Feasibility and Safety Study of Concomitant Left Bundle Branch Area Pacing and Atrioventricular Node Ablation with Same-Day Hospital Dismissal
Abstract
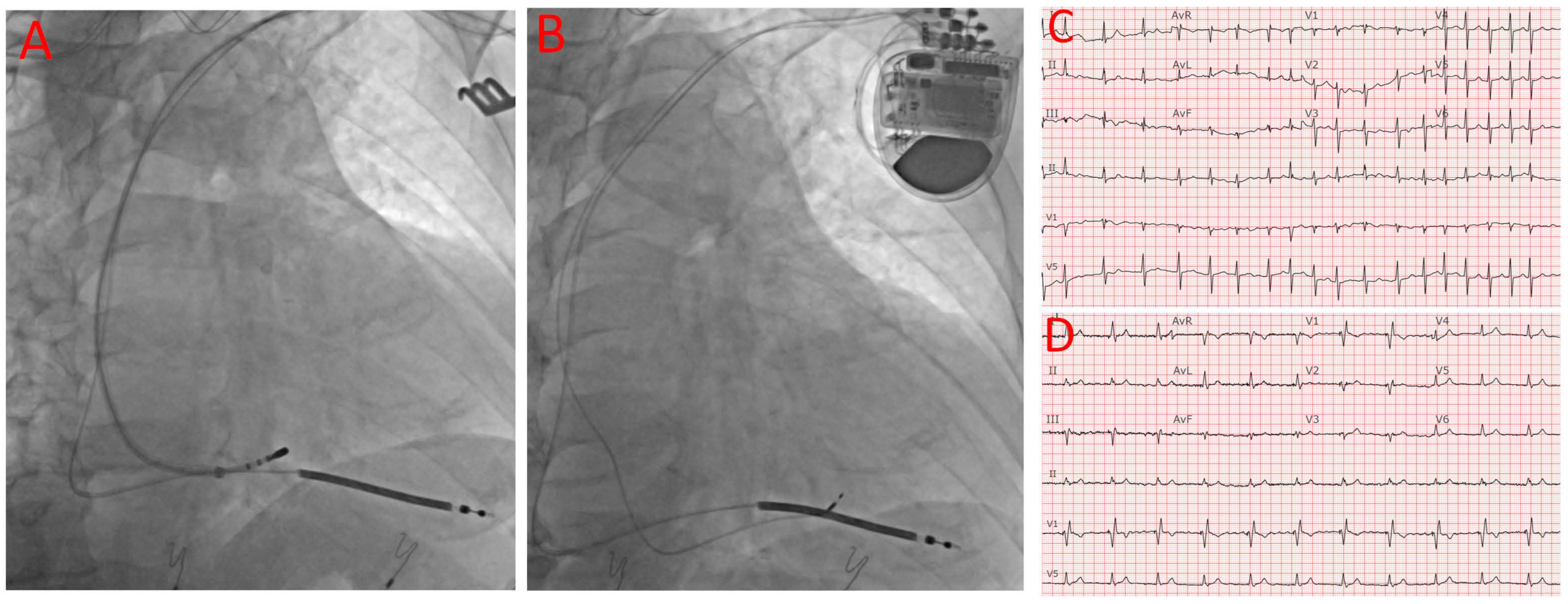
:1. Introduction
2. Methods
3. Results
4. Discussion
5. Limitation
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Deyell, M.W.; Leather, R.A.; Macle, L.; Forman, J.; Khairy, P.; Zhang, R.; Ding, L.; Chakrabarti, S.; Yeung-Lai-Wah, J.A.; Lane, C.; et al. Efficacy and Safety of Same-Day Discharge for Atrial Fibrillation Ablation. JACC Clin. Electrophysiol. 2020, 6, 609–619. [Google Scholar] [CrossRef] [PubMed]
- Archontakis, S.; Oikonomou, E.; Sideris, K.; Laina, A.; Tirovola, D.; Paraskevopoulou, D.; Kostakis, P.; Doundoulakis, I.; Arsenos, P.; Ntalakouras, I.; et al. Safety of same-day discharge versus overnight stay strategy following cardiac device implantations: A high-volume single-centre experience. J. Interv. Card. Electrophysiol. Int. J. Arrhythm. Pacing 2023, 66, 471–481. [Google Scholar] [CrossRef] [PubMed]
- Lim, K.T.; Davis, M.J.; Powell, A.; Arnolda, L.; Moulden, K.; Bulsara, M.; Weerasooriya, R. Ablate and pace strategy for atrial fibrillation: Long-term outcome of AIRCRAFT trial. Europace: European pacing, arrhythmias, and cardiac electrophysiology. Europace 2007, 9, 498–505. [Google Scholar] [CrossRef] [PubMed]
- January, C.T.; Wann, L.S.; Alpert, J.S.; Calkins, H.; Cigarroa, J.E.; Cleveland, J.C., Jr.; Conti, J.B.; Ellinor, P.T.; Ezekowitz, M.D.; Field, M.E.; et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J. Am. Coll. Cardiol. 2014, 64, e1–e76. [Google Scholar] [CrossRef]
- Liu, J.; Sun, F.; Wang, Z.; Sun, J.; Jiang, X.; Zhao, W.; Zhang, Z.; Liu, L.; Zhang, S. Left Bundle Branch Area Pacing vs. Biventricular Pacing for Cardiac Resynchronization Therapy: A Meta-Analysis. Front. Cardiovasc. Med. 2021, 8, 669301. [Google Scholar] [CrossRef]
- Sharma, P.S.; Patel, N.R.; Ravi, V.; Zalavadia, D.V.; Dommaraju, S.; Garg, V.; Larsen, T.R.; Naperkowski, A.M.; Wasserlauf, J.; Krishnan, K.; et al. Clinical outcomes of left bundle branch area pacing compared to right ventricular pacing: Results from the Geisinger-Rush Conduction System Pacing Registry. Heart Rhythm 2022, 19, 3–11. [Google Scholar] [CrossRef]
- Vijayaraman, P.; Ponnusamy, S.; Cano, O.; Sharma, P.S.; Naperkowski, A.; Subsposh, F.A.; Moskal, P.; Bednarek, A.; Dal Forno, A.R.; Young, W.; et al. Left Bundle Branch Area Pacing for Cardiac Resynchronization Therapy: Results From the International LBBAP Collaborative Study Group. JACC Clin. Electrophysiol. 2021, 7, 135–147. [Google Scholar] [CrossRef]
- Vijayaraman, P.; Subzposh, F.A.; Naperkowski, A.; Panikkath, R.; John, K.; Mascarenhas, V.; Bauch, T.D.; Huang, W. Prospective evaluation of feasibility and electrophysiologic and echocardiographic characteristics of left bundle branch area pacing. Heart Rhythm 2019, 16, 1774–1782. [Google Scholar] [CrossRef]
- Jin, Q.Q.; Zheng, C.; Wang, Y.J.; Lin, J.X.; Wu, D.Z.; Lin, J.F.; Guan, X.Q. Feasibility of Left Bundle Branch Area Pacing Combined with Atrioventricular Node Ablation in Atrial Fibrillation Patients with Heart Failure. J. Cardiovasc. Dev. Dis. 2022, 9, 338. [Google Scholar] [CrossRef]
- Rijks, J.H.J.; Lankveld, T.; Manusama, R.; Broers, B.; Stipdonk, A.; Chaldoupi, S.M.; Bekke, R.; Schotten, U.; Linz, D.; Luermans, J.; et al. Left Bundle Branch Area Pacing and Atrioventricular Node Ablation in a Single-Procedure Approach for Elderly Patients with Symptomatic Atrial Fibrillation. J. Clin. Med. 2023, 12, 4028. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, X. Successful zero fluoroscopy cardiac resynchronization therapy-defibrillator implantation with left bundle branch area pacing using an electroanatomic mapping system. HeartRhythm Case Rep. 2022, 8, 756–759. [Google Scholar] [CrossRef]
- Huang, W.; Su, L.; Wu, S.; Xu, L.; Xiao, F.; Zhou, X.; Ellenbogen, K.A. A Novel Pacing Strategy with Low and Stable Output: Pacing the Left Bundle Branch Immediately Beyond the Conduction Block. Can. J. Cardiol. 2017, 33, 1736.e1–1736.e3. [Google Scholar] [CrossRef]
- Burri, H.; Jastrzebski, M.; Cano, O.; Curila, K.; de Pooter, J.; Huang, W.; Israel, C.; Joza, J.; Romero, J.; Vernooy, K.; et al. EHRA clinical consensus statement on conduction system pacing implantation: Executive summary. Endorsed by the Asia-Pacific Heart Rhythm Society (APHRS), Canadian Heart Rhythm Society (CHRS) and Latin-American Heart Rhythm Society (LAHRS). Europace 2023, 25, 1237–1248. [Google Scholar] [CrossRef]
- Wang, G.; Zhao, Z.; Zhao, S.; Ding, S.; Shen, S.; Wang, L. Effect of cardiac resynchronization therapy on patients with heart failure and narrow QRS complexes: A meta-analysis of five randomized controlled trials. J. Interv. Card. Electrophysiol. Int. J. Arrhythm. Pacing 2015, 44, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Jabbour, R.J.; Shun-Shin, M.J.; Finegold, J.A.; Afzal Sohaib, S.M.; Cook, C.; Nijjer, S.S.; Whinnett, Z.I.; Manisty, C.H.; Brugada, J.; Francis, D.P. Effect of study design on the reported effect of cardiac resynchronization therapy (CRT) on quantitative physiological measures: Stratified meta-analysis in narrow-QRS heart failure and implications for planning future studies. J. Am. Heart Assoc. 2015, 4, e000896. [Google Scholar] [CrossRef] [PubMed]
- Acosta, H.; Viafara, L.M.; Hanif, N.; Acosta, S.; Pagadala, M.; Acosta, B.; Pothula, S.; Peckosh, C.; Bear, J.; Alzate, S.; et al. A Novel and Practical Method of Performing Atrioventricular Nodal Ablation via a Superior Approach in Patients with Refractory Atrial Fibrillation Undergoing Cardiac Resynchronization Device Implantation. J. Innov. Card Rhythm Manag. 2019, 10, 3924–3928. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Liu, X. Outcomes of Left Bundle Branch Area Pacing and Concomitant AV Node Ablation through the Same Veinous Access in Patients with Atrial Fibrillation and Systolic Heart Failure Who Require Non-Pharmacologic Rate Control. J. Atr. Fibrillation Electrophysiol. 2022, 15, 5. [Google Scholar]
- Pillai, A.; Kolominsky, J.; Koneru, J.N.; Kron, J.; Shepard, R.K.; Kalahasty, G.; Huang, W.; Verma, A.; Ellenbogen, K.A. Atrioventricular junction ablation in patients with conduction system pacing leads: A comparison of His-bundle vs. left bundle branch area pacing leads. Heart Rhythm 2022, 19, 1116–1123. [Google Scholar] [CrossRef]
- Linde, C.; Bongiorni, M.G.; Birgersdotter-Green, U.; Curtis, A.B.; Deisenhofer, I.; Furokawa, T.; Gillis, A.M.; Haugaa, K.H.; Lip, G.Y.H.; Van Gelder, I.; et al. Sex differences in cardiac arrhythmia: A consensus document of the European Heart Rhythm Association, endorsed by the Heart Rhythm Society and Asia Pacific Heart Rhythm Society. Europace 2018, 20, 1565–1565ao. [Google Scholar] [CrossRef]
- Jastrzebski, M.; Kielbasa, G.; Cano, O.; Curila, K.; Heckman, L.; De Pooter, J.; Chovanec, M.; Rademakers, L.; Huybrechts, W.; Grieco, D.; et al. Left bundle branch area pacing outcomes: The multicentre European MELOS study. Eur. Heart J. 2022, 43, 4161–4173. [Google Scholar] [CrossRef]
- Su, L.; Wang, S.; Wu, S.; Xu, L.; Huang, Z.; Chen, X.; Zheng, R.; Jiang, L.; Ellenbogen, K.A.; Whinnett, Z.I.; et al. Long-Term Safety and Feasibility of Left Bundle Branch Pacing in a Large Single-Center Study. Circ. Arrhythm. Electrophysiol. 2021, 14, e009261. [Google Scholar] [CrossRef] [PubMed]

| Baseline Characteristics (N = 24) | |
| Age ± SD (year old) | 78 ± 5 |
| Gender | |
| Male (%) | 9 (37%) |
| Female (%) | 15 (63%) |
| AF type | |
| Persistent (%) | 24 (100%) |
| Paroxysmal (%) | 0 (0%) |
| Type of device implanted | |
| CRT-D (%) | 6 (25%) |
| Dual-chamber PPM (%) | 18 (75%) |
| LVEF | 44 ± 14 |
| Pre-implantation QRS duration (ms) | 117 ± 32 |
| Comorbidities | |
| Diabetes (%) | 10 (42%) |
| Hypertension | 19 (79%) |
| Coronary artery disease | 11 (46%) |
| Chronic obstructive lung disease | 4 (17%) |
| Cerebrovascular accident | 3 (13%) |
| Medication | |
| Beta-blocker | 20 (83%) |
| Non-dihydropyridine calcium channel blocker | 10 (42%) |
| Digoxin | 5 (21%) |
| Class I C antiarrhythmic drug | 3 (13%) |
| Class III antiarrhythmic drug | 9 (38%) |
| Complication (N = 24, Duration of Follow up: 90 Days) | |
|---|---|
| Pocket hematoma, no intervention needed | 1 |
| Pocket hematoma requiring intervention | 0 |
| Pneumothorax | 0 |
| Cardiac perforation (including tamponade) | 0 |
| Lead dislodgement requiring revision | 0 |
| Infection | 0 |
| Post-Implantation Device Parameters: QRS Width and LVEF (N = 24) | |
|---|---|
| LBBA lead Capture Threshold (V@0.4 ms) | |
| Acute | 0.8 ± 0.3 |
| One week post-implantation | 0.7 ± 0.4 |
| Three months post-implantation | 0.6 ± 0.3 |
| LBBA Lead Sensing (mV) | |
| Acute | 9.9 ± 3.9 |
| One week post-implantation | 11.2 ± 4.7 |
| Three months post-implantation | 10.4 ± 4.1 |
| LBBA Lead Impedance (Ohm) | |
| Acute | 710 ± 216 |
| One week post-implantation | 654 ± 162 |
| Three months post-implantation | 544 ± 110 |
| Post-implantation QRS duration (ms) | 123 ± 14 |
| Post-implantation LVEF (%) | 46 ± 12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Liu, X. Feasibility and Safety Study of Concomitant Left Bundle Branch Area Pacing and Atrioventricular Node Ablation with Same-Day Hospital Dismissal. J. Clin. Med. 2023, 12, 7002. https://doi.org/10.3390/jcm12227002
Liu Z, Liu X. Feasibility and Safety Study of Concomitant Left Bundle Branch Area Pacing and Atrioventricular Node Ablation with Same-Day Hospital Dismissal. Journal of Clinical Medicine. 2023; 12(22):7002. https://doi.org/10.3390/jcm12227002
Chicago/Turabian StyleLiu, Zhigang, and Xiaoke Liu. 2023. "Feasibility and Safety Study of Concomitant Left Bundle Branch Area Pacing and Atrioventricular Node Ablation with Same-Day Hospital Dismissal" Journal of Clinical Medicine 12, no. 22: 7002. https://doi.org/10.3390/jcm12227002
APA StyleLiu, Z., & Liu, X. (2023). Feasibility and Safety Study of Concomitant Left Bundle Branch Area Pacing and Atrioventricular Node Ablation with Same-Day Hospital Dismissal. Journal of Clinical Medicine, 12(22), 7002. https://doi.org/10.3390/jcm12227002





