Advances in Stroke Neurorehabilitation
Abstract
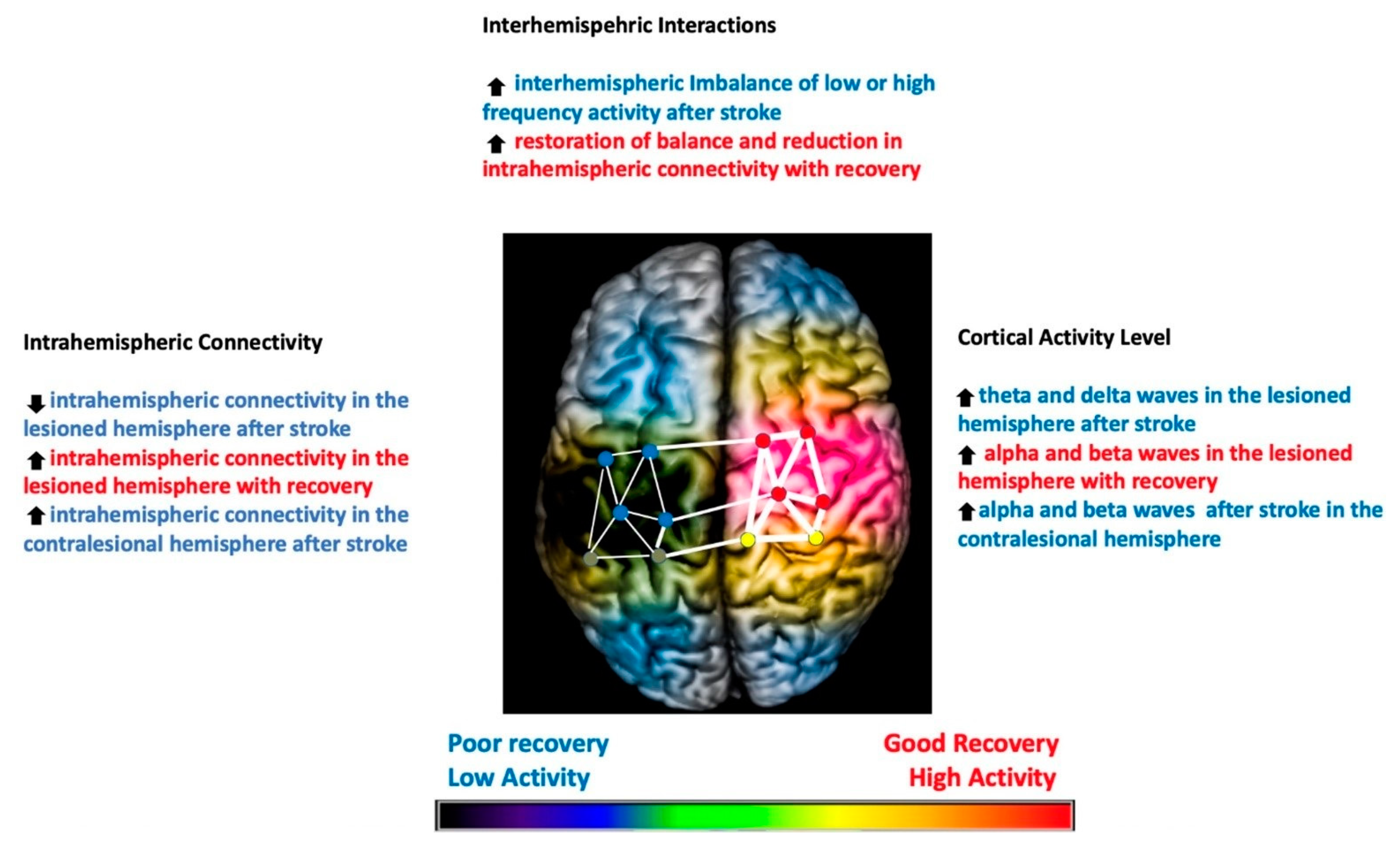
1. Introduction
2. Non-Invasive/Minimally Invasive Brain Stimulation
2.1. Vagus Nerve Stimulation
2.2. Transcranial Magnetic Stimulation
2.3. Transcranial Direct Current Stimulation
3. Activity-Based Therapies
3.1. Constraint Induced Movement Therapy
3.2. Robot-Assisted Therapies
3.3. Telerehabilitation
4. Brain–Computer Interfaces
5. Pharmacological and Cellular Therapies
5.1. Selective Serotonin Reuptake Inhibitors (SSRIs)
5.2. Maraviroc
5.3. Stem Cell Therapies
6. Cognitive-Based Therapies
6.1. Prism Adaptation Therapy
6.2. Virtual Reality
6.3. Motor Imagery and Action Observation Therapies
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Loubinoux, I.; Carel, C.; Pariente, J.; Dechaumont, S.; Albucher, J.-F.; Marque, P.; Manelfe, C.; Chollet, F. Correlation between cerebral reorganization and motor recovery after subcortical infarcts. NeuroImage 2003, 20, 2166–2180. [Google Scholar] [CrossRef]
- Nair, D.G.; Hutchinson, S.; Fregni, F.; Alexander, M.; Pascual-Leone, A.; Schlaug, G. Imaging correlates of motor recovery from cerebral infarction and their physiological significance in well-recovered patients. NeuroImage 2007, 34, 253–263. [Google Scholar] [CrossRef]
- Keser, Z.; Buchl, S.C.; Seven, N.A.; Markota, M.; Clark, H.M.; Jones, D.T.; Lanzino, G.; Brown, R.D.; Worrell, G.A.; Lundstrom, B.N. Electroencephalogram (EEG) With or Without Transcranial Magnetic Stimulation (TMS) as Biomarkers for Post-stroke Recovery: A Narrative Review. Front. Neurol. 2022, 13, 827866. [Google Scholar] [CrossRef] [PubMed]
- Fregni, F.; Pascual-Leone, A. Technology Insight: Noninvasive brain stimulation in neurology—Perspectives on the therapeutic potential of rTMS and tDCS. Nat. Clin. Pract. Neurol. 2007, 3, 383–393. [Google Scholar] [CrossRef]
- Bindman, L.J.; Lippold, O.C.J.; Redfearn, J.W.T. The action of brief polarizing currents on the cerebral cortex of the rat (1) during current flow and (2) in the production of long-lasting after-effects. J. Physiol. 1964, 172, 369–382. [Google Scholar] [CrossRef] [PubMed]
- Priori, A. Brain polarization in humans: A reappraisal of an old tool for prolonged non-invasive modulation of brain excitability. Clin. Neurophysiol. 2003, 114, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Dawson, J.; Liu, C.Y.; Francisco, G.E.; Cramer, S.C.; Wolf, S.L.; Dixit, A.; Alexander, J.; Ali, R.; Brown, B.L.; Feng, W.; et al. Vagus nerve stimulation paired with rehabilitation for upper limb motor function after ischaemic stroke (VNS-REHAB): A randomised, blinded, pivotal, device trial. Lancet 2021, 397, 1545–1553. [Google Scholar] [CrossRef]
- Fridriksson, J.; Rorden, C.; Elm, J.; Sen, S.; George, M.S.; Bonilha, L. Transcranial Direct Current Stimulation vs Sham Stimulation to Treat Aphasia after Stroke: A Randomized Clinal Trial. JAMA Neurol. 2018, 75, 1470–1476. [Google Scholar] [CrossRef]
- Harvey, R.L.; Edwards, D.; Dunning, K.; Fregni, F.; Stein, J.; Laine, J.; Rogers, L.M.; Vox, F.; Durand-Sanchez, A.; Bockbrader, M.; et al. Randomized Sham-Controlled Trial of Navigated Repetitive Transcranial Magnetic Stimulation for Motor Recovery in Stroke. Stroke 2018, 49, 2138–2146. [Google Scholar] [CrossRef]
- Vink, J.J.; van Lieshout, E.C.; Otte, W.M.; van Eijk, R.P.; Kouwenhoven, M.; Neggers, S.F.; van der Worp, H.B.; Visser-Meily, J.M.; Dijkhuizen, R.M. Continuous Theta-Burst Stimulation of the Contralesional Primary Motor Cortex for Promotion of Upper Limb Recovery After Stroke: A Randomized Controlled Trial. Stroke 2023, 54, 1962–1971. [Google Scholar] [CrossRef]
- Stockbridge, M.D.; Elm, J.; Breining, B.L.; Tippett, D.C.; Sebastian, R.; Cassarly, C.; Teklehaimanot, A.; Spell, L.A.; Sheppard, S.M.; Vitti, E.; et al. Transcranial Direct-Current Stimulation in Subacute Aphasia: A Randomized Controlled Trial. Stroke 2023, 54, 912–920. [Google Scholar] [CrossRef] [PubMed]
- Meyers, E.C.; Solorzano, B.R.; James, J.; Ganzer, P.D.; Lai, E.S.; Rennaker, R.L.; Kilgard, M.P.; Hays, S.A. Vagus Nerve Stimulation Enhances Stable Plasticity and Generalization of Stroke Recovery. Stroke 2018, 49, 710–717. [Google Scholar] [CrossRef] [PubMed]
- Dawson, J.; Pierce, D.; Dixit, A.; Kimberley, T.J.; Robertson, M.; Tarver, B.; Hilmi, O.; McLean, J.; Forbes, K.; Kilgard, M.P.; et al. Safety, Feasibility, and Efficacy of Vagus Nerve Stimulation Paired With Upper-Limb Rehabilitation after Ischemic Stroke. Stroke 2016, 47, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Kimberley, T.J.; Pierce, D.; Prudente, C.N.; Francisco, G.E.; Yozbatiran, N.; Smith, P.; Tarver, B.; Engineer, N.D.; Dickie, D.A.; Kline, D.K.; et al. Vagus Nerve Stimulation Paired With Upper Limb Rehabilitation After Chronic Stroke. Stroke 2018, 49, 2789–2792. [Google Scholar] [CrossRef]
- Capone, F.; Miccinilli, S.; Pellegrino, G.; Zollo, L.; Simonetti, D.; Bressi, F.; Florio, L.; Ranieri, F.; Falato, E.; Di Santo, A.; et al. Transcutaneous vagus nerve stimulation combined with robotic rehabilitation improves upper limb function after stroke. Neural Plast. 2017, 2017, 7876507. [Google Scholar] [CrossRef]
- Chang, J.L.; Coggins, A.N.; Saul, M.; Paget-Blanc, A.; Straka, M.; Wright, J.; Datta-Chaudhuri, T.; Zanos, S.; Volpe, B.T. Transcutaneous auricular vagus nerve stimulation (tavns) delivered during upper limb interactive robotic training demonstrates novel antagonist control for reaching movements following stroke. Front. Neurosci. 2021, 15, 767302. [Google Scholar] [CrossRef]
- Keser, Z.; Feng, W. Vagus Nerve Stimulation for Stroke Motor Recovery—What Is Next? Transl. Stroke Res. 2022, 14, 438–442. [Google Scholar] [CrossRef]
- Rossi, S.; Hallett, M.; Rossini, P.M.; Pascual-Leone, A. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin. Neurophysiol. 2009, 120, 2008–2039. [Google Scholar] [CrossRef]
- Fisicaro, F.; Lanza, G.; Grasso, A.A.; Pennisi, G.; Bella, R.; Paulus, W.; Pennisi, M. Repetitive transcranial magnetic stimulation in stroke rehabilitation: Review of the current evidence and pitfalls. Ther. Adv. Neurol. Disord. 2019, 12, 175628641987831. [Google Scholar] [CrossRef]
- Hsu, W.-Y.; Cheng, C.-H.; Liao, K.-K.; Lee, I.-H.; Lin, Y.-Y. Effects of Repetitive Transcranial Magnetic Stimulation on Motor Functions in Patients With Stroke. Stroke 2012, 43, 1849–1857. [Google Scholar] [CrossRef]
- Lefaucheur, J.-P.; Aleman, A.; Baeken, C.; Benninger, D.H.; Brunelin, J.; Di Lazzaro, V.; Filipović, S.R.; Grefkes, C.; Hasan, A.; Hummel, F.C.; et al. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS): An update (2014–2018). Clin. Neurophysiol. 2020, 131, 474–528. [Google Scholar] [CrossRef] [PubMed]
- Edwards, D.J.; Liu, C.Y.; Dunning, K.; Fregni, F.; Laine, J.; Leiby, B.E.; Rogers, L.M.; Harvey, R.L. Electric Field Navigated 1-Hz rTMS for Poststroke Motor Recovery: The E-FIT Randomized Controlled Trial. Stroke 2023, 54, 2254–2264. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.-S.; Kwon, B.S.; Gil Seo, H.; Park, J.; Paik, N.-J. Low-Frequency Repetitive Transcranial Magnetic Stimulation Over Contralesional Motor Cortex for Motor Recovery in Subacute Ischemic Stroke: A Randomized Sham-Controlled Trial. Neurorehabilit. Neural Repair 2020, 34, 856–867. [Google Scholar] [CrossRef] [PubMed]
- Di Pino, G.; Pellegrino, G.; Assenza, G.; Capone, F.; Ferreri, F.; Formica, D.; Ranieri, F.; Tombini, M.; Ziemann, U.; Rothwell, J.C.; et al. Modulation of brain plasticity in stroke: A novel model for neurorehabilitation. Nat. Rev. Neurol. 2014, 10, 597–608. [Google Scholar] [CrossRef] [PubMed]
- Postman-Caucheteux, W.A.; Birn, R.M.; Pursley, R.H.; Butman, J.A.; Solomon, J.M.; Picchioni, D.; McArdle, J.; Braun, A.R. Single-trial fMRI Shows Contralesional Activity Linked to Overt Naming Errors in Chronic Aphasic Patients. J. Cogn. Neurosci. 2010, 22, 1299–1318. [Google Scholar] [CrossRef] [PubMed]
- Keser, Z.; Sebastian, R.; Hasan, K.M.; Hillis, A.E. Right Hemispheric Homologous Language Pathways Negatively Predicts Poststroke Naming Recovery. Stroke 2020, 51, 1002–1005. [Google Scholar] [CrossRef]
- Khedr, E.M.; El-Fetoh, N.A.; Ali, A.M.; El-Hammady, D.H.; Khalifa, H.; Atta, H.; Karim, A.A. Dual-Hemisphere Repetitive Transcranial Magnetic Stimulation for Rehabilitation of Poststroke Aphasia: A Randomized, Double-Blind Clinical Trial. Neurorehabilit. Neural Repair 2014, 28, 740–750. [Google Scholar] [CrossRef]
- Oliveri, M.; Rossini, P.M.; Traversa, R.; Cicinelli, P.; Filippi, M.M.; Pasqualetti, P.; Tomaiuolo, F.; Caltagirone, C. Left frontal transcranial magnetic stimulation reduces contralesional extinction in patients with unilateral right brain damage. Brain 1999, 122, 1731–1739. [Google Scholar] [CrossRef][Green Version]
- Oliveri, M.; Rossini, P.M.; Filippi, M.M.; Traversa, R.; Cicinelli, P.; Palmieri, M.G.; Pasqualetti, P.; Caltagirone, C. Time-dependent activation of parieto-frontal networks for directing attention to tactile space: A study with paired transcranial magnetic stimulation pulses in right-brain-damaged patients with extinction. Brain 2000, 123, 1939–1947. [Google Scholar] [CrossRef]
- Kim, B.R.; Chun, M.H.; Kim, D.-Y.; Lee, S.J. Effect of high- and low-frequency repetitive transcranial magnetic stimulation on visuospatial neglect in patients with acute stroke: A double-blind, sham-controlled trial. Arch. Phys. Med. Rehabil. 2013, 94, 803–807. [Google Scholar] [CrossRef]
- Cazzoli, D.; Müri, R.M.; Schumacher, R.; von Arx, S.; Chaves, S.; Gutbrod, K.; Bohlhalter, S.; Bauer, D.; Vanbellingen, T.; Bertschi, M.; et al. Theta burst stimulation reduces disability during the activities of daily living in spatial neglect. Brain 2012, 135, 3426–3439. [Google Scholar] [CrossRef] [PubMed]
- Nyffeler, T.; Cazzoli, D.; Hess, C.W.; Müri, R.M. One session of repeated parietal theta burst stimulation trains induces long-lasting improvement of visual neglect. Stroke 2009, 40, 2791–2796. [Google Scholar] [CrossRef] [PubMed]
- Nyffeler, T.; Vanbellingen, T.; Kaufmann, B.C.; Pflugshaupt, T.; Bauer, D.; Frey, J.; Chechlacz, M.; Bohlhalter, S.; Müri, R.M.; Nef, T.; et al. Theta burst stimulation in neglect after stroke: Functional outcome and response variability origins. Brain 2019, 142, 992–1008. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.; Song, W.; Zhang, Y.; Yang, Y.; Huo, S.; Zhang, R.; Wang, M. Long-term effects of continuous theta-burst stimulation in visuospatial neglect. J. Int. Med. Res. 2015, 43, 196–203. [Google Scholar] [CrossRef]
- Rossini, P.M.; Burke, D.; Chen, R.; Cohen, L.G.; Daskalakis, Z.; Di Iorio, R.; Di Lazzaro, V.; Ferreri, F.; Fitzgerald, P.B.; George, M.S.; et al. Non-invasive electrical and magnetic stimulation of the brain, spinal cord, roots and peripheral nerves: Basic principles and procedures for routine clinical and research application. An updated report from an I.F.C.N. Committee. Clin. Neurophysiol. 2015, 126, 1071–1107. [Google Scholar] [CrossRef]
- Schlaug, G.; Renga, V.; Nair, D. Transcranial Direct Current Stimulation in Stroke Recovery. Arch. Neurol. 2008, 65, 1571–1576. [Google Scholar] [CrossRef]
- Bikson, M.; Grossman, P.; Thomas, C.; Zannou, A.L.; Jiang, J.; Adnan, T.; Mourdoukoutas, A.P.; Kronberg, G.; Truong, D.; Boggio, P.; et al. Safety of Transcranial Direct Current Stimulation: Evidence Based Update 2016. Brain Stimul. 2016, 9, 641–661. [Google Scholar] [CrossRef]
- Chhatbar, P.Y.; Ramakrishnan, V.; Kautz, S.; George, M.S.; Adams, R.J.; Feng, W. Transcranial Direct Current Stimulation Post-Stroke Upper Extremity Motor Recovery Studies Exhibit a Dose–Response Relationship. Brain Stimul. 2015, 9, 16–26. [Google Scholar] [CrossRef]
- Feng, W.; Wang, J.; Chhatbar, P.Y.; Doughty, C.; Landsittel, D.; Lioutas, V.; Kautz, S.A.; Schlaug, G.; Feng, W.; Wang, J.; et al. Corticospinal tract lesion load: An imaging biomarker for stroke motor outcomes. Ann. Neurol. 2015, 78, 860–870. [Google Scholar] [CrossRef]
- Baker, J.M.; Rorden, C.; Fridriksson, J. Using transcranial direct-current stimulation to treat stroke patients with aphasia. Stroke 2010, 41, 1229–1236. [Google Scholar] [CrossRef]
- Fridriksson, J.; Richardson, J.D.; Baker, J.M.; Rorden, C. Transcranial direct current stimulation improves naming reaction time in fluent aphasia: A double-blind, sham-controlled study. Stroke 2011, 42, 819–821. [Google Scholar] [CrossRef] [PubMed]
- Cherney, L.R.; Erickson, R.K.; Small, S.L. Epidural cortical stimulation as adjunctive treatment for non-fluent aphasia: Preliminary findings. J. Neurol. Neurosurg. Psychiatry 2010, 81, 1014–1021. [Google Scholar] [CrossRef] [PubMed]
- Kang, E.K.; Kim, Y.K.; Sohn, H.M.; Cohen, L.G.; Paik, N.-J. Improved picture naming in aphasia patients treated with cathodal tDCS to inhibit the right Broca’s homologue area. Restor. Neurol. Neurosci. 2011, 29, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Elsner, B.; Kugler, J.; Mehrholz, J. Transcranial direct current stimulation (tDCS) for improving aphasia after stroke: A systematic review with network meta-analysis of randomized controlled trials. J. Neuroeng. Rehabil. 2020, 17, 88. [Google Scholar] [CrossRef]
- Hayward, K.S.; Brauer, S.G.; Ruddy, K.L.; Lloyd, D.; Carson, R.G. Repetitive reaching training combined with transcranial Random Noise Stimulation in stroke survivors with chronic and severe arm paresis is feasible: A pilot, triple-blind, randomised case series. J. Neuroeng. Rehabil. 2017, 14, 46. [Google Scholar] [CrossRef]
- Arnao, V.; Riolo, M.; Carduccio, F.; Tuttolomondo, A.; D’amelio, M.; Brighina, F.; Gangitano, M.; Salemi, G.; Ragonese, P.; Aridon, P. Effects of transcranial random noise stimulation combined with Graded Repetitive Arm Supplementary Program (GRASP) on motor rehabilitation of the upper limb in sub-acute ischemic stroke patients: A randomized pilot study. J. Neural Transm. 2019, 126, 1701–1706. [Google Scholar] [CrossRef]
- Fedorov, A.; Chibisova, Y.; Szymaszek, A.; Alexandrov, M.; Gall, C.; Sabel, B.A. Non-invasive alternating current stimulation induces recovery from stroke. Restor. Neurol. Neurosci. 2010, 28, 825–833. [Google Scholar] [CrossRef]
- Wu, M.; Wu, Y.; Yu, Y.; Gao, J.; Meng, F.; He, F.; Shi, J.; Luo, B. Effects of theta burst stimulation of the left dorsolateral prefrontal cortex in disorders of consciousness. Brain Stimul. 2018, 11, 1382–1384. [Google Scholar] [CrossRef]
- Piccione, F.; Cavinato, M.; Manganotti, P.; Formaggio, E.; Storti, S.F.; Battistin, L.; Cagnin, A.; Tonin, P.; Dam, M. Behavioral and Neurophysiological Effects of Repetitive Transcranial Magnetic Stimulation on the Minimally Conscious State. Neurorehabilit. Neural Repair 2010, 25, 98–102. [Google Scholar] [CrossRef]
- Liu, P.; Gao, J.; Pan, S.; Meng, F.; Pan, G.; Li, J.; Luo, B. Effects of High-Frequency Repetitive Transcranial Magnetic Stimulation on Cerebral Hemodynamics in Patients with Disorders of Consciousness: A Sham-Controlled Study. Eur. Neurol. 2016, 76, 1–7. [Google Scholar] [CrossRef]
- Xia, X.; Liu, Y.; Bai, Y.; Liu, Z.; Yang, Y.; Guo, Y.; Xu, R.; Gao, X.; Li, X.; He, J. Long-lasting repetitive transcranial magnetic stimulation modulates electroencephalography oscillation in patients with disorders of consciousness. NeuroReport 2017, 28, 1022–1029. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Wang, Y.; Li, C.; Li, X.; He, J.; Bai, Y. Transcranial magnetic stimulation-evoked connectivity reveals modulation effects of repetitive transcranial magnetic stimulation on patients with disorders of consciousness. NeuroReport 2019, 30, 1307–1315. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Wu, M.; Meng, F.; Hu, Y.; Gao, J.; Chen, Z.; Bao, W.; Liu, K.; Luo, B.; Pan, G. Effects of 20 Hz Repetitive Transcranial Magnetic Stimulation on Disorders of Consciousness: A Resting-State Electroencephalography Study. Neural Plast. 2018, 2018, 5036184. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Liu, T.; Huang, Q.; Su, Y.; Chen, W.; Gao, D.; Tian, X.; Huang, T.; Zhen, Z.; Han, T.; et al. Electroencephalography and Functional Magnetic Resonance Imaging-Guided Simultaneous Transcranial Direct Current Stimulation and Repetitive Transcranial Magnetic Stimulation in a Patient With Minimally Conscious State. Front. Neurosci. 2019, 13, 746. [Google Scholar] [CrossRef]
- Bai, Y.; Xia, X.; Kang, J.; Yin, X.; Yang, Y.; He, J.; Li, X. Evaluating the Effect of Repetitive Transcranial Magnetic Stimulation on Disorders of Consciousness by Using TMS-EEG. Front. Neurosci. 2016, 10, 473. [Google Scholar] [CrossRef]
- Xia, X.; Bai, Y.; Zhou, Y.; Yang, Y.; Xu, R.; Gao, X.; Li, X.; He, J. Effects of 10 Hz Repetitive Transcranial Magnetic Stimulation of the Left Dorsolateral Prefrontal Cortex in Disorders of Consciousness. Front. Neurol. 2017, 8, 182. [Google Scholar] [CrossRef]
- Jang, S.H.; Kwon, Y.H. Effect of repetitive transcranial magnetic stimulation on the ascending reticular activating system in a patient with disorder of consciousness: A case report. BMC Neurol. 2020, 20, 37. [Google Scholar] [CrossRef]
- Naro, A.; Bramanti, A.; Leo, A.; Bramanti, P.; Calabrò, R.S. Metaplasticity: A Promising Tool to Disentangle Chronic Disorders of Consciousness Differential Diagnosis. Int. J. Neural Syst. 2018, 28, 1750059. [Google Scholar] [CrossRef]
- Bai, Y.; Xia, X.; Wang, Y.; Guo, Y.; Yang, Y.; He, J.; Li, X. Fronto-parietal coherence response to tDCS modulation in patients with disorders of consciousness. Int. J. Neurosci. 2018, 128, 587–594. [Google Scholar] [CrossRef]
- Martens, G.; Fregni, F.; Carrière, M.; Barra, A.; Laureys, S.; Thibaut, A. Single tDCS session of motor cortex in patients with disorders of consciousness: A pilot study. Brain Inj. 2019, 33, 1679–1683. [Google Scholar] [CrossRef]
- Bai, Y.; Xia, X.; Kang, J.; Yang, Y.; He, J.; Li, X. TDCS modulates cortical excitability in patients with disorders of consciousness. NeuroImage Clin. 2017, 15, 702–709. [Google Scholar] [CrossRef]
- Cavinato, M.; Genna, C.; Formaggio, E.; Gregorio, C.; Storti, S.F.; Manganotti, P.; Casanova, E.; Piperno, R.; Piccione, F. Behavioural and electrophysiological effects of tDCS to prefrontal cortex in patients with disorders of consciousness. Clin. Neurophysiol. 2018, 130, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Martens, G.; Lejeune, N.; O’Brien, A.T.; Fregni, F.; Martial, C.; Wannez, S.; Laureys, S.; Thibaut, A. Randomized controlled trial of home-based 4-week tDCS in chronic minimally conscious state. Brain Stimul. 2018, 11, 982–990. [Google Scholar] [CrossRef]
- Wang, X.; Guo, Y.; Zhang, Y.; Li, J.; Gao, Z.; Li, Y.; Zhou, T.; Zhang, H.; He, J.; Cong, F. Combined Behavioral and Mismatch Negativity Evidence for the Effects of Long-Lasting High-Definition tDCS in Disorders of Consciousness: A Pilot Study. Front. Neurosci. 2020, 14, 381. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Bai, Y.; Xia, X.; Li, J.; Wang, X.; Dai, Y.; Dang, Y.; He, J.; Liu, C.; Zhang, H. Effects of Long-Lasting High-Definition Transcranial Direct Current Stimulation in Chronic Disorders of Consciousness: A Pilot Study. Front. Neurosci. 2019, 13, 412. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Song, W.; Du, J.; Huo, S.; Shan, G.; Li, R. Transcranial Direct Current Stimulation in Patients with Prolonged Disorders of Consciousness: Combined Behavioral and Event-Related Potential Evidence. Front. Neurol. 2017, 8, 620. [Google Scholar] [CrossRef]
- Hermann, B.; Raimondo, F.; Hirsch, L.; Huang, Y.; Denis-Valente, M.; Pérez, P.; Engemann, D.; Faugeras, F.; Weiss, N.; Demeret, S.; et al. Combined behavioral and electrophysiological evidence for a direct cortical effect of prefrontal tDCS on disorders of consciousness. Sci. Rep. 2020, 10, 4323. [Google Scholar] [CrossRef]
- Zhang, Y.; Lu, J.; Du, J.; Huo, S.; Li, R.; Song, W. Neural correlates of different behavioral response to transcranial direct current stimulation between patients in the unresponsive wakefulness syndrome and minimally conscious state. Neurol. Sci. 2020, 41, 75–82. [Google Scholar] [CrossRef]
- Naro, A.; Bramanti, P.; Leo, A.; Russo, M.; Calabrò, R.S. Transcranial Alternating Current Stimulation in Patients with Chronic Disorder of Consciousness: A Possible Way to Cut the Diagnostic Gordian Knot? Brain Topogr. 2016, 29, 623–644. [Google Scholar] [CrossRef]
- Mancuso, M.; Abbruzzese, L.; Canova, S.; Landi, G.; Rossi, S.; Santarnecchi, E. Transcranial Random Noise Stimulation Does Not Improve Behavioral and Neurophysiological Measures in Patients with Subacute Vegetative-Unresponsive Wakefulness State (VS-UWS). Front. Hum. Neurosci. 2017, 11, 524. [Google Scholar] [CrossRef]
- Yu, Y.T.; Yang, Y.; Wang, L.B.; Fang, J.L.; Chen, Y.Y.; He, J.H.; Rong, P.J. Transcutaneous auricular vagus nerve stimulation in disorders of consciousness monitored by fMRI: The first case report. Brain Stimul. 2017, 10, 328–330. [Google Scholar] [CrossRef] [PubMed]
- Noé, E.; Ferri, J.; Colomer, C.; Moliner, B.; O’Valle, M.; Ugart, P.; Rodriguez, C.; Llorens, R. Feasibility, safety and efficacy of transauricular vagus nerve stimulation in a cohort of patients with disorders of consciousness. Brain Stimul. 2020, 13, 427–429. [Google Scholar] [CrossRef] [PubMed]
- Jeffers, M.S.; Karthikeyan, S.; Gomez-Smith, M.; Gasinzigwa, S.; Achenbach, J.; Feiten, A.; Corbett, D. Does Stroke Rehabilitation Really Matter? Part B: An Algorithm for Prescribing an Effective Intensity of Rehabilitation. Neurorehabilit. Neural Repair 2018, 32, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Birkenmeier, R.L.; Prager, E.M.; Lang, C.E. Translating Animal Doses of Task-Specific Training to People With Chronic Stroke in 1-Hour Therapy Sessions: A Proof-of-Concept Study. Neurorehabilit. Neural Repair 2010, 24, 620–635. [Google Scholar] [CrossRef]
- Lang, C.E.; Macdonald, J.R.; Reisman, D.S.; Boyd, L.; Kimberley, T.J.; Schindler-Ivens, S.M.; Hornby, T.G.; Ross, S.A.; Scheets, P.L. Observation of Amounts of Movement Practice Provided During Stroke Rehabilitation. Arch. Phys. Med. Rehabil. 2009, 90, 1692–1698. [Google Scholar] [CrossRef]
- Wolf, S.L.; Winstein, C.J.; Miller, J.P.; Taub, E.; Uswatte, G.; Morris, D.; Giuliani, C.; Light, K.E.; Nichols-Larsen, D. Effect of Constraint-Induced Movement Therapy on Upper Extremity Function 3 to 9 Months After Stroke: The EXCITE Randomized Clinical Trial. JAMA 2006, 296, 2095–2104. [Google Scholar] [CrossRef]
- Rodgers, H.; Bosomworth, H.; Krebs, H.I.; van Wijck, F.; Howel, D.; Wilson, N.; Aird, L.; Alvarado, N.; Andole, S.; Cohen, D.L.; et al. Robot assisted training for the upper limb after stroke (RATULS): A multicentre randomised controlled trial. Lancet 2019, 394, 51–62. [Google Scholar] [CrossRef]
- Cramer, S.C.; Dodakian, L.; Le, V.; See, J.; Augsburger, R.; McKenzie, A.; Zhou, R.J.; Chiu, N.L.; Heckhausen, J.; Cassidy, J.M.; et al. Efficacy of Home-Based Telerehabilitation vs In-Clinic Therapy for Adults After Stroke: A Randomized Clinical Trial. JAMA Neurol. 2019, 76, 1079–1087. [Google Scholar] [CrossRef]
- Kwakkel, G.; Veerbeek, J.M.; Van Wegen, E.E.H.; Wolf, S.L. Constraint-induced movement therapy after stroke. Lancet Neurol. 2015, 14, 224–234. [Google Scholar] [CrossRef]
- Ogden, R.; Franz, S.I. On cerebral motor control: The recovery from experimentally produced hemiplegia. Psychobiology 1917, 1, 33–49. [Google Scholar] [CrossRef][Green Version]
- Fritz, S.L.; Butts, R.J.; Wolf, S.L. Constraint-induced movement therapy: From history to plasticity. Expert Rev. Neurother. 2012, 12, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Tedla, J.S.; Gular, K.; Reddy, R.S.; Ferreira, A.d.S.; Rodrigues, E.C.; Kakaraparthi, V.N.; Gyer, G.; Sangadala, D.R.; Qasheesh, M.; Kovela, R.K.; et al. Effectiveness of constraint-induced movement therapy (cimt) on balance and functional mobility in the stroke population: A systematic review and meta-analysis. Healthcare 2022, 10, 495. [Google Scholar] [CrossRef] [PubMed]
- Liepert, J.; Miltner, W.; Bauder, H.; Sommer, M.; Dettmers, C.; Taub, E.; Weiller, C. Motor cortex plasticity during constraint-induced movement therapy in stroke patients. Neurosci. Lett. 1998, 250, 5–8. [Google Scholar] [CrossRef] [PubMed]
- Wittenberg, G.F.; Chen, R.; Ishii, K.; Bushara, K.O.; Taub, E.; Gerber, L.H.; Hallett, M.; Cohen, L.G. Constraint-Induced Therapy in Stroke: Magnetic-Stimulation Motor Maps and Cerebral Activation. Neurorehabilit. Neural Repair 2003, 17, 48–57. [Google Scholar] [CrossRef]
- Sawaki, L.; Butler, A.J.; Leng, X.; Wassenaar, P.A.; Mohammad, Y.M.; Blanton, S.; Sathian, K.; Nichols-Larsen, D.S.; Wolf, S.L.; Good, D.C.; et al. Constraint-Induced Movement Therapy Results in Increased Motor Map Area in Subjects 3 to 9 Months After Stroke. Neurorehabilit. Neural Repair 2008, 22, 505–513. [Google Scholar] [CrossRef]
- Takahashi, C.D.; Der-Yeghiaian, L.; Le, V.; Motiwala, R.R.; Cramer, S.C. Robot-based hand motor therapy after stroke. Brain 2014, 131, 425–437. [Google Scholar] [CrossRef]
- Lo, A.C.; Guarino, P.D.; Richards, L.G.; Haselkorn, J.K.; Wittenberg, G.F.; Federman, D.G.; Ringer, R.J.; Wagner, T.H.; Krebs, H.I.; Volpe, B.T.; et al. Robot-Assisted Therapy for Long-Term Upper-Limb Impairment after Stroke. N. Engl. J. Med. 2010, 362, 1772–1783. [Google Scholar] [CrossRef]
- Veerbeek, J.M.; Langbroek-Amersfoort, A.C.; van Wegen, E.; Meskers, C.; Kwakkel, G. Effects of Robot-Assisted Therapy for the Upper Limb After Stroke: A Systematic Review and Meta-analysis. Neurorehabilit. Neural Repair 2017, 31, 107–121. [Google Scholar] [CrossRef]
- Dromerick, A.W.; Geed, S.; Barth, J.; Brady, K.; Giannetti, M.L.; Mitchell, A.; Edwardson, M.A.; Tan, M.T.; Zhou, Y.; Newport, E.L.; et al. Critical Period After Stroke Study (CPASS): A phase II clinical trial testing an optimal time for motor recovery after stroke in humans. Proc. Natl. Acad. Sci. USA 2021, 118, e2026676118. [Google Scholar] [CrossRef]
- Edwards, D.; Kumar, S.; Brinkman, L.; Ferreira, I.C.; Esquenazi, A.; Nguyen, T.; Su, M.; Stein, S.; May, J.; Hendrix, A.; et al. Telerehabilitation Initiated Early in Post-Stroke Recovery: A Feasibility Study. Neurorehabilit. Neural Repair 2023, 37, 131–141. [Google Scholar] [CrossRef]
- Wolpaw, J.R. Brain–computer interfaces. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2013; pp. 67–74. [Google Scholar]
- Chaudhary, U.; Xia, B.; Silvoni, S.; Cohen, L.G.; Birbaumer, N. Brain–computer interface–based communication in the completely locked-in state. PLoS Biol. 2017, 15, e1002593. [Google Scholar] [CrossRef] [PubMed]
- Sitaram, R.; Ros, T.; Stoeckel, L.; Haller, S.; Scharnowski, F.; Lewis-Peacock, J.; Weiskopf, N.; Blefari, M.L.; Rana, M.; Oblak, E.; et al. Closed-loop brain training: The science of neurofeedback. Nat. Rev. Neurosci. 2016, 18, 86–100. [Google Scholar] [CrossRef] [PubMed]
- Mane, R.; Wu, Z.; Wang, D. Poststroke motor, cognitive and speech rehabilitation with brain–computer interface: A perspective review. Stroke Vasc. Neurol. 2022, 7, 541. [Google Scholar] [CrossRef] [PubMed]
- Cervera, M.A.; Soekadar, S.R.; Ushiba, J.; Millán, J.d.R.; Liu, M.; Birbaumer, N.; Garipelli, G. Brain-computer interfaces for post-stroke motor rehabilitation: A meta-analysis. Ann. Clin. Transl. Neurol. 2018, 5, 651–663. [Google Scholar] [CrossRef] [PubMed]
- Bai, Z.; Fong, K.N.K.; Zhang, J.J.; Chan, J.; Ting, K.H. Immediate and long-term effects of BCI-based rehabilitation of the upper extremity after stroke: A systematic review and meta-analysis. J. Neuroeng. Rehabil. 2020, 17, 57. [Google Scholar] [CrossRef]
- Lennon, O.; Tonellato, M.; Del Felice, A.; Di Marco, R.; Fingleton, C.; Korik, A.; Guanziroli, E.; Molteni, F.; Guger, C.; Otner, R.; et al. A systematic review establishing the current state-of-the-art, the limitations, and the DESIRED checklist in studies of direct neural interfacing with robotic gait devices in stroke rehabilitation. Front. Neurosci. 2020, 14, 578. [Google Scholar] [CrossRef]
- Lim, C.G.; Poh, X.W.W.; Fung, S.S.D.; Guan, C.; Bautista, D.; Cheung, Y.B.; Zhang, H.; Yeo, S.N.; Krishnan, R.; Lee, T.S. A randomized controlled trial of a brain-computer interface based attention training program for ADHD. PLoS ONE 2019, 14, e0216225. [Google Scholar] [CrossRef]
- Strehl, U.; Aggensteiner, P.; Wachtlin, D.; Brandeis, D.; Albrecht, B.; Arana, M.; Bach, C.; Banaschewski, T.; Bogen, T.; Flaig-Röhr, A.; et al. Neurofeedback of slow cortical potentials in children with attention-deficit/hyperactivity disorder: A multicenter randomized trial controlling for unspecific effects. Front. Hum. Neurosci. 2017, 11, 135. [Google Scholar] [CrossRef]
- Lee, T.-S.; Goh, S.J.A.; Quek, S.Y.; Phillips, R.; Guan, C.; Cheung, Y.B.; Feng, L.; Teng, S.S.W.; Wang, C.C.; Chin, Z.Y.; et al. A brain-computer interface based cognitive training system for healthy elderly: A randomized control pilot study for usability and preliminary efficacy. PLoS ONE 2013, 8, e79419. [Google Scholar] [CrossRef]
- Chollet, F.; Tardy, J.; Albucher, J.-F.; Thalamas, C.; Berard, E.; Lamy, C.; Bejot, Y.; Deltour, S.; Jaillard, A.; Niclot, P.; et al. Fluoxetine for motor recovery after acute ischaemic stroke (FLAME): A randomised placebo-controlled trial. Lancet Neurol. 2011, 10, 123–130. [Google Scholar] [CrossRef]
- Legg, L.A.; Tilney, R.; Hsieh, C.-F.; Wu, S.; Lundström, E.; Rudberg, A.-S.; Kutlubaev, M.A.; Dennis, M.; Soleimani, B.; Barugh, A.; et al. Selective serotonin reuptake inhibitors (SSRIs) for stroke recovery. Cochrane Database Syst. Rev. 2019, 11, CD009286. [Google Scholar] [CrossRef] [PubMed]
- Dennis, M.; Mead, G.; Forbes, J.; Graham, C.; Hackett, M.; Hankey, G.J.; House, A.; Lewis, S.; Lundström, E.; Sandercock, P.; et al. Effects of fluoxetine on functional outcomes after acute stroke (FOCUS): A pragmatic, double-blind, randomised, controlled trial. Lancet 2018, 393, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Lundström, E.; Isaksson, E.; Näsman, P.; Wester, P.; Mårtensson, B.; Norrving, B.; Wallén, H.; Borg, J.; Dennis, M.; Mead, G.; et al. Safety and efficacy of fluoxetine on functional recovery after acute stroke (EFFECTS): A randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2020, 19, 661–669. [Google Scholar] [CrossRef] [PubMed]
- Hankey, G.J.; Hackett, M.L.; Almeida, O.P.; Flicker, L.; Mead, G.E.; Dennis, M.S.; Etherton-Beer, C.; Ford, A.H.; Billot, L.; Jan, S.; et al. Twelve-Month Outcomes of the AFFINITY Trial of Fluoxetine for Functional Recovery After Acute Stroke: AFFINITY Trial Steering Committee on Behalf of the AFFINITY Trial Collaboration. Stroke 2021, 52, 2502–2509. [Google Scholar] [CrossRef] [PubMed]
- Stockbridge, M.D.; Fridriksson, J.; Sen, S.; Bonilha, L.; Hillis, A.E. Protocol for Escitalopram and Language Intervention for Subacute Aphasia (ELISA): A randomized, double blind, placebo-controlled trial. PLoS ONE 2021, 16, e0261474. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Greenhill, S.; Huang, S.; Silva, T.K.; Sano, Y.; Wu, S.; Cai, Y.; Nagaoka, Y.; Sehgal, M.; Cai, D.J.; et al. Author response: CCR5 is a suppressor for cortical plasticity and hippocampal learning and memory. eLife 2016, 5, e20985. [Google Scholar] [CrossRef]
- Joy, M.T.; Ben Assayag, E.; Shabashov-Stone, D.; Liraz-Zaltsman, S.; Mazzitelli, J.; Arenas, M.; Abduljawad, N.; Kliper, E.; Korczyn, A.D.; Thareja, N.S.; et al. CCR5 Is a Therapeutic Target for Recovery after Stroke and Traumatic Brain Injury. Cell 2019, 176, 1143–1157.e13. [Google Scholar] [CrossRef]
- Ben Assayag, E.; Korczyn, A.D.; Giladi, N.; Goldbourt, U.; Berliner, A.S.; Shenhar-Tsarfaty, S.; Kliper, E.; Hallevi, H.; Shopin, L.; Hendler, T.; et al. Predictors for poststroke outcomes: The Tel Aviv brain acute stroke cohort (TABASCO) study protocol. Int. J. Stroke 2011, 7, 341–347. [Google Scholar] [CrossRef]
- Mahla, R.S. Stem Cells Applications in Regenerative Medicine and Disease Therapeutics. Int. J. Cell Biol. 2016, 2016, 6940283. [Google Scholar] [CrossRef]
- Satani, N.; Cai, C.; Giridhar, K.; McGhiey, D.; George, S.; Parsha, K.; Nghiem, D.M.; Valenzuela, K.S.; Riecke, J.; Vahidy, F.S.; et al. World-Wide Efficacy of Bone Marrow Derived Mesenchymal Stromal Cells in Preclinical Ischemic Stroke Models: Systematic Review and Meta-Analysis. Front. Neurol. 2019, 10, 405. [Google Scholar] [CrossRef]
- Levy, M.L.; Crawford, J.R.; Dib, N.; Verkh, L.; Tankovich, N.; Cramer, S.C. Phase I/II Study of Safety and Preliminary Efficacy of Intravenous Allogeneic Mesenchymal Stem Cells in Chronic Stroke. Stroke 2019, 50, 2835–2841. [Google Scholar] [CrossRef] [PubMed]
- Jaillard, A.; Hommel, M.; Moisan, A.; Zeffiro, T.A.; Favre-Wiki, I.M.; Barbieux-Guillot, M.; Vadot, W.; Marcel, S.; Lamalle, L.; Grand, S.; et al. Autologous Mesenchymal Stem Cells Improve Motor Recovery in Subacute Ischemic Stroke: A Randomized Clinical Trial. Transl. Stroke Res. 2020, 11, 910–923. [Google Scholar] [CrossRef]
- Savitz, S.I.; Yavagal, D.; Rappard, G.; Likosky, W.; Rutledge, N.; Graffagnino, C.; Alderazi, Y.; Elder, J.A.; Chen, P.R.; Budzik, R.F., Jr.; et al. A Phase 2 Randomized, Sham-Controlled Trial of Internal Carotid Artery Infusion of Autologous Bone Marrow–Derived ALD-401 Cells in Patients With Recent Stable Ischemic Stroke (RECOVER-Stroke). Circulation 2019, 139, 192–205. [Google Scholar] [CrossRef] [PubMed]
- Muir, K.W.; Bulters, D.; Willmot, M.; Sprigg, N.; Dixit, A.; Ward, N.; Tyrrell, P.; Majid, A.; Dunn, L.; Bath, P.; et al. Intracerebral implantation of human neural stem cells and motor recovery after stroke: Multicentre prospective single-arm study (PISCES-2). J. Neurol. Neurosurg. Psychiatry 2020, 91, 396–401. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.O.; Barrett, A.M. Chapter 5—Right Brain Stroke Syndromes. In Stroke Rehabilitation; Wilson, R., Raghavan, P., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 71–89. [Google Scholar] [CrossRef]
- Weinberg, J.; Diller, L.; Gordon, W.A.; Gerstman, L.J.; Lieberman, A.; Lakin, P.; Hodges, G.; Ezrachi, O. Training sensory awareness and spatial organization in people with right brain damage. Arch. Phys. Med. Rehabil. 1979, 60, 491–496. [Google Scholar]
- Weinberg, J.; Diller, L.; Gordon, W.A.; Gerstman, L.J.; Lieberman, A.; Lakin, P.; Hodges, G.; Ezrachi, O. Visual scanning training effect on reading-related tasks in acquired right brain damage. Arch. Phys. Med. Rehabil. 1977, 58, 479–486. [Google Scholar]
- Garza, J.P.; Eslinger, P.J.; Barrett, A.M. Perceptual–attentional and motor-intentional bias in near and far space. Brain Cogn. 2008, 68, 9–14. [Google Scholar] [CrossRef]
- Barrett, A.M. Spatial Neglect and Anosognosia After Right Brain Stroke. Contin. Lifelong Learn. Neurol 2021, 27, 1624–1645. [Google Scholar] [CrossRef]
- Barrett, A.M.; Goedert, K.M.; Basso, J.C. Prism adaptation for spatial neglect after stroke: Translational practice gaps. Nat. Rev. Neurol. 2012, 8, 567–577. [Google Scholar] [CrossRef]
- Winstein, C.J.; Stein, J.; Arena, R.; Bates, B.; Cherney, L.R.; Cramer, S.C.; Deruyter, F.; Eng, J.J.; Fisher, B.; Harvey, R.L.; et al. Guidelines for Adult Stroke Rehabilitation and Recovery. Stroke 2016, 47, e98–e169. [Google Scholar] [CrossRef]
- Champod, A.S.; Frank, R.C.; Taylor, K.; Eskes, G.A. The effects of prism adaptation on daily life activities in patients with visuospatial neglect: A systematic review. Neuropsychol. Rehabil. 2018, 28, 491–514. [Google Scholar] [CrossRef] [PubMed]
- Fortis, P.; Chen, P.; Goedert, K.M.; Barrett, A.M. Effects of prism adaptation on motor-intentional spatial bias in neglect. NeuroReport 2011, 22, 700–705. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Hreha, K.; Gonzalez-Snyder, C.; Rich, T.J.; Gillen, R.W.; Parrott, D.; Barrett, A.M. Impacts of Prism Adaptation Treatment on Spatial Neglect and Rehabilitation Outcome: Dosage Matters. Neurorehabilit. Neural Repair 2022, 36, 500–513. [Google Scholar] [CrossRef] [PubMed]
- Goedert, K.M.; Chen, P.; Foundas, A.L.; Barrett, A.M. Frontal lesions predict response to prism adaptation treatment in spatial neglect: A randomised controlled study. Neuropsychol. Rehabil. 2020, 30, 32–53. [Google Scholar] [CrossRef] [PubMed]
- Weiss, P.L.; Rand, D.; Katz, N.; Kizony, R. Video capture virtual reality as a flexible and effective rehabilitation tool. J. Neuroeng. Rehabil. 2004, 1, 12. [Google Scholar] [CrossRef]
- Baniña, M.C.; Molad, R.; Solomon, J.M.; Berman, S.; Soroker, N.; Frenkel-Toledo, S.; Liebermann, D.G.; Levin, M.F. Exercise intensity of the upper limb can be enhanced using a virtual rehabilitation system. Disabil. Rehabil. Assist. Technol. 2020, 17, 100–106. [Google Scholar] [CrossRef]
- Perez-Marcos, D.; Chevalley, O.; Schmidlin, T.; Garipelli, G.; Serino, A.; Vuadens, P.; Tadi, T.; Blanke, O.; Millán, J.D.R. Increasing upper limb training intensity in chronic stroke using embodied virtual reality: A pilot study. J. Neuroeng. Rehabil. 2017, 14, 119. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, P.; Xing, L.; Mei, L.; Zhao, J.; Zhang, T. Leap Motion-based virtual reality training for improving motor functional recovery of upper limbs and neural reorganization in subacute stroke patients. Neural Regen. Res. 2017, 12, 1823. [Google Scholar]
- Aşkın, A.; Atar, E.; Koçyiğit, H.; Tosun, A. Effects of Kinect-based virtual reality game training on upper extremity motor recovery in chronic stroke. Somatosens. Mot. Res. 2018, 35, 25–32. [Google Scholar] [CrossRef]
- Bui, J.; Luauté, J.; Farnè, A. Enhancing Upper Limb Rehabilitation of Stroke Patients With Virtual Reality: A Mini Review. Front. Virtual Real. 2021, 2, 595771. [Google Scholar] [CrossRef]
- Hao, J.; He, Z.; Yu, X.; Remis, A. Comparison of immersive and non-immersive virtual reality for upper extremity functional recovery in patients with stroke: A systematic review and network meta-analysis. Neurol. Sci. 2023, 44, 2679–2697. [Google Scholar] [CrossRef] [PubMed]
- Juliano, J.M.; Spicer, R.P.; Vourvopoulos, A.; Lefebvre, S.; Jann, K.; Ard, T.; Santarnecchi, E.; Krum, D.M.; Liew, S.-L. Embodiment Is Related to Better Performance on a Brain–Computer Interface in Immersive Virtual Reality: A Pilot Study. Sensors 2020, 20, 1204. [Google Scholar] [CrossRef] [PubMed]
- Jeannerod, M. Neural Simulation of Action: A Unifying Mechanism for Motor Cognition. NeuroImage 2001, 14, S103–S109. [Google Scholar] [CrossRef]
- Decety, J. The neurophysiological basis of motor imagery. Behav. Brain Res. 1996, 77, 45–52. [Google Scholar] [CrossRef]
- Taube, W.; Mouthon, M.; Leukel, C.; Hoogewoud, H.-M.; Annoni, J.-M.; Keller, M. Brain activity during observation and motor imagery of different balance tasks: An fMRI study. Cortex 2015, 64, 102–114. [Google Scholar] [CrossRef] [PubMed]
- Page, S.J.; Levine, P.; Leonard, A.C. Effects of mental practice on affected limb use and function in chronic stroke. Arch. Phys. Med. Rehabil. 2005, 86, 399–402. [Google Scholar] [CrossRef] [PubMed]
- Dijkerman, H.C.; Ietswaart, M.; Johnston, M.; MacWalter, R.S. Does motor imagery training improve hand function in chronic stroke patients? A pilot study. Clin. Rehabil. 2004, 18, 538–549. [Google Scholar] [CrossRef]
- Ietswaart, M.; Johnston, M.; Dijkerman, H.C.; Joice, S.; Scott, C.L.; MacWalter, R.S.; Hamilton, S.J. Mental practice with motor imagery in stroke recovery: Randomized controlled trial of efficacy. Brain 2011, 134, 1373–1386. [Google Scholar] [CrossRef]
- Braun, S.M.; Beurskens, A.J.; Kleynen, M.; Oudelaar, B.; Schols, J.M.; Wade, D.T. A Multicenter Randomized Controlled Trial to Compare Subacute ‘Treatment as Usual’ With and Without Mental Practice Among Persons With Stroke in Dutch Nursing Homes. J. Am. Med. Dir. Assoc. 2012, 13, 85.e1–85.e7. [Google Scholar] [CrossRef]
- Guerra, Z.F.; Lucchetti, A.L.G.; Lucchetti, G. Motor Imagery Training After Stroke: A Systematic Review and Meta-analysis of Randomized Controlled Trials. J. Neurol. Phys. Ther. 2017, 41, 205–214. [Google Scholar] [CrossRef]
- Ertelt, D.; Small, S.; Solodkin, A.; Dettmers, C.; McNamara, A.; Binkofski, F.; Buccino, G. Action observation has a positive impact on rehabilitation of motor deficits after stroke. NeuroImage 2007, 36, T164–T173. [Google Scholar] [CrossRef] [PubMed]
- Franceschini, M.; Ceravolo, M.G.; Agosti, M.; Cavallini, P.; Bonassi, S.; Dall’armi, V.; Massucci, M.; Schifini, F.; Sale, P. Clinical Relevance of Action Observation in Upper-Limb Stroke Rehabilitation: A Possible Role in Recovery of Functional Dexterity. A Randomized Clinical Trial. Neurorehabilit. Neural Repair 2012, 26, 456–462. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Wei, W.; Luo, Z.; Gan, H.; Hu, X. Improving motor imagery practice with synchronous action observation in stroke patients. Top. Stroke Rehabil. 2016, 23, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Eaves, D.L.; Riach, M.; Holmes, P.S.; Wright, D.J. Motor Imagery during Action Observation: A Brief Review of Evidence, Theory and Future Research Opportunities. Front. Neurosci. 2016, 10, 514. [Google Scholar] [CrossRef]
| Author, Year | Time Since Stroke | Sample Size (Active/Sham) | Neuromodulation Intervention | Behavioral Intervention and Frequency | Outcome Metrics | Main Efficacy Outcomes |
|---|---|---|---|---|---|---|
| Dawson, 2021 [7] | 9 months–10 years | 53/55 | VNS | Standardized 7-tasks arm in-clinic training (18 sessions over 6 weeks) followed by home exercise | UEFM, WMFT, MAL, SIS, BDI, Euro-Qol-5D, SS-QOL | Clinically meaningful response (UEFM > 6 points) was seen in 47% in active vs. 24% in sham group (p < 0.01) at 90 days |
| Fridrikson, 2018 [8] | >6 months | 34/40 | tDCS | Computerized speech therapy | Correct Naming on Philadelphia Naming Test and 80 Trained Items | Relative 70% increase in correct naming for A-tDCS relative to sham and no futility. |
| Harvey, 2018 [9] | 3 to 12 months | 132/67 | rTMS | Task-oriented upper limb therapy (18 sessions over 6 weeks) | UEFM, WMFT, ARAT, SIS Euro-Qol-5D, PHQ-9 | Both groups had clinically meaningful response, without significant difference (p = 0.76) |
| Vink, 2023 [10] | Within 3 weeks | 29/31 | cTBS | Standard upper limb therapy (10 sessions) | ARAT, UEFM, SIS, Euro-Qol-5D | Significant improvement in active cTBS (9.6 points in ARAT, p = 0.0244) |
| Stockbridge 2023 [11] | <3 months | 30/28 | tDCS | Computer-delivered naming treatment | Philadelphia Naming Test | No significant difference (p = 0.54) |
| Author, Year | Time Since Stroke | Sample Size (Active/Control) | Study Intervention | Control Group | Outcome Metrics | Main Safety and Efficacy Outcomes |
|---|---|---|---|---|---|---|
| Wolf, 2006 [76] | 3 to 9 months | 106/116 | Constraint-induced movement therapy | Usual care | WMFT, MAL | Significant reduction in WMFT performance time (p < 0.001) |
| Rodgers, 2019 [77] | 1 week to 5 years | 257/259/254 | Robot-assisted training or enhanced upper limb therapy | Usual care | ARAT | No significant difference |
| Cramer, 2019 [78] | 4 to 36 weeks | 62/62 | Telerehabilitation | In-clinic therapy | UEFM | Non-inferiority of telerehabilitation |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gunduz, M.E.; Bucak, B.; Keser, Z. Advances in Stroke Neurorehabilitation. J. Clin. Med. 2023, 12, 6734. https://doi.org/10.3390/jcm12216734
Gunduz ME, Bucak B, Keser Z. Advances in Stroke Neurorehabilitation. Journal of Clinical Medicine. 2023; 12(21):6734. https://doi.org/10.3390/jcm12216734
Chicago/Turabian StyleGunduz, Muhammed Enes, Bilal Bucak, and Zafer Keser. 2023. "Advances in Stroke Neurorehabilitation" Journal of Clinical Medicine 12, no. 21: 6734. https://doi.org/10.3390/jcm12216734
APA StyleGunduz, M. E., Bucak, B., & Keser, Z. (2023). Advances in Stroke Neurorehabilitation. Journal of Clinical Medicine, 12(21), 6734. https://doi.org/10.3390/jcm12216734





