [Google Scholar] [CrossRef] [PubMed]
- Antonic, A.; Dottori, M.; Macleod, M.R.; Donnan, G.A.; Howells, D.W. NXY-059, a Failed Stroke Neuroprotectant, Offers No Protection to Stem Cell-Derived Human Neurons. J. Stroke Cerebrovasc. Dis. 2018, 27, 2158–2165. [Google Scholar] [CrossRef] [PubMed]
- Dirnagl, U.; Macleod, M.R. Stroke research at a road block: The streets from adversity should be paved with meta-analysis and good laboratory practice. Br. J. Pharmacol. 2009, 157, 1154–1156. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.H.; Cha, M.; Lee, B.H. Neuroprotective Effect of Antioxidants in the Brain. Int. J. Mol. Sci. 2020, 21, 7152. [Google Scholar] [CrossRef]
- Cherubini, A.; Polidori, M.C.; Bregnocchi, M.; Pezzuto, S.; Cecchetti, R.; Ingegni, T.; di Iorio, A.; Senin, U.; Mecocci, P. Antioxidant profile and early outcome in stroke patients. Stroke 2000, 31, 2295–2300. [Google Scholar] [CrossRef]
- Olney, J.W. Brain lesions, obesity, and other disturbances in mice treated with monosodium glutamate. Science 1969, 164, 719–721. [Google Scholar] [CrossRef]
- Rothman, S. Synaptic release of excitatory amino acid neurotransmitter mediates anoxic neuronal death. J. Neurosci. 1984, 4, 1884–1891. [Google Scholar] [CrossRef]
- Choi, D.W. Excitotoxicity: Still Hammering the Ischemic Brain in 2020. Front. Neurosci. 2020, 14, 579953. [Google Scholar] [CrossRef]
- Gill, R.; Foster, A.C.; Woodruff, G.N. Systemic administration of MK-801 protects against ischemia-induced hippocampal neurodegeneration in the gerbil. J. Neurosci. 1987, 7, 3343–3349. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, G.K.; Saleh, J.; Kunis, D. Delayed treatment with dextromethorphan and dextrorphan reduces cerebral damage after transient focal ischemia. Neurosci. Lett. 1988, 89, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Roundtable, Stroke Therapy Academic Industry. Recommendations for standards regarding preclinical neuroprotective and restorative drug development. Stroke 1999, 30, 2752–2758. [Google Scholar] [CrossRef] [PubMed]
- Saver, J.L.; Starkman, S.; Eckstein, M.; Stratton, S.J.; Pratt, F.D.; Hamilton, S.; Conwit, R.; Liebeskind, D.S.; Sung, G.; Kramer, I.; et al. Prehospital use of magnesium sulfate as neuroprotection in acute stroke. N. Engl. J. Med. 2015, 372, 528–536. [Google Scholar] [CrossRef]
- Ibanez, L.; Heitsch, L.; Carrera, C.; Farias, F.H.G.; Del Aguila, J.L.; Dhar, R.; Budde, J.; Bergmann, K.; Bradley, J.; Harari, O.; et al. Multi-ancestry GWAS reveals excitotoxicity associated with outcome after ischaemic stroke. Brain 2022, 145, 2394–2406. [Google Scholar] [CrossRef] [PubMed]
- Anrather, J.; Iadecola, C. Inflammation and Stroke: An Overview. Neurotherapeutics 2016, 13, 661–670. [Google Scholar] [CrossRef]
- Kim, J.Y.; Park, J.; Chang, J.Y.; Kim, S.H.; Lee, J.E. Inflammation after Ischemic Stroke: The Role of Leukocytes and Glial Cells. Exp. Neurobiol. 2016, 25, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Jayaraj, R.L.; Azimullah, S.; Beiram, R.; Jalal, F.Y.; Rosenberg, G.A. Neuroinflammation: Friend and foe for ischemic stroke. J. Neuroinflamm. 2019, 16, 142. [Google Scholar] [CrossRef]
- Widner, H.; Brundin, P. Immunological aspects of grafting in the mammalian central nervous system. A review and speculative synthesis. Brain Res. 1988, 472, 287–324. [Google Scholar] [CrossRef]
- Louveau, A.; Harris, T.H.; Kipnis, J. Revisiting the Mechanisms of CNS Immune Privilege. Trends Immunol. 2015, 36, 569–577. [Google Scholar] [CrossRef]
- del Zoppo, G.J.; Schmid-Schönbein, G.W.; Mori, E.; Copeland, B.R.; Chang, C.M. Polymorphonuclear leukocytes occlude capillaries following middle cerebral artery occlusion and reperfusion in baboons. Stroke 1991, 22, 1276–1283. [Google Scholar] [CrossRef] [PubMed]
- Prestigiacomo, C.J.; Kim, S.C.; Connolly, E.S., Jr.; Liao, H.; Yan, S.F.; Pinsky, D.J. CD18-mediated neutrophil recruitment contributes to the pathogenesis of reperfused but not nonreperfused stroke. Stroke 1999, 30, 1110–1117. [Google Scholar] [CrossRef] [PubMed]
- Barone, F.C.; Schmidt, D.B.; Hillegass, L.M.; Price, W.J.; White, R.F.; Feuerstein, G.Z.; Clark, R.K.; Lee, E.V.; Griswold, D.E.; Sarau, H.M. Reperfusion increases neutrophils and leukotriene B4 receptor binding in rat focal ischemia. Stroke 1992, 23, 1337–1347; discussion 47–48. [Google Scholar] [CrossRef] [PubMed]
- Beuker, C.; Strecker, J.K.; Rawal, R.; Schmidt-Pogoda, A.; Ruck, T.; Wiendl, H.; Klotz, L.; Schäbitz, W.R.; Sommer, C.J.; Minnerup, H.; et al. Immune Cell Infiltration into the Brain After Ischemic Stroke in Humans Compared to Mice and Rats: A Systematic Review and Meta-Analysis. Transl. Stroke Res. 2021, 12, 976–990. [Google Scholar] [CrossRef]
- Neumann, J.; Henneberg, S.; von Kenne, S.; Nolte, N.; Müller, A.J.; Schraven, B.; Görtler, M.W.; Reymann, K.G.; Gunzer, M.; Riek-Burchardt, M. Beware the intruder: Real time observation of infiltrated neutrophils and neutrophil-Microglia interaction during stroke in vivo. PLoS ONE 2018, 13, e0193970. [Google Scholar] [CrossRef]
- Enzmann, G.; Mysiorek, C.; Gorina, R.; Cheng, Y.J.; Ghavampour, S.; Hannocks, M.J.; Prinz, V.; Dirnagl, U.; Endres, M.; Prinz, M.; et al. The neurovascular unit as a selective barrier to polymorphonuclear granulocyte (PMN) infiltration into the brain after ischemic injury. Acta Neuropathol. 2013, 125, 395–412. [Google Scholar] [CrossRef]
- Otxoa-de-Amezaga, A.; Gallizioli, M.; Pedragosa, J.; Justicia, C.; Miró-Mur, F.; Salas-Perdomo, A.; Díaz-Marugan, L.; Gunzer, M.; Planas, A.M. Location of Neutrophils in Different Compartments of the Damaged Mouse Brain After Severe Ischemia/Reperfusion. Stroke 2019, 50, 1548–1557. [Google Scholar] [CrossRef]
- Erdener, Ş.E.; Tang, J.; Kılıç, K.; Postnov, D.; Giblin, J.T.; Kura, S.; Chen, I.A.; Vayisoğlu, T.; Sakadžić, S.; Schaffer, C.B.; et al. Dynamic capillary stalls in reperfused ischemic penumbra contribute to injury: A hyperacute role for neutrophils in persistent traffic jams. J. Cereb. Blood Flow Metab. 2021, 41, 236–252. [Google Scholar] [CrossRef]
- Denorme, F.; Portier, I.; Rustad, J.L.; Cody, M.J.; de Araujo, C.V.; Hoki, C.; Alexander, M.D.; Grandhi, R.; Dyer, M.R.; Neal, M.D.; et al. Neutrophil extracellular traps regulate ischemic stroke brain injury. J. Clin. Investig. 2022, 132, e154225. [Google Scholar] [CrossRef] [PubMed]
- Kollikowski, A.M.; Schuhmann, M.K.; Nieswandt, B.; Müllges, W.; Stoll, G.; Pham, M. Local Leukocyte Invasion during Hyperacute Human Ischemic Stroke. Ann. Neurol. 2020, 87, 466–479. [Google Scholar] [CrossRef]
- Bui, T.M.; Wiesolek, H.L.; Sumagin, R. ICAM-1: A master regulator of cellular responses in inflammation, injury resolution, and tumorigenesis. J. Leukoc. Biol. 2020, 108, 787–799. [Google Scholar] [CrossRef]
- Zhang, R.L.; Chopp, M.; Jiang, N.; Tang, W.X.; Prostak, J.; Manning, A.M.; Anderson, D.C. Anti-intercellular adhesion molecule-1 antibody reduces ischemic cell damage after transient but not permanent middle cerebral artery occlusion in the Wistar rat. Stroke 1995, 26, 1438–1442; discussion 43. [Google Scholar] [CrossRef]
- Muller, W.A. Mechanisms of leukocyte transendothelial migration. Annu. Rev. Pathol. 2011, 6, 323–344. [Google Scholar] [CrossRef] [PubMed]
- Bowes, M.P.; Zivin, J.A.; Rothlein, R. Monoclonal antibody to the ICAM-1 adhesion site reduces neurological damage in a rabbit cerebral embolism stroke model. Exp. Neurol. 1993, 119, 215–219. [Google Scholar] [CrossRef]
- Enlimomab Acute Stroke Trial Investigators. Use of anti-ICAM-1 therapy in ischemic stroke: Results of the Enlimomab Acute Stroke Trial. Neurology 2001, 57, 1428–1434. [Google Scholar] [CrossRef]
- Furuya, K.; Ginis, I.; Takeda, H.; Chen, Y.; Hallenbeck, J.M. Cell permeable exogenous ceramide reduces infarct size in spontaneously hypertensive rats supporting in vitro studies that have implicated ceramide in induction of tolerance to ischemia. J. Cereb. Blood Flow Metab. 2001, 21, 226–232. [Google Scholar] [CrossRef]
- Rudick, R.; Polman, C.; Clifford, D.; Miller, D.; Steinman, L. Natalizumab: Bench to bedside and beyond. JAMA Neurol. 2013, 70, 172–182. [Google Scholar] [CrossRef]
- Benakis, C.; Simats, A.; Tritschler, S.; Heindl, S.; Besson-Girard, S.; Llovera, G.; Pinkham, K.; Kolz, A.; Ricci, A.; Theis, F.J.; et al. T cells modulate the microglial response to brain ischemia. eLife 2022, 11, e82031. [Google Scholar] [CrossRef]
- Liesz, A.; Zhou, W.; Mracskó, É.; Karcher, S.; Bauer, H.; Schwarting, S.; Sun, L.; Bruder, D.; Stegemann, S.; Cerwenka, A.; et al. Inhibition of lymphocyte trafficking shields the brain against deleterious neuroinflammation after stroke. Brain 2011, 134, 704–720. [Google Scholar] [CrossRef]
- Elkind, M.S.V.; Veltkamp, R.; Montaner, J.; Johnston, S.C.; Singhal, A.B.; Becker, K.; Lansberg, M.G.; Tang, W.; Kasliwal, R.; Elkins, J. Natalizumab in acute ischemic stroke (ACTION II): A randomized, placebo-controlled trial. Neurology 2020, 95, e1091–e1104. [Google Scholar] [CrossRef]
- Woollard, S.M.; Kanmogne, G.D. Maraviroc: A review of its use in HIV infection and beyond. Drug Des. Dev. Ther. 2015, 9, 5447–5468. [Google Scholar] [CrossRef]
- Joy, M.T.; Ben Assayag, E.; Shabashov-Stone, D.; Liraz-Zaltsman, S.; Mazzitelli, J.; Arenas, M.; Abduljawad, N.; Kliper, E.; Korczyn, A.D.; Thareja, N.S.; et al. CCR5 Is a Therapeutic Target for Recovery after Stroke and Traumatic Brain Injury. Cell 2019, 176, 1143–1157.e13. [Google Scholar] [CrossRef] [PubMed]
- Kawabori, M.; Shichinohe, H.; Kuroda, S.; Houkin, K. Clinical Trials of Stem Cell Therapy for Cerebral Ischemic Stroke. Int. J. Mol. Sci. 2020, 21, 7380. [Google Scholar] [CrossRef]
- Stroncek, D.F.; Jin, P.; McKenna, D.H.; Takanashi, M.; Fontaine, M.J.; Pati, S.; Schäfer, R.; Peterson, E.; Benedetti, E.; Reems, J.A. Human Mesenchymal Stromal Cell (MSC) Characteristics Vary Among Laboratories When Manufactured From the Same Source Material: A Report by the Cellular Therapy Team of the Biomedical Excellence for Safer Transfusion (BEST) Collaborative. Front. Cell Dev. Biol. 2020, 8, 458. [Google Scholar] [CrossRef] [PubMed]
- Kawabori, M.; Kuroda, S.; Sugiyama, T.; Ito, M.; Shichinohe, H.; Houkin, K.; Houkin, K.; Kuge, Y.; Tamaki, N. Intracerebral, but not intravenous, transplantation of bone marrow stromal cells enhances functional recovery in rat cerebral infarct: An optical imaging study. Neuropathology 2012, 32, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Barbosa da Fonseca, L.M.; Gutfilen, B.; Rosado de Castro, P.H.; Battistella, V.; Goldenberg, R.C.; Kasai-Brunswick, T.; Chagas, C.L.; Wajnberg, E.; Maiolino, A.; Salles Xavier, S.; et al. Migration and homing of bone-marrow mononuclear cells in chronic ischemic stroke after intra-arterial injection. Exp. Neurol. 2010, 221, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, M.A.; Martins, M.P.; Araújo, M.D.; Klamt, C.; Vedolin, L.; Garicochea, B.; Raupp, E.F.; Sartori El Ammar, J.; Machado, D.C.; Costa, J.C.; et al. Intra-arterial infusion of autologous bone marrow mononuclear cells in patients with moderate to severe middle cerebral artery acute ischemic stroke. Cell Transplant. 2012, 21 (Suppl. S1), S13–S21. [Google Scholar] [CrossRef]
- Moniche, F.; Gonzalez, A.; Gonzalez-Marcos, J.R.; Carmona, M.; Piñero, P.; Espigado, I.; Garcia-Solis, D.; Cayuela, A.; Montaner, J.; Boada, C.; et al. Intra-arterial bone marrow mononuclear cells in ischemic stroke: A pilot clinical trial. Stroke 2012, 43, 2242–2244. [Google Scholar] [CrossRef]
- Bhatia, V.; Gupta, V.; Khurana, D.; Sharma, R.R.; Khandelwal, N. Randomized Assessment of the Safety and Efficacy of Intra-Arterial Infusion of Autologous Stem Cells in Subacute Ischemic Stroke. AJNR Am. J. Neuroradiol. 2018, 39, 899–904. [Google Scholar] [CrossRef]
- Hess, D.C.; Wechsler, L.R.; Clark, W.M.; Savitz, S.I.; Ford, G.A.; Chiu, D.; Yavagal, D.R.; Uchino, K.; Liebeskind, D.S.; Auchus, A.P.; et al. Safety and efficacy of multipotent adult progenitor cells in acute ischaemic stroke (MASTERS): A randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Neurol. 2017, 16, 360–368. [Google Scholar] [CrossRef]
- Lee, J.S.; Hong, J.M.; Moon, G.J.; Lee, P.H.; Ahn, Y.H.; Bang, O.Y. A long-term follow-up study of intravenous autologous mesenchymal stem cell transplantation in patients with ischemic stroke. Stem Cells 2010, 28, 1099–1106. [Google Scholar] [CrossRef]
- Bang, O.Y.; Lee, J.S.; Lee, P.H.; Lee, G. Autologous mesenchymal stem cell transplantation in stroke patients. Ann. Neurol. 2005, 57, 874–882. [Google Scholar] [CrossRef]
- Savitz, S.I.; Yavagal, D.; Rappard, G.; Likosky, W.; Rutledge, N.; Graffagnino, C.; Alderazi, Y.; Elder, J.A.; Chen, P.R.; Budzik, R.F., Jr.; et al. A Phase 2 Randomized, Sham-Controlled Trial of Internal Carotid Artery Infusion of Autologous Bone Marrow-Derived ALD-401 Cells in Patients With Recent Stable Ischemic Stroke (RECOVER-Stroke). Circulation 2019, 139, 192–205. [Google Scholar] [CrossRef]
- Jaillard, A.; Hommel, M.; Moisan, A.; Zeffiro, T.A.; Favre-Wiki, I.M.; Barbieux-Guillot, M.; Vadot, W.; Marcel, S.; Lamalle, L.; Grand, S.; et al. Autologous Mesenchymal Stem Cells Improve Motor Recovery in Subacute Ischemic Stroke: A Randomized Clinical Trial. Transl. Stroke Res. 2020, 11, 910–923. [Google Scholar] [CrossRef]
- Prasad, K.; Sharma, A.; Garg, A.; Mohanty, S.; Bhatnagar, S.; Johri, S.; Singh, K.K.; Nair, V.; Sarkar, R.S.; Gorthi, S.P.; et al. Intravenous autologous bone marrow mononuclear stem cell therapy for ischemic stroke: A multicentric, randomized trial. Stroke 2014, 45, 3618–3624. [Google Scholar] [CrossRef]
- Chung, J.W.; Chang, W.H.; Bang, O.Y.; Moon, G.J.; Kim, S.J.; Kim, S.K.; Lee, J.S.; Sohn, S.I.; Kim, Y.H.; STARTING-2 Collaborators. Efficacy and Safety of Intravenous Mesenchymal Stem Cells for Ischemic Stroke. Neurology 2021, 96, e1012–e1023. [Google Scholar] [CrossRef]
- Lee, J.; Chang, W.H.; Chung, J.W.; Kim, S.J.; Kim, S.K.; Lee, J.S.; Sohn, S.I.; Kim, Y.H.; Bang, O.Y.; STARTING-2 Collaborators. Efficacy of Intravenous Mesenchymal Stem Cells for Motor Recovery After Ischemic Stroke: A Neuroimaging Study. Stroke 2022, 53, 20–28. [Google Scholar] [CrossRef]
- Chiu, T.L.; Baskaran, R.; Tsai, S.T.; Huang, C.Y.; Chuang, M.H.; Syu, W.S.; Harn, H.J.; Lin, Y.C.; Chen, C.H.; Huang, P.C.; et al. Intracerebral transplantation of autologous adipose-derived stem cells for chronic ischemic stroke: A phase I study. J. Tissue Eng. Regen. Med. 2022, 16, 3–13. [Google Scholar] [CrossRef]
- Xu, S.; Qiu, Y.; Tao, J. The challenges and optimization of cell-based therapy for cardiovascular disease. J. Transl. Int. Med. 2021, 9, 234–238. [Google Scholar] [CrossRef]
- Gwag, B.J.; Lee, Y.A.; Ko, S.Y.; Lee, M.J.; Im, D.S.; Yun, B.S.; Lim, H.R.; Park, S.M.; Byun, H.Y.; Son, S.J.; et al. Marked prevention of ischemic brain injury by Neu2000, an NMDA antagonist and antioxidant derived from aspirin and sulfasalazine. J. Cereb. Blood Flow Metab. 2007, 27, 1142–1151. [Google Scholar] [CrossRef]
- Vane, J.R.; Botting, R.M. The mechanism of action of aspirin. Thromb. Res. 2003, 110, 255–258. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.M.; Lee, J.S.; Lee, Y.B.; Shin, D.H.; Shin, D.I.; Hwang, Y.H.; Ahn, S.H.; Kim, J.G.; Sohn, S.I.; Kwon, S.U.; et al. Nelonemdaz for Patients With Acute Ischemic Stroke Undergoing Endovascular Reperfusion Therapy: A Randomized Phase II Trial. Stroke 2022, 53, 3250–3259. [Google Scholar] [CrossRef]
- Lee, J.S.; Lee, J.S.; Gwag, B.J.; Choi, D.W.; An, C.S.; Kang, H.G.; Song, T.J.; Ahn, S.H.; Kim, C.H.; Shin, D.I.; et al. The Rescue on Reperfusion Damage in Cerebral Infarction by Nelonemdaz (RODIN) Trial: Protocol for a Double-Blinded Clinical Trial of Nelonemdaz in Patients with Hyperacute Ischemic Stroke and Endovascular Thrombectomy. J. Stroke 2023, 25, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Hoh, B.L.; Ko, N.U.; Amin-Hanjani, S.; Chou, S.-Y.; Cruz-Flores, S.; Dangayach, N.S.; Derdeyn, C.P.; Du, R.; Hänggi, D.; Hetts, S.W.; et al. 2023 Guideline for the Management of Patients With Aneurysmal Subarachnoid Hemorrhage: A Guideline From the American Heart Association/American Stroke Association. Stroke 2023, 54, e314–e370. [Google Scholar] [CrossRef] [PubMed]
- Laskowitz, D.T.; Kolls, B.J. Neuroprotection in subarachnoid hemorrhage. Stroke 2010, 41 (Suppl. S1), S79–S84. [Google Scholar] [CrossRef]
- Carlson, A.P.; Hänggi, D.; Macdonald, R.L.; Shuttleworth, C.W. Nimodipine Reappraised: An Old Drug With a Future. Curr. Neuropharmacol. 2020, 18, 65–82. [Google Scholar] [CrossRef]
- Horn, J.; de Haan, R.J.; Vermeulen, M.; Limburg, M. Very Early Nimodipine Use in Stroke (VENUS): A randomized, double-blind, placebo-controlled trial. Stroke 2001, 32, 461–465. [Google Scholar] [CrossRef]
- Ahmed, N.; Näsman, P.; Wahlgren, N.G. Effect of intravenous nimodipine on blood pressure and outcome after acute stroke. Stroke 2000, 31, 1250–1255. [Google Scholar] [CrossRef]
- Wahlgren, N.G.M.D.; De Keyser, J.; Indredavik, B.; Ryman, T. The Intravenous Nimodipine West European Trial (INWEST) of nimodipine in the treat-ment of acute ischemic stroke. Cerebrovasc. Dis. 1994, 4, 204–210. [Google Scholar] [CrossRef]
- Janovák, L.; Turcsányi, Á.; Bozó, É.; Deák, Á.; Mérai, L.; Sebők, D.; Juhász, Á.; Csapó, E.; Abdelghafour, M.M.; Farkas, E.; et al. Preparation of novel tissue acidosis-responsive chitosan drug nanoparticles: Characterization and in vitro release properties of Ca2+ channel blocker nimodipine drug molecules. Eur. J. Pharm. Sci. 2018, 123, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Oesterle, A.; Laufs, U.; Liao, J.K. Pleiotropic Effects of Statins on the Cardiovascular System. Circ. Res. 2017, 120, 229–243. [Google Scholar] [CrossRef]
- Scandinavian Simvastatin Survival Study Group. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: The Scandinavian Simvastatin Survival Study (4S). Lancet 1994, 344, 1383–1389. [Google Scholar]
- Sacks, F.M.; Pfeffer, M.A.; Moye, L.A.; Rouleau, J.L.; Rutherford, J.D.; Cole, T.G.; Brown, L.; Warnica, J.W.; Arnold, J.M.; Wun, C.C.; et al. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events Trial investigators. N. Engl. J. Med. 1996, 335, 1001–1009. [Google Scholar] [CrossRef]
- Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: A randomised placebo-controlled trial. Lancet 2002, 360, 7–22. [Google Scholar] [CrossRef] [PubMed]
- Flint, A.C.; Kamel, H.; Navi, B.B.; Rao, V.A.; Faigeles, B.S.; Conell, C.; Klingman, J.G.; Sidney, S.; Hills, N.K.; Sorel, M.; et al. Statin use during ischemic stroke hospitalization is strongly associated with improved poststroke survival. Stroke 2012, 43, 147–154. [Google Scholar] [CrossRef]
- Katsanos, A.H.; Lioutas, V.A.; Charidimou, A.; Catanese, L.; Ng, K.K.H.; Perera, K.; de Sa Boasquevisque, D.; Tsivgoulis, G.; Smith, E.E.; Sharma, M.; et al. Statin treatment and accrual of covert cerebral ischaemia on neuroimaging: A systematic review and meta-analysis of randomized trials. Eur. J. Neurol. 2020, 27, 1023–1027. [Google Scholar] [CrossRef]
- Amarenco, P.; Bogousslavsky, J.; Callahan, A., 3rd; Goldstein, L.B.; Hennerici, M.; Rudolph, A.E.; Sillesen, H.; Simunovic, L.; Szarek, M.; Welch, K.M.; et al. High-dose atorvastatin after stroke or transient ischemic attack. N. Engl. J. Med. 2006, 355, 549–559. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, X.; Dong, L.; Wen, Y.; Cui, L. The many roles of statins in ischemic stroke. Curr. Neuropharmacol. 2014, 12, 564–574. [Google Scholar] [CrossRef] [PubMed]
- Vitturi, B.K.; Gagliardi, R.J. Effectiveness of statins in patients with stroke due to cervical artery dissection: A preliminary study. Med. Clínica 2021, 157, 313–317. [Google Scholar] [CrossRef] [PubMed]
- Kusznir Vitturi, B.; José Gagliardi, R. The role of statins in cardioembolic stroke. J. Clin. Neurosci. 2020, 72, 174–179. [Google Scholar] [CrossRef]
- Kim, J.; Lee, H.S.; Nam, C.M.; Heo, J.H. Effects of Statin Intensity and Adherence on the Long-Term Prognosis After Acute Ischemic Stroke. Stroke 2017, 48, 2723–2730. [Google Scholar] [CrossRef]
- Harper, C.R.; Jacobson, T.A. The broad spectrum of statin myopathy: From myalgia to rhabdomyolysis. Curr. Opin. Lipidol. 2007, 18, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Basiak, M.; Kosowski, M.; Cyrnek, M.; Bułdak, Ł.; Maligłówka, M.; Machnik, G.; Okopień, B. Pleiotropic Effects of PCSK-9 Inhibitors. Int. J. Mol. Sci. 2021, 22, 3144. [Google Scholar] [CrossRef]
- Hess, D.C.; Blauenfeldt, R.A.; Andersen, G.; Hougaard, K.D.; Hoda, M.N.; Ding, Y.; Ji, X. Remote ischaemic conditioning-a new paradigm of self-protection in the brain. Nat. Rev. Neurol. 2015, 11, 698–710. [Google Scholar] [CrossRef]
- Zhao, W.; Li, S.; Ren, C.; Meng, R.; Jin, K.; Ji, X. Remote ischemic conditioning for stroke: Clinical data, challenges, and future directions. Ann. Clin. Transl. Neurol. 2019, 6, 186–196. [Google Scholar] [CrossRef] [PubMed]
- Blauenfeldt, R.A.; Hjort, N.; Valentin, J.B.; Homburg, A.M.; Modrau, B.; Sandal, B.F.; Gude, M.F.; Hougaard, K.D.; Damgaard, D.; Poulsen, M.; et al. Remote Ischemic Conditioning for Acute Stroke: The RESIST Randomized Clinical Trial. JAMA 2023, 330, 1236–1246. [Google Scholar] [CrossRef]
- Chen, H.S.; Cui, Y.; Li, X.Q.; Wang, X.H.; Ma, Y.T.; Zhao, Y.; Han, J.; Deng, C.Q.; Hong, M.; Bao, Y.; et al. Effect of Remote Ischemic Conditioning vs Usual Care on Neurologic Function in Patients With Acute Moderate Ischemic Stroke: The RICAMIS Randomized Clinical Trial. JAMA 2022, 328, 627–636. [Google Scholar] [CrossRef]
- Hougaard, K.D.; Hjort, N.; Zeidler, D.; Sørensen, L.; Nørgaard, A.; Hansen, T.M.; von Weitzel-Mudersbach, P.; Simonsen, C.Z.; Damgaard, D.; Gottrup, H.; et al. Remote ischemic perconditioning as an adjunct therapy to thrombolysis in patients with acute ischemic stroke: A randomized trial. Stroke 2014, 45, 159–167. [Google Scholar] [CrossRef]
- England, T.J.; Hedstrom, A.; O’Sullivan, S.; Donnelly, R.; Barrett, D.A.; Sarmad, S.; Sprigg, N.; Bath, P.M. RECAST (Remote Ischemic Conditioning After Stroke Trial): A Pilot Randomized Placebo Controlled Phase II Trial in Acute Ischemic Stroke. Stroke 2017, 48, 1412–1415. [Google Scholar] [CrossRef]
- Veldema, J.; Gharabaghi, A. Non-invasive brain stimulation for improving gait, balance, and lower limbs motor function in stroke. J. Neuroeng. Rehabil. 2022, 19, 84. [Google Scholar] [CrossRef]
- Azad, T.D.; Veeravagu, A.; Steinberg, G.K. Neurorestoration after stroke. Neurosurg. Focus 2016, 40, E2. [Google Scholar] [CrossRef]
- Fregni, F.; El-Hagrassy, M.M.; Pacheco-Barrios, K.; Carvalho, S.; Leite, J.; Simis, M.; Brunelin, J.; Nakamura-Palacios, E.M.; Marangolo, P.; Venkatasubramanian, G.; et al. Evidence-Based Guidelines and Secondary Meta-Analysis for the Use of Transcranial Direct Current Stimulation in Neurological and Psychiatric Disorders. Int. J. Neuropsychopharmacol. 2021, 24, 256–313. [Google Scholar] [CrossRef]
- Kleim, J.A.; Bruneau, R.; VandenBerg, P.; MacDonald, E.; Mulrooney, R.; Pocock, D. Motor cortex stimulation enhances motor recovery and reduces peri-infarct dysfunction following ischemic insult. Neurol. Res. 2003, 25, 789–793. [Google Scholar] [CrossRef]
- Levy, R.; Ruland, S.; Weinand, M.; Lowry, D.; Dafer, R.; Bakay, R. Cortical stimulation for the rehabilitation of patients with hemiparetic stroke: A multicenter feasibility study of safety and efficacy. J. Neurosurg. 2008, 108, 707–714. [Google Scholar] [CrossRef]
- Dawson, J.; Pierce, D.; Dixit, A.; Kimberley, T.J.; Robertson, M.; Tarver, B.; Hilmi, O.; McLean, J.; Forbes, K.; Kilgard, M.P.; et al. Safety, Feasibility, and Efficacy of Vagus Nerve Stimulation Paired With Upper-Limb Rehabilitation After Ischemic Stroke. Stroke 2016, 47, 143–150. [Google Scholar] [CrossRef]
- Kimberley, T.J.; Pierce, D.; Prudente, C.N.; Francisco, G.E.; Yozbatiran, N.; Smith, P.; Tarver, B.; Engineer, N.D.; Alexander Dickie, D.; Kline, D.K.; et al. Vagus Nerve Stimulation Paired With Upper Limb Rehabilitation After Chronic Stroke. Stroke 2018, 49, 2789–2792. [Google Scholar] [CrossRef] [PubMed]
- Chavez, L.M.; Huang, S.S.; MacDonald, I.; Lin, J.G.; Lee, Y.C.; Chen, Y.H. Mechanisms of Acupuncture Therapy in Ischemic Stroke Rehabilitation: A Literature Review of Basic Studies. Int. J. Mol. Sci. 2017, 18, 2270. [Google Scholar] [CrossRef]
- Zhu, S.; Meng, B.; Jiang, J.; Wang, X.; Luo, N.; Liu, N.; Shen, H.; Wang, L.; Li, Q. The Updated Role of Transcranial Ultrasound Neuromodulation in Ischemic Stroke: From Clinical and Basic Research. Front. Cell. Neurosci. 2022, 16, 839023. [Google Scholar] [CrossRef]
- Caglayan, A.B.; Beker, M.C.; Caglayan, B.; Yalcin, E.; Caglayan, A.; Yulug, B.; Hanoglu, L.; Kutlu, S.; Doeppner, T.R.; Hermann, D.M.; et al. Acute and Post-acute Neuromodulation Induces Stroke Recovery by Promoting Survival Signaling, Neurogenesis, and Pyramidal Tract Plasticity. Front. Cell. Neurosci. 2019, 13, 144. [Google Scholar] [CrossRef]
- Lyden, P.D.; Bosetti, F.; Diniz, M.A.; Rogatko, A.; Koenig, J.I.; Lamb, J.; Nagarkatti, K.A.; Cabeen, R.P.; Hess, D.C.; Kamat, P.K.; et al. The Stroke Preclinical Assessment Network: Rationale, Design, Feasibility, and Stage 1 Results. Stroke 2022, 53, 1802–1812. [Google Scholar] [CrossRef]
- Koellhoffer, E.C.; McCullough, L.D. The effects of estrogen in ischemic stroke. Transl. Stroke Res. 2013, 4, 390–401. [Google Scholar] [CrossRef]
- Schallert, T. Behavioral tests for preclinical intervention assessment. NeuroRx 2006, 3, 497–504. [Google Scholar] [CrossRef]
- Sansing, L. Primary Results of the Stroke Preclinical Assessment Network (SPAN). In Proceedings of the International Stroke Conference, Dallas, TX, USA, 8–10 February 2023. [Google Scholar]
- Morais, A.; Locascio, J.J.; Sansing, L.H.; Lamb, J.; Nagarkatti, K.; Imai, T.; van Leyen, K.; Aronowski, J.; Koenig, J.I.; Bosetti, F.; et al. Embracing Heterogeneity in The Multicenter Stroke Preclinical Assessment Network (SPAN) Trial. Stroke 2023, 54, 620–631. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Schappell, L.E.; Polizu, C.; DiPersio, J.; Tsirka, S.E.; Halterman, M.W.; Nadkarni, N.A. Evolving Clinical–Translational Investigations of Cerebroprotection in Ischemic Stroke. J. Clin. Med. 2023, 12, 6715. https://doi.org/10.3390/jcm12216715
Li Y, Schappell LE, Polizu C, DiPersio J, Tsirka SE, Halterman MW, Nadkarni NA. Evolving Clinical–Translational Investigations of Cerebroprotection in Ischemic Stroke. Journal of Clinical Medicine. 2023; 12(21):6715. https://doi.org/10.3390/jcm12216715
Chicago/Turabian StyleLi, Yinghui, Laurel E. Schappell, Claire Polizu, James DiPersio, Stella E. Tsirka, Marc W. Halterman, and Neil A. Nadkarni. 2023. "Evolving Clinical–Translational Investigations of Cerebroprotection in Ischemic Stroke" Journal of Clinical Medicine 12, no. 21: 6715. https://doi.org/10.3390/jcm12216715
APA StyleLi, Y., Schappell, L. E., Polizu, C., DiPersio, J., Tsirka, S. E., Halterman, M. W., & Nadkarni, N. A. (2023). Evolving Clinical–Translational Investigations of Cerebroprotection in Ischemic Stroke. Journal of Clinical Medicine, 12(21), 6715. https://doi.org/10.3390/jcm12216715






