Postoperative Complications Associated with Non-Steroidal Anti-Inflammatory Combinations Used Status-Post Total Hip and Knee Arthroplasty
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Source and Population
2.2. Identification of Study Cohorts
2.3. Statistical Analysis
3. Results
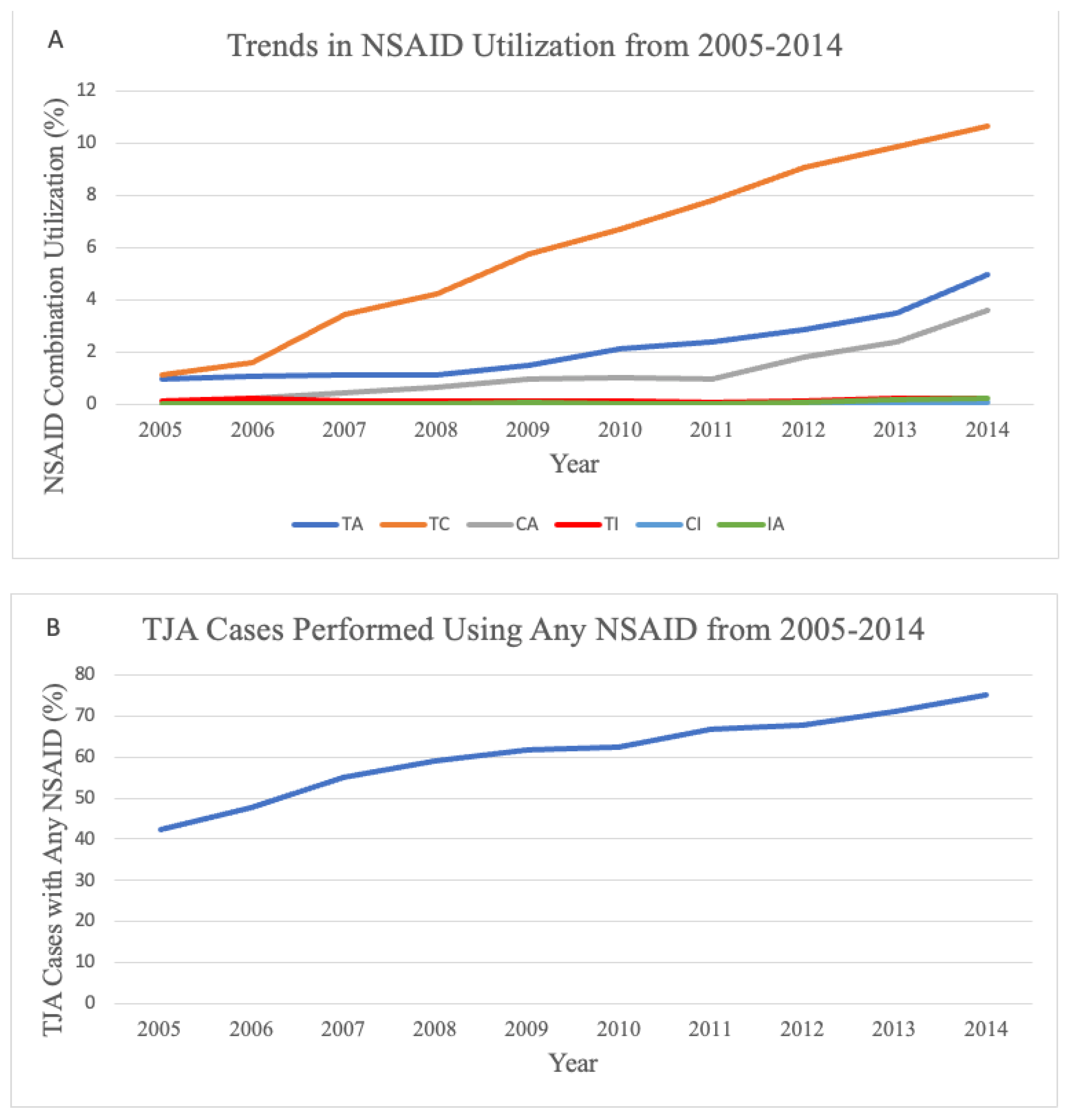
3.1. Trends in Type of NSAID Ordered
3.2. Patient and Hospital Demographics
3.3. Patient Comorbidities
3.4. Primary Endpoints: Acute Kidney Injury, Stroke, and Gastrointestinal Bleeding
3.5. Secondary Endpoints: Other Postoperative Complications
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Golladay, G.J.; Balch, K.R.; Dalury, D.F.; Satpathy, J.; Jiranek, W.A. Oral Multimodal Analgesia for Total Joint Arthroplasty. J. Arthroplast. 2017, 32, S69–S73. [Google Scholar] [CrossRef] [PubMed]
- Halawi, M.J.; Vovos, T.J.; Green, C.L.; Wellman, S.S.; Attarian, D.E.; Bolognesi, M.P. Opioid-Based Analgesia: Impact on Total Joint Arthroplasty. J. Arthroplast. 2015, 30, 2360–2363. [Google Scholar] [CrossRef] [PubMed]
- Gaffney, C.J.; Pelt, C.E.; Gililland, J.M.; Peters, C.L. Perioperative Pain Management in Hip and Knee Arthroplasty. Orthop. Clin. N. Am. 2017, 48, 407–419. [Google Scholar] [CrossRef]
- Mörwald, E.E.; Olson, A.; Cozowicz, C.; Poeran, J.; Mazumdar, M.; Memtsoudis, S.G. Association of Opioid Prescription and Perioperative Complications in Obstructive Sleep Apnea Patients Undergoing Total Joint Arthroplasties. Sleep Breath. 2018, 22, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Fleischman, A.N.; Tarabichi, M.; Foltz, C.; Makar, G.; Hozack, W.J.; Austin, M.S.; Chen, A.F. Opioid Prescription in Orthopedic Surgery after Discharge Research Group Cluster-Randomized Trial of Opiate-Sparing Analgesia after Discharge from Elective Hip Surgery. J. Am. Coll. Surg. 2019, 229, 335–345.e5. [Google Scholar] [CrossRef] [PubMed]
- Carr, D.B.; Goudas, L.C. Acute Pain. Lancet 1999, 353, 2051–2058. [Google Scholar] [CrossRef] [PubMed]
- FitzGerald, G.A.; Patrono, C. The Coxibs, Selective Inhibitors of Cyclooxygenase-2. N. Engl. J. Med. 2001, 345, 433–442. [Google Scholar] [CrossRef]
- Atkinson, T.J.; Fudin, J. Nonsteroidal Antiinflammatory Drugs for Acute and Chronic Pain. Phys. Med. Rehabil. Clin. N. Am. 2020, 31, 219–231. [Google Scholar] [CrossRef]
- Bresalier, R.S.; Sandler, R.S.; Quan, H.; Bolognese, J.A.; Oxenius, B.; Horgan, K.; Lines, C.; Riddell, R.; Morton, D.; Lanas, A.; et al. Cardiovascular Events Associated with Rofecoxib in a Colorectal Adenoma Chemoprevention Trial. N. Engl. J. Med. 2005, 352, 1092–1102. [Google Scholar] [CrossRef]
- Solomon, S.D.; McMurray, J.J.V.; Pfeffer, M.A.; Wittes, J.; Fowler, R.; Finn, P.; Anderson, W.F.; Zauber, A.; Hawk, E.; Bertagnolli, M.; et al. Cardiovascular Risk Associated with Celecoxib in a Clinical Trial for Colorectal Adenoma Prevention. N. Engl. J. Med. 2005, 352, 1071–1080. [Google Scholar] [CrossRef]
- Lucas, G.N.C.; Leitão, A.C.C.; Alencar, R.L.; Xavier, R.M.F.; Daher, E.D.F.; da Silva Junior, G.B. Pathophysiological Aspects of Nephropathy Caused by Non-Steroidal Anti-Inflammatory Drugs. J. Bras. Nefrol. 2019, 41, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Pitchon, D.N.; Dayan, A.C.; Schwenk, E.S.; Baratta, J.L.; Viscusi, E.R. Updates on Multimodal Analgesia for Orthopedic Surgery. Anesthesiol. Clin. 2018, 36, 361–373. [Google Scholar] [CrossRef] [PubMed]
- Fleischman, A.N.; Li, W.T.; Luzzi, A.J.; Van Nest, D.S.; Torjman, M.C.; Schwenk, E.S.; Arnold, W.A.; Parvizi, J. Risk of Gastrointestinal Bleeding with Extended Use of Nonsteroidal Anti-Inflammatory Drug Analgesia After Joint Arthroplasty. J. Arthroplast. 2021, 36, 1921–1925. [Google Scholar] [CrossRef]
- Grosso, M.J.; Kozaily, E.; Parvizi, J.; Austin, M.S. Aspirin Is Safe for Venous Thromboembolism Prophylaxis for Patients with a History of Gastrointestinal Issues. J. Arthroplast. 2021, 36, S332–S336. [Google Scholar] [CrossRef]
- Madhusudhan, T.R.; Rangan, A.; Gregg, P.J. Gastric Protection and Gastrointestinal Bleeding with Aspirin Thromboprophylaxis in Hip and Knee Joint Replacements. Ann. R. Coll. Surg. Engl. 2008, 90, 332–335. [Google Scholar] [CrossRef]
- Lalmohamed, A.; Vestergaard, P.; Javaid, M.K.; de Boer, A.; Leufkens, H.G.M.; van Staa, T.P.; de Vries, F. Risk of Gastrointestinal Bleeding in Patients Undergoing Total Hip or Knee Replacement Compared with Matched Controls: A Nationwide Cohort Study. Am. J. Gastroenterol. 2013, 108, 1277–1285. [Google Scholar] [CrossRef]
- Varas-Lorenzo, C.; Riera-Guardia, N.; Calingaert, B.; Castellsague, J.; Pariente, A.; Scotti, L.; Sturkenboom, M.; Perez-Gutthann, S. Stroke Risk and NSAIDs: A Systematic Review of Observational Studies. Pharmacoepidemiol. Drug Saf. 2011, 20, 1225–1236. [Google Scholar] [CrossRef] [PubMed]
- Ungprasert, P.; Cheungpasitporn, W.; Crowson, C.S.; Matteson, E.L. Individual Non-Steroidal Anti-Inflammatory Drugs and Risk of Acute Kidney Injury: A Systematic Review and Meta-Analysis of Observational Studies. Eur. J. Intern. Med. 2015, 26, 285–291. [Google Scholar] [CrossRef]
- Mittal, A.; Tamer, P.; Shah, I.; Cortes, A.; Hinman, A.D. Postoperative Acute Kidney Injury with Dual NSAID Use after Outpatient Primary Total Joint Arthroplasty. J. Am. Acad. Orthop. Surg. 2022, 30, 676–681. [Google Scholar] [CrossRef]
- Premier Applied Sciences. Premier Healthcare Database: Data That Informs and Performs; Premier Inc.: Charlotte, NC, USA, 2020. [Google Scholar]
- Zhang, J.; Ding, E.L.; Song, Y. Adverse Effects of Cyclooxygenase 2 Inhibitors on Renal and Arrhythmia Events: Meta-Analysis of Randomized Trials. JAMA 2006, 296, 1619–1632. [Google Scholar] [CrossRef]
- Chou, C.-I.; Shih, C.-J.; Chen, Y.-T.; Ou, S.-M.; Yang, C.-Y.; Kuo, S.-C.; Chu, D. Adverse Effects of Oral Nonselective and Cyclooxygenase-2-Selective NSAIDs on Hospitalization for Acute Kidney Injury: A Nested Case-Control Cohort Study. Medicine 2016, 95, e2645. [Google Scholar] [CrossRef] [PubMed]
- Hermann, M.; Shaw, S.; Kiss, E.; Camici, G.; Bühler, N.; Chenevard, R.; Lüscher, T.F.; Gröne, H.J.; Ruschitzka, F. Selective COX-2 Inhibitors and Renal Injury in Salt-Sensitive Hypertension. Hypertension 2005, 45, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Höcherl, K.; Endemann, D.; Kammerl, M.C.; Grobecker, H.F.; Kurtz, A. Cyclo-Oxygenase-2 Inhibition Increases Blood Pressure in Rats. Br. J. Pharmacol. 2002, 136, 1117–1126. [Google Scholar] [CrossRef] [PubMed]
- Richter, C.M.; Godes, M.; Wagner, C.; Maser-Gluth, C.; Herzfeld, S.; Dorn, M.; Priem, F.; Slowinski, T.; Bauer, C.; Schneider, W.; et al. Chronic Cyclooxygenase-2 Inhibition Does Not Alter Blood Pressure and Kidney Function in Renovascular Hypertensive Rats. J. Hypertens. 2004, 22, 191–198. [Google Scholar] [CrossRef]
- Fosslien, E. Cardiovascular Complications of Non-Steroidal Anti-Inflammatory Drugs. Ann. Clin. Lab. Sci. 2005, 35, 347–385. [Google Scholar]
- Rothwell, P.M.; Algra, A.; Chen, Z.; Diener, H.-C.; Norrving, B.; Mehta, Z. Effects of Aspirin on Risk and Severity of Early Recurrent Stroke after Transient Ischaemic Attack and Ischaemic Stroke: Time-Course Analysis of Randomised Trials. Lancet 2016, 388, 365–375. [Google Scholar] [CrossRef]
- Brill, J.B.; Calvo, R.Y.; Wallace, J.D.; Lewis, P.R.; Bansal, V.; Sise, M.J.; Shackford, S.R. Aspirin as Added Prophylaxis for Deep Vein Thrombosis in Trauma: A Retrospective Case-Control Study. J. Trauma Acute Care Surg. 2016, 80, 625–630. [Google Scholar] [CrossRef]
- Eikelboom, J.W.; Hirsh, J.; Spencer, F.A.; Baglin, T.P.; Weitz, J.I. Antiplatelet Drugs: Antithrombotic Therapy and Prevention of Thrombosis, 9th Ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012, 141, e89S–e119S. [Google Scholar] [CrossRef]
- Lotke, P.A.; Lonner, J.H. The Benefit of Aspirin Chemoprophylaxis for Thromboembolism after Total Knee Arthroplasty. Clin. Orthop. Relat. Res. 2006, 452, 175–180. [Google Scholar] [CrossRef]
- Berend, K.R.; Lombardi, A.V., Jr. Multimodal Venous Thromboembolic Disease Prevention for Patients Undergoing Primary or Revision Total Joint Arthroplasty: The Role of Aspirin. Am. J. Orthop. 2006, 35, 24–29. [Google Scholar]
- Pulmonary Embolism Prevention (PEP) Trial Collaborative Group. Prevention of Pulmonary Embolism and Deep Vein Thrombosis with Low Dose Aspirin: Pulmonary Embolism Prevention (PEP) Trial. Lancet 2000, 355, 1295–1302. [Google Scholar] [CrossRef]
- Ohnuma, T.; Raghunathan, K.; Fuller, M.; Ellis, A.R.; JohnBull, E.A.; Bartz, R.R.; Stefan, M.S.; Lindenauer, P.K.; Horn, M.E.; Krishnamoorthy, V. Trends in Comorbidities and Complications Using ICD-9 and ICD-10 in Total Hip and Knee Arthroplasties. J. Bone Jt. Surg. Am. 2021, 103, 696–704. [Google Scholar] [CrossRef] [PubMed]
- Angerett, N.R.; Yevtukh, A.; Ferguson, C.M.; Kahan, M.E.; Ali, M.; Hallock, R.H. Improving Postoperative Acute Kidney Injury Rates Following Primary Total Joint Arthroplasty. J. Arthroplast. 2022, 37, S1004–S1009. [Google Scholar] [CrossRef] [PubMed]
| T + C | A + C | T + A | T + I | C + I | I + A | p-Value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total Patients (n) | 121,393 (62.0%) | 26,345 (13.5%) | 43,854 (22.4%) | 2547 (1.3%) | 395 (0.2%) | 1299 (0.7%) | |||||||
| Age (years) | 65.04 ± 0.03 | 65.44 ± 0.08 | 65.79 ± 0.05 | 65.69 ± 0.26 | 64.57 ± 0.53 | 64.83 ± 0.28 | <0.001 | ||||||
| Length of Stay (days) | 2.66 ± 0.00 | 2.48 ± 0.01 | 2.86 ± 0.01 | 2.82 ± 0.04 | 2.96 ± 0.17 | 1.81 ± 0.04 | <0.001 | ||||||
| Cost of Hospitalization (USD) | 16,053.97 ± 20.19 | 15,237.62 ± 42.18 | 15,436.36 ± 55.00 | 15,688.10 ± 148.28 | 16,445.46 ± 573.6 | 13,884.32 ± 112.62 | <0.001 | ||||||
| n | % | n | % | n | % | n | % | n | % | n | % | ||
| Sex | <0.001 | ||||||||||||
| Female | 68,462 | 56.4 | 10,645 | 40.4 | 24,772 | 56.5 | 1093 | 42.9 | 210 | 53.2 | 718 | 55.3 | |
| Race/Ethnicity | <0.001 | ||||||||||||
| White | 99,667 | 82.1 | 22,220 | 84.34 | 36,247 | 82.65 | 1770 | 69.49 | 306 | 77.47 | 687 | 52.9 | |
| Black | 8330 | 6.9 | 2047 | 7.77 | 3227 | 7.36 | 122 | 4.79 | 31 | 7.85 | 69 | 5.3 | |
| Hispanic | 909 | 0.8 | 92 | 0.35 | 109 | 0.25 | 9 | 0.35 | 0 | 0.00 | 3 | 0.2 | |
| Other | 12,304 | 10.1 | 1916 | 7.27 | 4134 | 9.43 | 644 | 25.28 | 58 | 14.68 | 538 | 41.4 | |
| Unspecified | 183 | 0.2 | 70 | 0.27 | 137 | 0.31 | 2 | 0.08 | 0 | 0.00 | 2 | 0.2 | |
| Insurance Type | <0.0021 | ||||||||||||
| Medicare | 62,552 | 51.5 | 9999 | 52.2 | 12,106 | 57.5 | 1431 | 56.18 | 200 | 50.63 | 727 | 56.0 | |
| Medicaid | 3407 | 2.8 | 600 | 2.6 | 545 | 2.6 | 84 | 3.30 | 17 | 4.30 | 42 | 3.2 | |
| Managed Care | 41,060 | 33.8 | 6637 | 33.1 | 5957 | 28.3 | 791 | 31.06 | 136 | 34.43 | 416 | 32.0 | |
| Private | 9258 | 7.6 | 1441 | 7.5 | 1454 | 6.9 | 118 | 4.63 | 30 | 7.59 | 48 | 3.7 | |
| Other | 5116 | 4.2 | 982 | 4.9 | 982 | 4.7 | 123 | 4.83 | 12 | 3.04 | 66 | 5.1 | |
| Geographic Region | <0.001 | ||||||||||||
| Midwest | 25,997 | 21.4 | 4640 | 17.61 | 9456 | 21.56 | 257 | 10.09 | 108 | 27.3 | 39 | 3.0 | |
| Northeast | 15,267 | 12.6 | 5594 | 21.23 | 6479 | 14.77 | 615 | 24.15 | 59 | 14.9 | 102 | 7.9 | |
| South | 53,031 | 43.7 | 11,582 | 43.96 | 21,787 | 49.68 | 1408 | 55.28 | 129 | 32.7 | 1124 | 86.5 | |
| West | 27,098 | 22.3 | 4529 | 17.19 | 6132 | 13.98 | 267 | 10.48 | 99 | 25.1 | 34 | 2.6 | |
| Teaching Status | <0.001 | ||||||||||||
| Teaching | 49,983 | 41.2 | 11,683 | 44.3 | 18,724 | 42.7 | 355 | 13.9 | 219 | 55.4 | 150 | 11.5 | |
| Urban vs. Rural | <0.001 | ||||||||||||
| Rural | 13,077 | 10.8 | 1919 | 7.3 | 4593 | 10.5 | 286 | 11.2 | 74 | 18.7 | 29 | 2.2 | |
| T + C | A + C | T + A | T + I | C + I | I + A | p-Value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | n | % | n | % | ||
| Diabetes | 1209 | 1.00 | 264 | 1.00 | 603 | 1.38 | 16 | 0.63 | 4 | 1.01 | 9 | 0.69 | 0.521 |
| Hypertension | 61,972 | 51.1 | 12,127 | 46.0 | 28,324 | 64.6 | 1105 | 43.4 | 222 | 56.2 | 692 | 53.3 | <0.001 |
| Renal Failure | 3059 | 2.52 | 870 | 3.30 | 1613 | 3.68 | 32 | 1.26 | 12 | 3.04 | 25 | 1.92 | <0.001 |
| Valvular Disease | 3320 | 2.73 | 732 | 2.78 | 1670 | 3.81 | 69 | 2.71 | 9 | 2.28 | 17 | 1.31 | <0.001 |
| HIV/AIDS | 36 | 0.03 | 2 | 0.01 | 28 | 0.06 | 0 | 0.00 | 0 | 0.00 | 1 | 0.08 | 0.198 |
| CHF | 1895 | 1.56 | 376 | 1.43 | 953 | 2.17 | 32 | 1.26 | 14 | 3.54 | 25 | 1.92 | 0.096 |
| Hypothyroidism | 6828 | 5.62 | 17,966 | 68.2 | 3978 | 9.07 | 341 | 13.4 | 63 | 15.9 | 118 | 9.08 | <0.001 |
| Liver Disease | 353 | 0.29 | 1041 | 3.95 | 230 | 0.52 | 23 | 0.90 | 2 | 0.51 | 6 | 0.46 | <0.001 |
| Peripheral Vascular Disease | 914 | 0.75 | 2242 | 8.51 | 562 | 1.28 | 35 | 1.37 | 5 | 1.27 | 23 | 1.77 | <0.001 |
| Pulmonary Circulation Disorders | 337 | 0.28 | 787 | 2.99 | 168 | 0.38 | 16 | 0.63 | 5 | 1.27 | 4 | 0.31 | <0.001 |
| Chronic PUD | 3 | 0.00 | 12 | 0.05 | 7 | 0.02 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0.531 |
| Chronic Pulmonary Disease | 6194 | 5.10 | 16,241 | 61.6 | 3456 | 7.88 | 319 | 12.5 | 54 | 13.7 | 147 | 11.3 | <0.001 |
| Univariate Analysis | Model 1 | Model 2 | Model 3 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Complication | Combination | n | % | OR | CI | p-Value | aOR | CI | p-Value | aOR | CI | p-Value | aOR | CI | p-Value |
| AKI | T + C | 1664 | 1.37% | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | ||||
| A + C | 476 | 1.81% | 1.32 | 1.19–1.47 | <0.001 | 1.22 | 1.1–1.36 | 0.000 | 1.08 | 0.97–1.20 | 0.000 | 1.20 | 1.09–1.34 | 0 | |
| T + A | 608 | 1.39% | 0.82 | 0.75–0.91 | <0.001 | 0.81 | 0.73–0.88 | 0.000 | 0.75 | 0.68–0.83 | 0.000 | 0.76 | 0.69–0.83 | 0 | |
| T + I | 28 | 1.10% | 0.79 | 0.55–1.16 | 0.24 | 0.75 | 0.52–1.10 | 0.140 | 0.80 | 0.55–1.17 | 0.247 | 0.78 | 0.53–1.14 | 0.195 | |
| C + I | 10 | 2.53% | 1.87 | 0.99–3.50 | 0.05 | 1.90 | 1.01–3.58 | 0.047 | 1.71 | 0.90–3.24 | 0.100 | 1.69 | 0.89–3.2 | 0.109 | |
| I + A | 15 | 0.09% | 0.84 | 0.50–1.40 | 0.505 | 0.76 | 0.45–1.26 | 0.286 | 0.95 | 0.56–1.59 | 0.833 | 0.93 | 0.55–1.57 | 0.786 | |
| Stroke | T + C | 62 | 0.05% | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | ||||
| A + C | 27 | 0.10% | 2.01 | 1.28–3.16 | 0.015 | 1.80 | 1.15–2.83 | 0.011 | 1.71 | 1.08–2.69 | 0.021 | 1.80 | 1.15–2.84 | 0.011 | |
| T + A | 34 | 0.08% | 1.51 | 1.00–2.31 | 0.280 | 1.18 | 0.77–1.79 | 0.440 | 1.14 | 0.75–1.73 | 0.500 | 1.15 | 0.76–1.76 | 0.500 | |
| T + I | 4 | 0.16% | 3.07 | 1.12–8.45 | 0.030 | 3.00 | 1.09–8.26 | 0.034 | 3.09 | 1.12–8.51 | 0.030 | 3.48 | 1.25–9.73 | 0.017 | |
| C + I | 1 | 0.25% | 4.97 | 0.69–35.9 | 0.140 | 5.24 | 0.72–37.98 | 0.101 | 4.71 | 0.65–34.34 | 0.126 | 4.82 | 0.66–35.14 | 0.121 | |
| I + A | 3 | 0.22% | 4.53 | 1.42–14.5 | 0.010 | 3.31 | 0.80–13.65 | 0.098 | 3.93 | 0.95–16.26 | 0.050 | 4.29 | 1.06–17.89 | 0.046 | |
| GIB | T + C | 31 | 6.86% | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | ||||
| A + C | 2 | 0.44% | / | / | 0.040 | / | / | / | / | / | / | / | / | / | |
| T + A | 8 | 1.77% | 0.71 | 0.33–1.55 | 0.182 | 0.46 | 0.14–1.51 | 0.201 | 0.47 | 0.14–1.55 | 0.216 | 0.47 | 1.56–220.0 | 0.144 | |
| T + I | 0 | 0.00% | / | / | 0.390 | / | / | / | / | / | / | / | / | / | |
| C + I | 0 | 0.00% | / | / | 0.735 | / | / | / | / | / | / | / | / | / | |
| I + A | 0 | 0.00% | / | / | 0.540 | / | / | / | / | / | / | / | / | / | |
| Complication | Combination | Univariate | Model 1 | Model 2 | Model 3 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | % of NSAID Group | OR | CI | p-Value | aOR | CI | p-Value | aOR | CI | p-Value | aOR | CI | p-Value | ||
| PJI | T + C | 23 | 0.02 | Reference | |||||||||||
| A + C | 6 | 0.02 | 1.2 | 0.50–2.95 | 0.690 | 1.11 | 0.45–2.70 | 0.816 | 1.10 | 0.40–2.70 | 0.840 | 1.20 | 0.49–2.90 | 0.700 | |
| T + A | 9 | 0.02 | 1.08 | 0.50–2.30 | 0.839 | 0.89 | 0.40–1.90 | 0.700 | 0.87 | 0.40–1.90 | 0.700 | 0.90 | 0.40–1.90 | 0.750 | |
| T + I | 1 | 0.04 | 2.07 | 0.30–15.4 | 0.482 | 2.02 | 0.27–15.0 | 0.492 | 2.18 | 0.30–16.0 | 0.445 | 2.30 | 0.30–17.4 | 0.419 | |
| C + I | 1 | 0.25 | 13.4 | 1.80–99.3 | 0.011 | 13.8 | 1.90–102.6 | 0.010 | 12.1 | 1.60–91.6 | 0.015 | 10.3 | 1.35–78.3 | 0.024 | |
| I + A | 1 | 0.08 | 4.1 | 0.55–30.1 | 0.170 | 3.56 | 0.50–26.6 | 0.216 | 4.00 | 0.53–30.1 | 0.177 | 5.50 | 0.71–42.0 | 0.103 | |
| DVT | T + C | 235 | 0.19 | Reference | |||||||||||
| A + C | 46 | 0.17 | 0.90 | 0.66–1.24 | 0.520 | 0.84 | 0.60–1.15 | 0.294 | 0.92 | 0.60–1.13 | 0.242 | 0.80 | 0.60–1.1 | 0.220 | |
| T + A | 83 | 0.19 | 0.98 | 0.76–1.30 | 0.177 | 0.79 | 0.61–1.01 | 0.066 | 0.78 | 0.60–1.00 | 0.052 | 0.76 | 0.60–0.98 | 0.039 | |
| T + I | 2 | 0.08 | 0.40 | 0.10–1.63 | 0.202 | 0.40 | 0.100–1.60 | 0.192 | 0.41 | 0.10–1.66 | 0.212 | 0.43 | 0.11–1.74 | 0.237 | |
| C + I | 0 | 0.00 | - | - | - | - | - | - | - | - | - | - | - | - | |
| I + A | 0 | 0.00 | - | - | - | - | - | - | - | - | - | - | - | - | |
| PE | T + C | 203 | 0.17 | Reference | |||||||||||
| A + C | 58 | 0.22 | 1.30 | 0.98–1.76 | 0.065 | 1.23 | 0.92–1.64 | 0.167 | 1.21 | 0.90–1.62 | 0.200 | 1.20 | 0.90–1.60 | 0.232 | |
| T + A | 107 | 0.24 | 1.46 | 1.16–1.85 | 0.002 | 1.16 | 0.92–1.47 | 0.196 | 1.15 | 0.91–1.46 | 0.233 | 1.13 | 0.90–1.43 | 0.300 | |
| T + I | 2 | 0.08 | 0.47 | 0.11–1.88 | 0.285 | 0.45 | 0.11–1.80 | 0.260 | 0.47 | 0.12–1.88 | 0.283 | 0.47 | 0.12–1.88 | 0.285 | |
| C + I | 1 | 0.25 | 1.50 | 0.21–10.8 | 0.680 | 1.50 | 0.21–10.8 | 0.680 | 1.40 | 0.20–10.2 | 0.728 | 1.35 | 0.19–9.70 | 0.763 | |
| I + A | 0 | 0.00 | - | - | - | - | - | - | - | - | - | - | - | - | |
| Readmission | T + C | 5623 | 4.63 | Reference | |||||||||||
| A + C | 960 | 3.64 | 0.78 | 0.73–0.83 | <0.001 | 0.73 | 0.68–0.78 | <0.001 | 0.72 | 0.67–0.77 | 0.000 | 0.71 | 0.67–0.76 | <0.001 | |
| T + A | 1824 | 4.16 | 0.89 | 0.85–0.94 | <0.001 | 0.73 | 0.69–0.77 | <0.001 | 0.72 | 0.68–0.76 | 0.000 | 0.71 | 0.68–0.75 | <0.001 | |
| T + I | 101 | 3.97 | 0.85 | 0.70–1.04 | 0.109 | 0.83 | 0.68–1.02 | 0.074 | 0.85 | 0.70–1.05 | 0.127 | 0.97 | 0.79–1.18 | 0.743 | |
| C + I | 19 | 4.81 | 1.04 | 0.66–1.65 | 0.870 | 1.05 | 0.66–1.67 | 0.834 | 1.03 | 0.64–1.63 | 0.916 | 1.00 | 0.63–1.6 | 0.993 | |
| I + A | 50 | 3.85 | 0.82 | 0.62–1.09 | 0.181 | 0.81 | 0.60–1.07 | 0.143 | 0.85 | 0.64–1.12 | 0.250 | 0.96 | 0.70–1.27 | 0.762 | |
| Hematoma | T + C | 162 | 0.13 | Reference | |||||||||||
| A + C | 19 | 0.07 | 0.54 | 0.34–0.87 | 0.005 | 0.51 | 0.31–0.81 | 0.005 | 0.50 | 0.31–0.81 | 0.005 | 0.50 | 0.31–0.81 | 0.005 | |
| T + A | 45 | 0.10 | 0.77 | 0.55–1.07 | 0.119 | 0.63 | 0.45–0.88 | 0.006 | 0.63 | 0.45–0.87 | 0.006 | 0.63 | 0.45–0.88 | 0.007 | |
| T + I | 1 | 0.04 | 0.30 | 0.04–2.10 | 0.221 | 0.29 | 0.04–2.05 | 0.213 | 0.30 | 0.04–2.07 | 0.216 | 0.30 | 0.42–2.17 | 0.235 | |
| C + I | 0 | 0.00 | - | - | - | - | - | - | - | - | - | - | - | - | |
| I + A | 0 | 0.00 | - | - | - | - | - | - | - | - | - | - | - | - | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakata, H.; Shelby, T.; Wang, J.C.; Bouz, G.J.; Mayfield, C.K.; Oakes, D.A.; Lieberman, J.R.; Christ, A.B.; Heckmann, N.D. Postoperative Complications Associated with Non-Steroidal Anti-Inflammatory Combinations Used Status-Post Total Hip and Knee Arthroplasty. J. Clin. Med. 2023, 12, 6969. https://doi.org/10.3390/jcm12226969
Nakata H, Shelby T, Wang JC, Bouz GJ, Mayfield CK, Oakes DA, Lieberman JR, Christ AB, Heckmann ND. Postoperative Complications Associated with Non-Steroidal Anti-Inflammatory Combinations Used Status-Post Total Hip and Knee Arthroplasty. Journal of Clinical Medicine. 2023; 12(22):6969. https://doi.org/10.3390/jcm12226969
Chicago/Turabian StyleNakata, Haley, Tara Shelby, Jennifer C. Wang, Gabriel J. Bouz, Cory K. Mayfield, Daniel A. Oakes, Jay R. Lieberman, Alexander B. Christ, and Nathanael D. Heckmann. 2023. "Postoperative Complications Associated with Non-Steroidal Anti-Inflammatory Combinations Used Status-Post Total Hip and Knee Arthroplasty" Journal of Clinical Medicine 12, no. 22: 6969. https://doi.org/10.3390/jcm12226969
APA StyleNakata, H., Shelby, T., Wang, J. C., Bouz, G. J., Mayfield, C. K., Oakes, D. A., Lieberman, J. R., Christ, A. B., & Heckmann, N. D. (2023). Postoperative Complications Associated with Non-Steroidal Anti-Inflammatory Combinations Used Status-Post Total Hip and Knee Arthroplasty. Journal of Clinical Medicine, 12(22), 6969. https://doi.org/10.3390/jcm12226969






