The Management of Coronary Artery Disease in TAVR Patients
Abstract
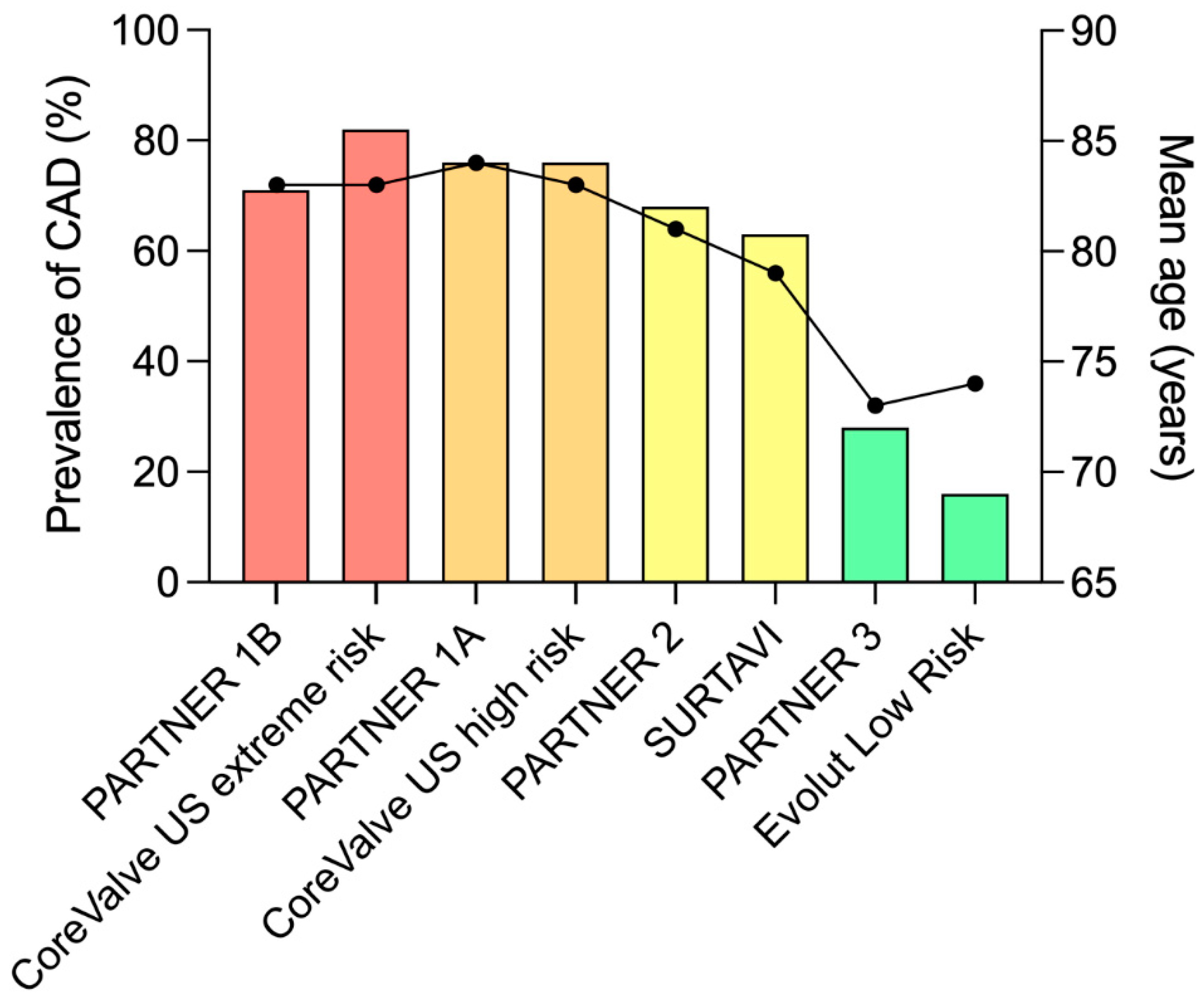
:1. Introduction
2. Prognostic Impact of CAD in TAVR Candidates
3. Coronary Evaluation Pre-TAVR
4. Coronary Revascularization Pre-TAVR
5. Timing of Coronary Revascularization Pre-TAVR
6. Coronary Management during TAVR
7. Acute Coronary Syndrome after TAVR
- -
- All STEMI management performance indicators were significantly worse in the population with a history of TAVR, with a 33% longer door-to-balloon time, but also a significantly longer procedure time, fluoroscopy time, and higher contrast volume and dosimetry level, reflecting increased procedural complexity.
- -
- The PCI failure rate was four times higher in the population of patients with a history of TAVR (16% versus 4%), with particularly high rates of coronary cannulation failure (6%) and lesion crossing failure (5%).
- -
- Revascularization failure was an independent predictor of poor outcomes.
- -
- In addition to the classical atherothrombotic mechanism, other alternative mechanisms were involved such as coronary embolism (complicating the TAVR procedure itself, or related to TAVR valve thrombosis), late migration of the TAVR valve resulting in delayed coronary occlusion (particularly with self-expanding valves), or restenosis/thrombosis of stents implanted in the work-up pre-TAVR.
8. Coronary Access after TAVR
9. Limitations
10. Conclusions and Future Directions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACS | acute coronary syndrome |
| CAD | coronary artery disease |
| CCTA | cardiac computed tomography angiography |
| FFR | fractional flow reserve |
| iFR | instantaneous wave-free ration |
| MACCE | major adverse cardiovascular and cerebrovascular event |
| PCI | percutaneous coronary intervention |
| SAVR | surgical aortic valve replacement |
| TAVR | transcatheter aortic valve replacement |
References
- Stewart, B.F.; Siscovick, D.; Lind, B.K.; Gardin, J.M.; Gottdiener, J.S.; Smith, V.E.; Kitzman, D.W.; Otto, C.M. Clinical factors associated with calcific aortic valve disease. Cardiovascular Health Study. J. Am. Coll. Cardiol. 1997, 29, 630–634. [Google Scholar] [CrossRef]
- Faroux, L.; Guimaraes, L.; Wintzer-Wehekind, J.; Junquera, L.; Ferreira-Neto, A.N.; Del Val, D.; Muntané-Carol, G.; Mohammadi, S.; Paradis, J.M.; Rodés-Cabau, J. Coronary Artery Disease and Transcatheter Aortic Valve Replacement: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2019, 74, 362–372. [Google Scholar] [CrossRef]
- Leon, M.B.; Smith, C.R.; Mack, M.; Miller, D.C.; Moses, J.W.; Svensson, L.G.; Tuzcu, E.M.; Webb, J.G.; Fontana, G.P.; Makkar, R.R.; et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N. Engl. J. Med. 2010, 363, 1597–1607. [Google Scholar] [CrossRef]
- Popma, J.J.; Adams, D.H.; Reardon, M.J.; Yakubov, S.J.; Kleiman, N.S.; Heimansohn, D.; Hermiller, J., Jr.; Hughes, G.C.; Harrison, J.K.; Coselli, J.; et al. Transcatheter aortic valve replacement using a self-expanding bioprosthesis in patients with severe aortic stenosis at extreme risk for surgery. J. Am. Coll. Cardiol. 2014, 63, 1972–1981. [Google Scholar] [CrossRef]
- Smith, C.R.; Leon, M.B.; Mack, M.J.; Miller, D.C.; Moses, J.W.; Svensson, L.G.; Tuzcu, E.M.; Webb, J.G.; Fontana, G.P.; Makkar, R.R.; et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N. Engl. J. Med. 2011, 364, 2187–2198. [Google Scholar] [CrossRef]
- Adams, D.H.; Popma, J.J.; Reardon, M.J.; Yakubov, S.J.; Coselli, J.S.; Deeb, G.M.; Gleason, T.G.; Buchbinder, M.; Hermiller, J., Jr.; Kleiman, N.S.; et al. Transcatheter aortic-valve replacement with a self-expanding prosthesis. N. Engl. J. Med. 2014, 370, 1790–1798. [Google Scholar] [CrossRef]
- Leon, M.B.; Smith, C.R.; Mack, M.J.; Makkar, R.R.; Svensson, L.G.; Kodali, S.K.; Thourani, V.H.; Tuzcu, E.M.; Miller, D.C.; Herrmann, H.C.; et al. Transcatheter or Surgical Aortic-Valve Replacement in Intermediate-Risk Patients. N. Engl. J. Med. 2016, 374, 1609–1620. [Google Scholar] [CrossRef]
- Reardon, M.J.; Van Mieghem, N.M.; Popma, J.J.; Kleiman, N.S.; Søndergaard, L.; Mumtaz, M.; Adams, D.H.; Deeb, G.M.; Maini, B.; Gada, H.; et al. Surgical or Transcatheter Aortic-Valve Replacement in Intermediate-Risk Patients. N. Engl. J. Med. 2017, 376, 1321–1331. [Google Scholar] [CrossRef]
- Mack, M.J.; Leon, M.B.; Thourani, V.H.; Makkar, R.; Kodali, S.K.; Russo, M.; Kapadia, S.R.; Malaisrie, S.C.; Cohen, D.J.; Pibarot, P.; et al. Transcatheter Aortic-Valve Replacement with a Balloon-Expandable Valve in Low-Risk Patients. N. Engl. J. Med. 2019, 380, 1695–1705. [Google Scholar] [CrossRef]
- Popma, J.J.; Deeb, G.M.; Yakubov, S.J.; Mumtaz, M.; Gada, H.; O’Hair, D.; Bajwa, T.; Heiser, J.C.; Merhi, W.; Kleiman, N.S.; et al. Transcatheter Aortic-Valve Replacement with a Self-Expanding Valve in Low-Risk Patients. N. Engl. J. Med. 2019, 380, 1706–1715. [Google Scholar] [CrossRef]
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 2022, 43, 561–632. [Google Scholar] [CrossRef]
- van den Boogert, T.P.W.; Vendrik, J.; Claessen, B.E.P.M.; Baan, J.; Beijk, M.A.; Limpens, J.; Boekholdt, S.A.M.; Hoek, R.; Planken, R.N.; Henriques, J.P. CTCA for detection of significant coronary artery disease in routine TAVI work-up: A systematic review and meta-analysis. Neth. Heart J. 2018, 26, 591–599. [Google Scholar] [CrossRef]
- Chieffo, A.; Giustino, G.; Spagnolo, P.; Panoulas, V.F.; Montorfano, M.; Latib, A.; Figini, F.; Agricola, E.; Gerli, C.; Franco, A.; et al. Routine Screening of Coronary Artery Disease With Computed Tomographic Coronary Angiography in Place of Invasive Coronary Angiography in Patients Undergoing Transcatheter Aortic Valve Replacement. Circ. Cardiovasc. Interv. 2015, 8, e002025. [Google Scholar] [CrossRef]
- Neumann, F.J.; Sousa-Uva, M.; Ahlsson, A.; Alfonso, F.; Banning, A.P.; Benedetto, U.; Byrne, R.A.; Collet, J.P.; Falk, V.; Head, S.J.; et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur. Heart J. 2019, 40, 87–165. [Google Scholar] [CrossRef]
- Ahmad, Y.; Götberg, M.; Cook, C.; Howard, J.P.; Malik, I.; Mikhail, G.; Frame, A.; Petraco, R.; Rajkumar, C.; Demir, O.; et al. Coronary Hemodynamics in Patients With Severe Aortic Stenosis and Coronary Artery Disease Undergoing Transcatheter Aortic Valve Replacement: Implications for Clinical Indices of Coronary Stenosis Severity. JACC Cardiovasc. Interv. 2018, 11, 2019–2031. [Google Scholar] [CrossRef]
- Yamanaka, F.; Shishido, K.; Ochiai, T.; Moriyama, N.; Yamazaki, K.; Sugitani, A.; Tani, T.; Tobita, K.; Mizuno, S.; Tanaka, Y.; et al. Instantaneous Wave-Free Ratio for the Assessment of Intermediate Coronary Artery Stenosis in Patients With Severe Aortic Valve Stenosis: Comparison With Myocardial Perfusion Scintigraphy. JACC Cardiovasc. Interv. 2018, 11, 2032–2040. [Google Scholar] [CrossRef]
- Writing Committee Members Otto, C.M.; Nishimura, R.A.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P., 3rd; Gentile, F.; Jneid, H.; Krieger, E.V.; Mack, M.; McLeod, C.; et al. 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2021, 77, 450–500. [Google Scholar]
- Faroux, L.; Campelo-Parada, F.; Munoz-Garcia, E.; Nombela-Franco, L.; Fischer, Q.; Donaint, P.; Serra, V.; Veiga, G.; Gutiérrez, E.; Vilalta, V.; et al. Procedural Characteristics and Late Outcomes of Percutaneous Coronary Intervention in the Workup Pre-TAVR. JACC Cardiovasc. Interv. 2020, 13, 2601–2613. [Google Scholar] [CrossRef]
- Witberg, G.; Regev, E.; Chen, S.; Assali, A.; Barbash, I.M.; Planer, D.; Vaknin-Assa, H.; Guetta, V.; Vukasinovic, V.; Orvin, K.; et al. The Prognostic Effects of Coronary Disease Severity and Completeness of Revascularization on Mortality in Patients Undergoing Transcatheter Aortic Valve Replacement. JACC Cardiovasc. Interv. 2017, 10, 1428–1435. [Google Scholar] [CrossRef]
- Costa, G.; Pilgrim, T.; Amat Santos, I.J.; De Backer, O.; Kim, W.K.; Barbosa Ribeiro, H.; Saia, F.; Bunc, M.; Tchetche, D.; Garot, P.; et al. Management of Myocardial Revascularization in Patients With Stable Coronary Artery Disease Undergoing Transcatheter Aortic Valve Implantation. Circ. Cardiovasc. Interv. 2022, 15, e012417. [Google Scholar] [CrossRef]
- Patterson, T.; Clayton, T.; Dodd, M.; Khawaja, Z.; Morice, M.C.; Wilson, K.; Kim, W.K.; Meneveau, N.; Hambrecht, R.; Byrne, J.; et al. ACTIVATION (PercutAneous Coronary inTervention prIor to transcatheter aortic VAlve implantaTION): A Randomized Clinical Trial. JACC Cardiovasc. Interv. 2021, 14, 1965–1974. [Google Scholar] [CrossRef] [PubMed]
- Alperi, A.; Mohammadi, S.; Campelo-Parada, F.; Munoz-Garcia, E.; Nombela-Franco, L.; Faroux, L.; Veiga, G.; Serra, V.; Fischer, Q.; Pascual, I.; et al. Transcatheter Versus Surgical Aortic Valve Replacement in Patients With Complex Coronary Artery Disease. JACC Cardiovasc. Interv. 2021, 14, 2490–2499. [Google Scholar] [CrossRef] [PubMed]
- van Rosendael, P.J.; van der Kley, F.; Kamperidis, V.; Katsanos, S.; Al Amri, I.; Regeer, M.; Schalij, M.J.; Ajmone Marsan, N.; Bax, J.J.; Delgado, V. Timing of staged percutaneous coronary intervention before transcatheter aortic valve implantation. Am. J. Cardiol. 2015, 115, 1726–1732. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Rodriguez, A.P.; Thakkar, B.; Patel, N.J.; Ghatak, A.; Badheka, A.O.; Alfonso, C.E.; de Marchena, E.; Sakhuja, R.; Inglessis-Azuaje, I.; et al. Comparison of Outcomes of Transcatheter Aortic Valve Replacement Plus Percutaneous Coronary Intervention Versus Transcatheter Aortic Valve Replacement Alone in the United States. Am. J. Cardiol. 2016, 118, 1698–1704. [Google Scholar] [CrossRef]
- Vilalta, V.; Asmarats, L.; Ferreira-Neto, A.N.; Maes, F.; de Freitas Campos Guimarães, L.; Couture, T.; Paradis, J.M.; Mohammadi, S.; Dumont, E.; Kalavrouziotis, D.; et al. Incidence, Clinical Characteristics, and Impact of Acute Coronary Syndrome Following Transcatheter Aortic Valve Replacement. JACC Cardiovasc. Interv. 2018, 11, 2523–2533. [Google Scholar] [CrossRef]
- Faroux, L.; Munoz-Garcia, E.; Serra, V.; Alperi, A.; Nombela-Franco, L.; Fischer, Q.; Veiga, G.; Donaint, P.; Asmarats, L.; Vilalta, V.; et al. Acute Coronary Syndrome Following Transcatheter Aortic Valve Replacement. Circ. Cardiovasc. Interv. 2020, 13, e008620. [Google Scholar] [CrossRef]
- Faroux, L.; Lhermusier, T.; Vincent, F.; Nombela-Franco, L.; Tchétché, D.; Barbanti, M.; Abdel-Wahab, M.; Windecker, S.; Auffret, V.; Campanha-Borges, D.C.; et al. ST-Segment Elevation Myocardial Infarction Following Transcatheter Aortic Valve Replacement. J. Am. Coll. Cardiol. 2021, 77, 2187–2199. [Google Scholar] [CrossRef]
- Yudi, M.B.; Sharma, S.K.; Tang, G.H.L.; Kini, A. Coronary Angiography and Percutaneous Coronary Intervention After Transcatheter Aortic Valve Replacement. J. Am. Coll. Cardiol. 2018, 71, 1360–1378. [Google Scholar] [CrossRef]
- Faroux, L.; Couture, T.; Guimaraes, C.; Junquera, L.; Del Val, D.; Muntané-Carol, G.; Wintzer-Wehekind, J.; Mohammadi, S.; Paradis, J.M.; Delarochellière, R.; et al. Interaction Between Balloon-Expandable Valves and Coronary Ostia: Angiographic Analysis and Impact on Coronary Access. J. Invasive Cardiol. 2020, 32, 235–242. [Google Scholar]
- Rogers, T.; Greenspun, B.C.; Weissman, G.; Torguson, R.; Craig, P.; Shults, C.; Gordon, P.; Ehsan, A.; Wilson, S.R.; Goncalves, J.; et al. Feasibility of Coronary Access and Aortic Valve Reintervention in Low-Risk TAVR Patients. JACC Cardiovasc. Interv. 2020, 13, 726–735. [Google Scholar] [CrossRef]
- Couture, T.; Faroux, L.; Junquera, L.; Del Val, D.; Muntané-Carol, G.; Wintzer-Wehekind, J.; Alperi, A.; Mohammadi, S.; Paradis, J.M.; Delarochellière, R.; et al. Interaction Between Self-Expanding Transcatheter Heart Valves and Coronary Ostia: An Angiographically Based Analysis of the Evolut R/Pro Valve System. J. Invasive Cardiol. 2020, 32, 123–128. [Google Scholar]
- Ochiai, T.; Chakravarty, T.; Yoon, S.H.; Kaewkes, D.; Flint, N.; Patel, V.; Mahani, S.; Tiwana, R.; Sekhon, N.; Nakamura, M.; et al. Coronary Access After TAVR. JACC Cardiovasc. Interv. 2020, 13, 693–705. [Google Scholar] [CrossRef] [PubMed]
- Barbanti, M.; Costa, G.; Picci, A.; Criscione, E.; Reddavid, C.; Valvo, R.; Todaro, D.; Deste, W.; Condorelli, A.; Scalia, M.; et al. Coronary Cannulation After Transcatheter Aortic Valve Replacement: The RE-ACCESS Study. JACC Cardiovasc. Interv. 2020, 13, 2542–2555. [Google Scholar] [CrossRef] [PubMed]
- Tang, G.H.L.; Zaid, S.; Michev, I.; Ahmad, H.; Kaple, R.; Undemir, C.; Cohen, M.; Lansman, S.L. “Cusp-Overlap” View Simplifies Fluoroscopy-Guided Implantation of Self-Expanding Valve in Transcatheter Aortic Valve Replacement. JACC Cardiovasc. Interv. 2018, 11, 1663–1665. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; De Backer, O.; Bieliauskas, G.; Wong, I.; Bajoras, V.; Xiong, T.Y.; Zhang, Y.; Kofoed, K.F.; Chen, M.; Sondergaard, L. Cusp Symmetry and Coronary Ostial Eccentricity and its Impact on Coronary Access Following TAVR. JACC Cardiovasc. Interv. 2022, 15, 123–134. [Google Scholar] [CrossRef]





| Evolut Low Risk | Unprotected left main coronary artery, or |
| Multivessel coronary artery disease with a SYNTAX score >22 | |
| PARTNER 3 | Unprotected left main coronary artery, or |
| SYNTAX score >32 (in the absence of prior revascularization), or | |
| Heart Team assessment that optimal revascularization cannot be performed |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faroux, L.; Villecourt, A.; Metz, D. The Management of Coronary Artery Disease in TAVR Patients. J. Clin. Med. 2023, 12, 7126. https://doi.org/10.3390/jcm12227126
Faroux L, Villecourt A, Metz D. The Management of Coronary Artery Disease in TAVR Patients. Journal of Clinical Medicine. 2023; 12(22):7126. https://doi.org/10.3390/jcm12227126
Chicago/Turabian StyleFaroux, Laurent, Aurélien Villecourt, and Damien Metz. 2023. "The Management of Coronary Artery Disease in TAVR Patients" Journal of Clinical Medicine 12, no. 22: 7126. https://doi.org/10.3390/jcm12227126
APA StyleFaroux, L., Villecourt, A., & Metz, D. (2023). The Management of Coronary Artery Disease in TAVR Patients. Journal of Clinical Medicine, 12(22), 7126. https://doi.org/10.3390/jcm12227126







