Abstract
Left ventricular global longitudinal strain (GLS) has an important role in the diagnosis of cancer therapy-related cardiac dysfunction (CTRCD). Little is known about the role of atrial function in diagnosing CTRCD. The aim of our study was to assess the impact of anti-cancer drugs on atrial function measured by speckle-tracking echocardiography in breast cancer women. A prospective multicenter study was conducted enrolling 169 breast cancer women treated with anthracyclines. A cardiological evaluation including an electrocardiogram and echocardiogram with an analysis of GLS, left atrial (LA) strain, and LA stiffness (LASi) was performed at baseline (T0), 3 (T1), and 6 months (T2) after starting chemotherapy. The patients were divided into two groups: patients with asymptomatic mild cardiotoxicity at T1 (with a relative reduction in GLS > 15%; Group 1) and those without (Group 2). We did not find a significant change in left ventricular ejection fraction (LVEF) at T1 and T2; we found a significant change in GLS (p-value < 0.0001) in the peak atrial longitudinal strain (PALS) and in LASi (p-value < 0.0001). Impairment of atrial function was greater in Group 1 compared to Group 2. A PALS variation > 20.8% identified patients who were most likely to develop asymptomatic mild cardiotoxicity [AUC 0.62; CI (0.51–0.73) p = 0.06, sensitivity 45%, specificity 69.5%]. Conclusions: PALS and LASi significantly change during chemotherapy in association with GLS. Atrial strain is an additional parameter that could be measured together with GLS to detect cardiotoxicity early.
1. Introduction
It is known that anti-cancer drugs can cause cardiovascular complications such as left ventricular dysfunction, heart failure, arrhythmias, myocardial ischemia, and arterial hypertension. The mechanisms underlying cardiotoxicity may involve direct and/or indirect mechanisms of cardiomyocytes. Anthracyclines are associated with a high incidence of heart failure as a consequence of direct damage to cardiomyocytes which, once their function is altered, could lead to cell death. Other mechanisms underlying cardiotoxicity may include vascular dysfunction and thromboembolic ischemia. Other adverse effects result from impaired signaling of cardioprotective factors for cardiomyocytes, such as Neuregulin-1 (NRG1), or for other cardiac cell populations, such as vascular endothelial growth factor (VEGF) and platelet-derived growth factor (PDGF) [1,2,3,4]. Several definitions of cardiotoxicity have been previously proposed [5,6]. The 2022 ESC guidelines on cardio-oncology have optimized the practical management of the cardio-oncology patient and have proposed a universal definition of cardiac dysfunction related to anti-tumor therapy (CTRCD), distinguishing it into symptomatic and asymptomatic [7,8]. Asymptomatic mild CTRCD is defined as the presence of preserved LVEF ≥ 50% but a new relative decline in left ventricular global longitudinal strain (GLS) > 15% from baseline and/or a new rise in cardiac biomarkers [7]. Several studies conducted in the field of cardio-oncology have shown that LVEF alters late during anti-cancer therapy [9], whereas GLS alters earlier by identifying subclinical cardiac damage [10]. Cardiac biomarkers (natriuretic peptides and high-sensitivity troponins) can also detect cardiotoxic damage at an early phase and possibly predict the future development of CTRCD [11,12]. A recent systematic review and meta-analysis showed that measuring GLS after initiating potentially cardiotoxic chemotherapy with anthracyclines with or without trastuzumab had good prognostic performance for subsequent CTRCD [13]. Thus, GLS should be assessed before starting chemotherapy and during chemotherapy to detect early cardiac damage and to start cardioprotective therapy [14]. While the role of GLS in cardio-oncology is well established, little is known about the role of left atrial (LA) strain. Few studies have analyzed changes in atrial function in cancer patients. LA strain is known to have clinical utility in a variety of settings. For example, LA reservoir strain was introduced as a standard parameter in the diagnostic algorithm of diastolic function in HFpEF [15]. Indeed, LA strain is known to be an early and sensitive marker of myocardial fibrosis and elevated left ventricular (LV) filling pressures [16]. LA strain also has a prognostic role in patients with heart failure. Mandoli et al. showed that the loss of left atrial contractile function predicts worse outcomes in heart failure patients with reduced LVEF (HFrEF) [17]. Even in patients with cardiac amyloidosis and HFpEF, a loss of atrial contraction and atrial electromechanical dissociation in patients with sinus rhythm (SR) was associated with a poorer prognosis than in patients with SR and effective mechanical contraction [18]. There is a close correlation between GLS and LA strain. LA reservoir and pump strain are mainly determined by GLS and filling pressure, as demonstrated by Inoue et al. [16]. Thus, considering the close correlation between GLS and LA strain and the changes in GLS during chemotherapy, the aim of our study was to assess the impact of anthracyclines and trastuzumab on atrial function measured by speckle-tracking echocardiography (STE) in breast cancer women.
2. Materials and Methods
2.1. Centers Involved
A prospective multicenter study was carried out enrolling 169 women with newly diagnosed breast cancer, scheduled to receive anthracyclines (ANT) therapy followed by paclitaxel plus trastuzumab. Eight different Italian centers were involved: University Hospital “Paolo Giaccone”—Palermo (64 patients), ASST Metropolitan Hospital “Niguarda”—Milan (28 patients), University Hospital “Ospedali Riuniti”—Foggia (26 patients), University Hospital “San Giovanni di Dio”—Cagliari (23 patients), University Hospital “Borgo Trento”—Verona (16 patients), University Hospital “Gaetano Martino”—Messina (12 patients), University Hospital of Pisa, and University Hospital of Siena.
2.2. Criteria for Enrolling Patients in the Study
Inclusion criteria were newly diagnosed breast cancer, age between 18 and 80 years old, and treatment with anthracyclines for at least 4–6 cycles followed by trastuzumab. Exclusion criteria were prior chemotherapy or radiotherapy; ischemic heart disease (prior myocardial infarction and/or coronary revascularization), severe valve diseases, baseline LVEF < 50%, atrial fibrillation and sustained ventricular arrhythmias, life expectancy < 1 year, poor acoustic window, and unfeasible STE.
2.3. Follow-Up and Study Methodology
After approval by the local ethics committee and after providing written informed consent, all the patients underwent cardiological evaluation at baseline (T0), 3 (T1), and 6 (T2) months after starting chemotherapy. Clinical and anamnestic data were collected. An electrocardiogram and an echocardiogram with STE analysis were performed at baseline and during follow-up. The diagnosis of CTRCD was made according to the 2022 ESC guidelines on cardio-oncology, based on the LVEF and/or GLS reduction from baseline. Patients were divided into 2 groups: patients with mild asymptomatic cardiotoxicity, defined as a relative reduction in GLS > 15% at T1 compared to baseline (Group 1), and those without (Group 2).
2.4. Echocardiographic Examination
Echocardiographic examination was performed according to EACVI/American Society of Echocardiography (ASE) recommendations for chamber quantification [19], using a General Electric (GE) Vivid E95 ultrasound system prime echocardiography machine and a 4Vc-D (1.4–5.2 MHz) linear transducer. LV systolic and diastolic function were assessed. LVEF was assessed using the biplane-modified Simpson method from the apical 4- and 2-chamber views. Diastolic function was assessed according to the EACVI recommendations on diastolic function [13,14,15,16,17,18,19]. LA volume was measured using the modified Simpson biplane method and was indexed to the body surface to obtain the LA volume index (LAVi). Early diastolic (E) and late diastolic (A) velocities were assessed by trans-mitral pulsed wave Doppler to calculate the E/A ratio; septal and lateral peak systolic (S’), early diastolic (E’), and late diastolic (A’) annular velocities were obtained by Tissue Doppler Imaging (TDI). The mean E/E’ ratio was calculated and used as an index of the LV filling pressure. The maximum velocity of tricuspid regurgitation was sampled to estimate systolic pulmonary pressure (sPAP) and assess the degree of diastolic dysfunction in addition to other parameters. Right ventricular function was assessed by tricuspid annular plane systolic excursion (TAPSE) and by TDI (peak systolic S’).
Speckle-Tracking Echocardiography (STE)
STE analysis was performed offline to assess GLS and LA strain using semiautomated 2D Strain GE software (Echopac V.202, GE Healthcare, Horten, Norway, version for LA strain analysis: 2.02—release 34.0). The operators analyzing the images were not aware of the clinical characteristics of the patients. The GLS was obtained by recording apical 2-, 3-, and 4-chamber views with a frame rate between 60 and 80 fps and a stable electrocardiographic trace. The analysis was performed offline by two experienced and independent echocardiographers. Normal reference values of GLS were considered: −21.5 ± 2%, with a lower limit of normal of—18% [20,21]. For the evaluation of LA strain, STE analysis was performed on apical 2- and 4-chamber views optimized in terms of orientation, depth, and gain to avoid LA foreshortening and to visualize the entire LA throughout the cardiac cycle. The LA border was traced manually in these views, delineating a region of interest (ROI). The software divided the ROI into 6 segments and automatically calculated the LA reservoir strain (PALS—peak atrial longitudinal strain), peak atrial conduit strain, and peak atrial contraction strain (PACS). Indeed, the LA strain can be used to measure all LA functions during the cardiac cycle. QRS was used as the starting point to measure LA strain. LA strain was usually calculated as the average of all LA segments in 4- and 2-chamber views; only in a few patients with a non-optimal view of the left atrium in the 2-chamber view, it was calculated as the average of the segments in the 4-chamber view. The left atrial stiffness index (LASi) was obtained from the ratio between the E/E’ average and PALS. The following values were considered as reference ranges, according to the EACVI NORRE study [22,23]:
- ○
- PALS: 42.5% (IQR 36.1–48.0%), LLN 26.1 ± 0.7%
- ○
- LA conduit strain: −25.7% (IQR 20.4–31.8%), LLN—12.0 ± 0.5%
- ○
- PACS: −16.3% (IQR 12.9–19.5%), LLN—7.7 ± 0.3%
- ○
- LASi:
- 0.12 (IQR 0.10–0.15) for ages 20–40, with LLN 0.22 ± 0.01
- 0.13–0.22) for ages 40–60, with LLN 0.42 ± 0.04
- 0.24 (0.18–0.29) for age > 60 years, with LLN 0.55 ± 0.09
2.5. Statistical Analysis
Continuous variables were expressed as means and standard deviations or medians with interquartile ranges as appropriate. The Student’s t-test or, when necessary, the Mann–Whitney test was used to compare the two groups. Categorical variables were evaluated based on percentages of the total population and compared using the χ2 test and Fisher’s exact test. A one-way ANOVA test was used to determine if there was a statistically significant difference between the means of three or more groups. After adjustment for lack of sphericity in repeated measures, the ANOVA was performed with the Greenhouse–Geisser correction. ROC analysis was performed to evaluate the predictive ability of the percentage reduction in LA strain value between T1 and T0 on the development of subclinical dysfunction, as assessed by a reduction in GLS > 15% from T0 to T1.
A two-tailed p-value < 0.05 was considered statistically significant. Bonferroni’s correction was used to correct the significance of multiple tests. All the statistical analyses were performed using RStudio software (version 1.4.1103 2009–2021 RStudio).
3. Results
After collecting and analyzing data from the different centers involved, the “total study population” consisted of 169 patients (100% women, mean age: 55 ± 10.8 years) with newly diagnosed breast cancer, “HER2 receptor-positive”. According to the TNM classification, most patients were in stage IIA. More precisely, the anatomical classification according to TNM is shown in Table 1. As regards baseline cardiovascular risk factors, 22% (37 patients) had diabetes mellitus, 34% (57 patients) had arterial hypertension, 29% (50 patients) had obesity, and 33% (55 patients) were smokers. The hypertensive patients were treated with angiotensin-converting enzyme (ACE) inhibitors/angiotensin receptor blockers (ARBs), beta-blockers, or calcium channel blockers. Other basic clinical data of the study population are summarized in Table 1.

Table 1.
General characteristics of the study population and subgroups.
As for echocardiographic parameters at baseline, our cohort showed normal values of LVEF (60 ± 1.7%), GLS (−20.7 ± 2.1%), PALS (36 ± 8.9%), and normal values of LAVi and normal right ventricular function. Regarding the assessment of diastolic function in all the population at baseline, we found that at T0, 37% of patients had a normal diastolic function, 62% had grade I diastolic dysfunction, and only 1 patient had grade II diastolic dysfunction; no patients had grade 3 diastolic dysfunction. Table 2 summarizes key echocardiographic data from the total study population at baseline and during follow-up.

Table 2.
Echocardiographic characteristics of the study population at baseline and during monitoring.
3.1. Follow-Up
In the whole population after 3 (T1) and 6 (T2) months of anti-cancer therapy, we did not observe a significant reduction in LVEF (60 ± 1.7 vs. 59.7 ± 2.8 vs. 59 ± 3.4; p-value > 0.05). Analyzing diastolic function, the percentages of diastolic dysfunction present at baseline (T0) remained unchanged at T1; at T2, only 1 patient had grade II diastolic dysfunction, 74% of the patients had grade I diastolic dysfunction, and 25% of the patients had no diastolic dysfunction. In addition, during the follow-up, the E/A ratio changed significantly (p-value = 0.01), but the mean E/E’ ratio (p-value = 0.3) and pulmonary systolic pressure did not change significantly (p-value = 0.4); see Table 2 and Figure 1. Conversely, all STE parameters changed significantly during the follow-up at T1 and T2. We observed a significant reduction in GLS (−20.7 ± 2.1 vs. −19.2 ± 2.5 vs. −18.9 ± 2.4 p-value < 0.0001) and in PALS (4-chamber LA reservoir strain: 37 ± 9.3 vs. 32.7 ± 9 vs. 29.6 ± 7.6, p ≤ 0.0001; 2-chamber LA reservoir strain: 36 ± 9.1 vs. 32 ± 7.7 vs. 27.8 ± 5.9, p = 0.0003; and average LA reservoir strain: 36 ± 8.9 vs. 32.4 ± 7.7 vs. 28.7 ± 6.3, p = 0.0002). Furthermore, LASi worsened during follow-up (0.19 ± 0.07 vs. 0.25 ± 0.08 vs. 0.30 ± 0.09; p < 0.0001); see Table 2. We also found a significant increase in LAVi during follow-up (p-value = 0.03). We evaluated the development of CTRCD during follow-up, and we found that no patients developed symptomatic CTRCD or severe asymptomatic CTRCD. One patient developed moderate asymptomatic CTRCD at T1, and two patients developed moderate asymptomatic CTRCD at T2. A total of 28 patients (17% of our total population) developed asymptomatic mild CTRCD.
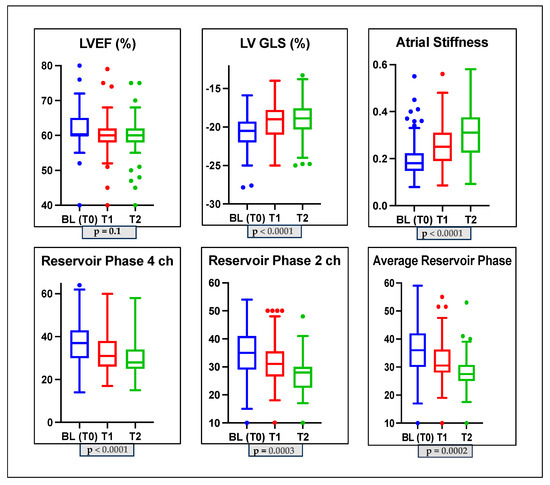
Figure 1.
Box plots showing echocardiographic characteristics of the study population at baseline and during monitoring. LVGLS: left ventricle global longitudinal strain; LVEF: left ventricle ejection fraction; 2CH: two-chamber; 4CH: four-chamber.
3.2. Patients with and without Mild CTRCD
In Table 3 and Table 4, the main echocardiographic changes that occurred in patients with mild asymptomatic CTRCD (Group 1) and without CTRCD (Group 2), respectively, are summarized whose baseline characteristics are highlighted in Table 1.

Table 3.
Echocardiographic characteristics in the subgroup of patients with asymptomatic mild CTRCD (Group 1).

Table 4.
Echocardiographic characteristics in the subgroup of patients without asymptomatic mild CTRCD (Group 2).
Specifically, we found in Group 1 a non-significant reduction in LVEF during the follow-up (p-value = 0.30); a significant reduction in GLS over time (p-value < 0.0001); a significant reduction in PALS (4-ch: 37.5 ± 7.6 vs. 30.4 ± 8.3 vs. 29.3 ± 7.3 p-value < 0.0001; 2-ch: 35.5 ± 9.1 vs. 27.3 ± 4.8 vs. 26 ± 4.6, p-value = 0.05; average PALS: 35.9 ± 8.7 vs. 27.3 ± 4.4 vs. 25.6 ± 4.4 p-value = 0.029); and a significant increase in LASi (0.21 ± 0.06 vs. 0.29 ± 0.09 vs. 0.32 ± 0.08 p-value < 0.0001). The LAVi and mean E/e’ ratio did not change significantly (p-value > 0.05); the E/A ratio changed significantly (p-value = 0.04); see Table 3 and Figure 2.
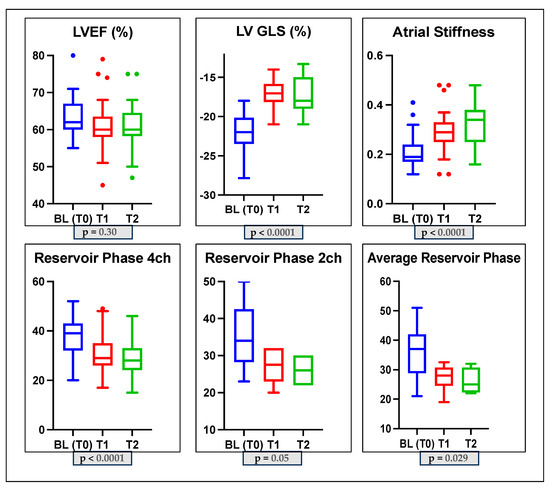
Figure 2.
Box plots showing echocardiographic characteristics of the subclinical dysfunction group (Group 1) at baseline and during monitoring. LVGLS: left ventricle global longitudinal strain; LVEF: left ventricle ejection fraction; 2CH: two-chamber; 4CH: four-chamber.
Analyzing Group 2 (patients without asymptomatic mild CTRCD), we did not find significant changes in LVEF. We also observed significant changes in GLS (p-value = 0.0030), PALS (p-value < 0.0001), LASi (p-value < 0.0001), and mean E/e’ ratio (p-value = 0.01; see Table 4). However, it is worthy of notice that impairment of ventricular and atrial function was greater in Group 1 compared to Group 2 over time (Table 5).

Table 5.
LVEF, GLS, atrial strain, and atrial stiffness changes in Group 1 compared to Group 2.
In particular, we found a 20% reduction in PALS in Group 1 at T1 compared to baseline (Table 5). This reduction could be used as a cut-off to delimit patients who are more likely to develop subclinical cardiac dysfunction, as assessed by a relative reduction in GLS > 15% while taking anticancer drugs. In fact, we found in the ROC curve analysis that PALS variation > 20.8% was fairly accurate in identifying patients who were most likely to develop asymptomatic mild cardiotoxicity [AUC 0.62; CI (0.51–0.73) p = 0.06, sensitivity 45%, specificity 69.5%].
In addition, when comparing the two groups at baseline, we found no significant differences in baseline PALS value or other echocardiographic parameters; only the baseline GLS and mean E/e’ ratio differed significantly but remained within normal values (Table 6).

Table 6.
Echocardiographic characteristics at baseline in the 2 subgroups.
4. Discussion
The present multicenter study was one of the first to evaluate LA function by STE in cardio-oncology. Our study showed the cardiotoxic effects of anthracyclines and trastuzumab on atrial function evaluated by STE, and it identified a percent change in PALS able to detect patients at higher risk of CTRCD. In our study, GLS and PALS changed significantly in all the patients treated with anti-cancer drugs, LASi increased significantly, and LVEF did not change during follow-up. Thus, in agreement with the literature data, our study reconfirms the ability of deformation imaging parameters such as GLS to identify signs of cardiotoxicity. In fact, GLS is more sensitive than LVEF and other echocardiographic parameters such as TDI for detecting early cardiac damage [24]. For a long time, LVEF has been used as the only tool for diagnosing cardiotoxicity. Beyond LVEF, research has moved towards finding new parameters that could help predict subclinical dysfunction. Among the methods developed in the search for subclinical cardiotoxicity, STE is certainly one of the most interesting and promising, because it allows a post-processing evaluation through the use of dedicated software [25]. Furthermore, the low costs, the reduced intra- and inter-operator variability (inter-examination variability of about 6% for GLS versus 10% for LVEF), together with the standardization of cut-off values and measurement methods promoted by the main scientific societies (EACVI, ASE, ESC) and by the manufacturers have allowed the considerable development of this method and its wide diffusion in echocardiography laboratories [26]. Myocardial work and other echocardiographic parameters are also emerging as early markers of cardiotoxicity [27,28,29]. Few studies have evaluated LA strain as a marker of early cardiac dysfunction without clear results on the usefulness of this parameter in predicting cardiotoxicity [30]. A study in the adult cancer population found no significant changes in LA strain during anthracycline chemotherapy [31]. On the other hand, another study found a significant and frequent reduction in LA strain after doxorubicin therapy and even suggested LA strain as being a more sensitive marker than LVEF or GLS in detecting CTRCD [32,33]. In particular, Laufer-Perl et al. showed that reduction in LA reservoir strain and LA conduit strain is frequent and occurs early in the course of anthracycline therapy, showing a significant correlation with routine echocardiographic diastolic parameters [32]. Other studies in cardio-oncology have shown significant changes in diastolic function; abnormal or worsening diastolic function precedes systolic dysfunction, and it is associated with an increased risk of CTRCD in patients with breast cancer [34]. GLS and LA strain are closely related parameters. The evaluation of left ventricular function cannot be separated from the evaluation of atrial function. Indeed, the LA function modulates LV filling and plays an important role in the diastolic phase of the cardiac cycle, while the LV function affects LA contraction and relaxation. Thus, changes in the intracavitary pressures in one chamber also affect the other [35]. In our study, we observed in all the patients a reduction in GLS and PALS and an increase in LASi in the presence of normal LVEF and normal left ventricular filling pressures. The changes in PALS and LASi were greater in the patients with CTRCD. A reduction in PALS > 20.8% (assessed only in the 4-chamber view) has been identified as a cut-off to detect patients at higher risk of CTRCD, with the best sensitivity and specificity. Decreased GLS and worsening LVEF are known to be associated with increased left ventricular filling pressures and decreased PALS. PALS has many advantages: it is angle-independent, overcoming Doppler limitations, and it provides a reproducible measurement of the LA deformation. It also helps to define indeterminate cases of diastolic dysfunctions [36]. In addition, the LA function can be considered a reliable index to estimate the diastolic function of the left ventricle [37]. Moreover, it is a strong predictor of functional capacity and an important prognostic marker in various diseases such as atrial fibrillation, mitral regurgitation, and HFpEF [38,39,40]. We believe that cardiotoxicity induced by antineoplastic drugs is a comprehensive phenomenon involving, contemporaneously, the whole heart (either ventricle or atria); accordingly, we detected by speckle-tracking analysis an impairment of either atrial or ventricular function. According to our results, LA function assessed by STE could be used, not as a substitute but as an adjunctive parameter to GLS in the assessment of patients undergoing treatment with anti-cancer drugs to identify early signs of cardiotoxicity. The main limitation of our study was the short follow-up time, which did not allow us to establish the real prognostic power of the detected left atrial function abnormalities in terms of LVEF decline and the occurrence of adverse events. A second limitation of the study was the low-risk profile of the study population, which, however, was an inclusion criterion. Another limitation of our study was the lack of determination of biomarkers to assess cardiotoxicity, due to the variability of the methods used for determination in the various centers. In addition, other limitations were the use of non-dedicated software for the analysis of LA strain and the use of the same software for the left ventricle. Moreover, for the PALS assessment, we employed QRS, and not P wave, as the reference point given that it represents the most used method.
5. Conclusions
Our multicenter study highlights the usefulness of LA function assessment using STE in women with breast cancer treated with chemotherapy because atrial function also changes significantly during anticancer treatment. Particularly in our study, the impairment of LA strain was significantly higher in patients who developed asymptomatic mild CTRCD. A cut-off of reduction in PALS of 20.8% was identified to detect patients at higher risk of cardiotoxicity. Thus, the assessment of PALS in addition to GLS could be considered to discover subclinical cardiotoxicity more accurately. In daily clinical practice, the use of this parameter could help in identifying cancer patients with a greater risk of cardiotoxicity to more precisely manage cardioprotective therapy and design tailored cardiological follow-ups.
Author Contributions
Conceptualization, G.N., M.C. and R.M.; methodology, G.N., M.C., R.M., C.Z., C.B., C.C.D., G.C. and A.M.; software, D.D.L. and C.M.; formal analysis; C.M., M.C. and R.M.; investigation, G.N., C.Z., C.B., C.C.D., G.C. and A.M.; resources, G.N., M.C., R.M., C.Z., C.B., C.C.D., G.C. and A.M.; data curation, C.M.; writing—original draft preparation, D.D.L. and G.N.; writing—review and editing, G.N., D.D.L., M.C., R.M., C.Z., C.B., C.C.D., G.C. and A.M.; supervision, G.N., M.C., R.M., C.Z., C.B., C.C.D., G.C. and A.M.; project administration, G.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Palermo Ethics Committee 1 A.O.U.P. “P. Giaccone” of University of Palermo (Report no. 5; Approval Date: 20 May 2020).
Informed Consent Statement
Not applicable.
Data Availability Statement
Data will be available upon reasonable request.
Acknowledgments
Thanks to Rita Cristina Myriam Intravaia, Roberta Manganaro, Girolamo Manno, Giulia Passavanti, Ludovico Rossetto, Luigi Rubino, and Oreste Fabio Triolo for their contribution to collecting data.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zamorano, J.L.; Gottfridsson, C.; Asteggiano, R.; Atar, D.; Badimon, L.; Bax, J.J.; Cardinale, D.; Cardone, A.; Feijen, E.A.M.; Ferdinandy, P.; et al. The cancer patient and cardiology. Eur. J. Heart Fail. 2020, 22, 2290–2309. [Google Scholar] [CrossRef]
- Morelli, M.B.; Bongiovanni, C.; Da Pra, S.; Miano, C.; Sacchi, F.; Lauriola, M.; D’Uva, G. Cardiotoxicity of Anticancer Drugs: Molecular Mechanisms and Strategies for Cardioprotection. Front. Cardiovasc. Med. 2022, 9, 847012. [Google Scholar] [CrossRef]
- Novo, G.; Santoro, C.; Manno, G.; Di Lisi, D.; Esposito, R.; Mandoli, G.E.; Evola, V.; Pastore, M.C.; Sperlongano, S.; D’Andrea, A.; et al. Usefulness of Stress Echocardiography in the Management of Patients Treated with Anticancer Drugs. J. Am. Soc. Echocardiogr. 2021, 34, 107–116. [Google Scholar] [CrossRef]
- López-Sendón, J.; Álvarez-Ortega, C.; Zamora Auñon, P.; Buño Soto, A.; Lyon, A.R.; Farmakis, D.; Cardinale, D.; Canales Albendea, M.; Feliu Batlle, J.; Rodríguez, I.; et al. Classification, prevalence, and outcomes of anticancer therapy-induced cardiotoxicity: The CARDIOTOX registry. Eur. Heart J. 2020, 41, 1720–1729. [Google Scholar] [CrossRef]
- Zamorano, J.L.; Lancellotti, P.; Rodriguez Muñoz, D.; Aboyans, V.; Asteggiano, R.; Galderisi, M.; Habib, G.; Lenihan, D.J.; Lip, G.Y.H.; Lyon, A.R.; et al. 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: The Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). Eur. Heart J. 2016, 37, 2768–2801. [Google Scholar] [CrossRef]
- Herrmann, J.; Lenihan, D.; Armenian, S.; Barac, A.; Blaes, A.; Cardinale, D.; Carver, J.; Dent, S.; Ky, B.; Lyon, A.R.; et al. Defining cardiovascular toxicities of cancer therapies: An International Cardio-Oncology Society (IC-OS) consensus statement. Eur. Heart J. 2022, 43, 280–299. [Google Scholar] [CrossRef]
- Lyon, A.R.; López-Fernández, T.; Couch, L.S.; Asteggiano, R.; Aznar, M.C.; Bergler-Klein, J.; Boriani, G.; Cardinale, D.; Cordoba, R.; Cosyns, B.; et al. 2022 ESC Guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS). Eur. Heart J. Cardiovasc. Imaging 2022, 23, e333–e465. [Google Scholar] [CrossRef]
- Leszek, P.; Klotzka, A.; Bartuś, S.; Burchardt, P.; Czarnecka, A.M.; Długosz-Danecka, M.; Gierlotka, M.; Koseła-Paterczyk, H.; Krawczyk-Ożóg, A.; Kubiatowski, T.; et al. A practical approach to the ESC 2022 cardio-oncology guidelines. Comments by a team of experts: Cardiologists and oncologists. Kardiol. Pol. 2023. Epub ahead of print. [Google Scholar] [CrossRef]
- Cardinale, D.; Colombo, A.; Bacchiani, G.; Tedeschi, I.; Meroni, C.A.; Veglia, F.; Civelli, M.; Lamantia, G.; Colombo, N.; Curigliano, G.; et al. Early detection of anthracycline cardiotoxicity and improvement with heart failure therapy. Circulation 2015, 131, 1981–1988. [Google Scholar] [CrossRef]
- Negishi, K.; Negishi, T.; Hare, J.L.; Haluska, B.A.; Plana, J.C.; Marwick, T.H. Independent and incremental value of deformation indices for prediction of trastuzumab-induced cardiotoxicity. J. Am. Soc. Echocardiogr. 2013, 26, 493–498. [Google Scholar] [CrossRef]
- Cardinale, D.; Sandri, M.T.; Colombo, A.; Colombo, N.; Boeri, M.; Lamantia, G.; Civelli, M.; Peccatori, F.; Martinelli, G.; Fiorentini, C.; et al. Prognostic value of troponin I in cardiac risk stratification of cancer patients undergoing high-dose chemotherapy. Circulation 2004, 109, 2749–2754. [Google Scholar] [CrossRef]
- Cardinale, D.; Colombo, A.; Torrisi, R.; Sandri, M.T.; Civelli, M.; Salvatici, M.; Lamantia, G.; Colombo, N.; Cortinovis, S.; Dessanai, M.A.; et al. Trastuzumab-induced cardiotoxicity: Clinical and prognostic implications of troponin I evaluation. J. Clin. Oncol. 2010, 28, 3910–3916. [Google Scholar] [CrossRef]
- Oikonomou, E.K.; Kokkinidis, D.G.; Kampaktsis, P.N.; Amir, E.A.; Marwick, T.H.; Gupta, D.; Thavendiranathan, P. Assessment of Prognostic Value of Left Ventricular Global Longitudinal Strain for Early Prediction of Chemotherapy-Induced Cardiotoxicity: A Systematic Review and Meta-analysis. JAMA Cardiol. 2019, 4, 1007–1018. [Google Scholar] [CrossRef]
- Liu, J.E.; Barac, A.; Thavendiranathan, P.; Scherrer-Crosbie, M. Strain Imaging in Cardio-Oncology. JACC CardioOncol. 2020, 2, 677–689. [Google Scholar] [CrossRef]
- Smiseth, O.A.; Morris, D.A.; Cardim, N.; Cikes, M.; Delgado, V.; Donal, E.; Flachskampf, F.A.; Galderisi, M.; Gerber, B.L.; Gimelli, A.; et al. Multimodality imaging in patients with heart failure and preserved ejection fraction: An expert consensus document of the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc Imaging 2022, 23, e34–e61. [Google Scholar] [CrossRef]
- Inoue, K.; Khan, F.H.; Remme, E.W.; Ohte, N.; García-Izquierdo, E.; Chetrit, M.; Moñivas-Palomero, V.; Mingo-Santos, S.; Andersen, Ø.S.; Gude, E.; et al. Determinants of left atrial reservoir and pump strain and use of atrial strain for evaluation of left ventricular filling pressure. Eur. Heart J. Cardiovasc. Imaging 2021, 23, 61–70. [Google Scholar] [CrossRef]
- Mandoli, G.E.; Pastore, M.C.; Benfari, G.; Setti, M.; Maritan, L.; Diviggiano, E.E.; D’Ascenzi, F.; Focardi, M.; Cavigli, L.; Valente, S.; et al. The loss of left atrial contractile function predicts a worse outcome in HFrEF patients. Front. Cardiovasc. Med. 2023, 9, 1079632. [Google Scholar] [CrossRef]
- Bandera, F.; Martone, R.; Chacko, L.; Ganesananthan, S.; Gilbertson, J.A.; Ponticos, M.; Lane, T.; Martinez-Naharro, A.; Whelan, C.; Quarta, C.; et al. Clinical Importance of Left Atrial Infiltration in Cardiac Transthyretin Amyloidosis. JACC Cardiovasc. Imaging 2022, 15, 17–29. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 233–270. [Google Scholar] [CrossRef]
- Galderisi, M.; Cosyns, B.; Edvardsen, T.; Cardim, N.; Delgado, V.; Di Salvo, G.; Donal, E.; Sade, L.E.; Ernande, L.; Garbi, M.; et al. Standardization of adult transthoracic echocardiography reporting in agreement with recent chamber quantification, diastolic function, and heart valve disease recommendations: An expert consensus document of the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2017, 18, 1301–1310. [Google Scholar]
- Nyberg, J.; Jakobsen, E.O.; Østvik, A.; Holte, E.; Stølen, S.; Lovstakken, L.; Grenne, B.; Dalen, H. Echocardiographic Reference Ranges of Global Longitudinal Strain for All Cardiac Chambers Using Guideline-Directed Dedicated Views. JACC Cardiovasc. Imaging 2023. Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Cameli, M.; Caputo, M.; Mondillo, S.; Ballo, P.; Palmerini, E.; Lisi, M.; Marino, E.; Galderisi, M. Feasibility and reference values of left atrial longitudinal strain imaging by two-dimensional speckle tracking. Cardiovasc. Ultrasound 2009, 7, 6. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, T.; Robinet, S.; Dulgheru, R.; Bernard, A.; Ilardi, F.; Contu, L.; Addetia, K.; Caballero, L.; Kacharava, G.; Athanassopoulos, G.D.; et al. Echocardiographic reference ranges for normal left atrial function parameters: Results from the EACVI NORRE study. Eur. Heart J. Cardiovasc. Imaging 2018, 19, 630–638. [Google Scholar] [CrossRef] [PubMed]
- Thavendiranathan, P.; Poulin, F.; Lim, K.D.; Plana, J.C.; Woo, A.; Marwick, T.H. Use of myocardial strain imaging by echocardiography for the early detection of cardiotoxicity in patients during and after cancer chemotherapy: A systematic review. J. Am. Coll Cardiol. 2014, 63 (25 Pt A), 2751–2768. [Google Scholar] [CrossRef] [PubMed]
- Santoro, C.; Arpino, G.; Esposito, R.; Lembo, M.; Paciolla, I.; Cardalesi, C.; de Simone, G.; Trimarco, B.; De Placido, S.; Galderisi, M. 2D and 3D strain for detection of subclinical anthracycline cardiotoxicity in breast cancer patients: A balance with feasibility. Eur. Heart J. Cardiovasc. Imaging 2017, 18, 930–936. [Google Scholar] [CrossRef]
- Voigt, J.U.; Pedrizzetti, G.; Lysyansky, P.; Marwick, T.H.; Houle, H.; Baumann, R.; Pedri, S.; Ito, Y.; Abe, Y.; Metz, S.; et al. Definitions for a common standard for 2D speckle tracking echocardiography: Consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. J. Am. Soc. Echocardiogr. 2015, 28, 183–193. [Google Scholar] [CrossRef]
- Calvillo-Argüelles, O.; Thampinathan, B.; Somerset, E.; Shalmon, T.; Amir, E.; Steve Fan, C.P.; Moon, S.; Abdel-Qadir, H.; Thevakumaran, Y.; Day, J.; et al. Diagnostic and Prognostic Value of Myocardial Work Indices for Identification of Cancer Therapy-Related Cardiotoxicity. JACC Cardiovasc. Imaging 2022, 15, 1361–1376. [Google Scholar] [CrossRef]
- Di Lisi, D.; Manno, G.; Novo, G. Subclinical Cardiotoxicity: The Emerging Role of Myocardial Work and Other Imaging Techniques. Curr. Probl. Cardiol. 2021, 46, 100818. [Google Scholar] [CrossRef]
- Novo, G.; Di Lisi, D.; Manganaro, R.; Manno, G.; Lazzara, S.; Immordino, F.A.; Madaudo, C.; Carerj, S.; Russo, A.; Incorvaia, L.; et al. Arterial Stiffness: Effects of Anticancer Drugs Used for Breast Cancer Women. Front. Physiol. 2021, 12, 661464. [Google Scholar] [CrossRef]
- Timóteo, A.T.; Moura Branco, L.; Filipe, F.; Galrinho, A.; Rio, P.; Portugal, G.; Oliveira, S.; Ferreira, R.C. Cardiotoxicity in breast cancer treatment: What about left ventricular diastolic function and left atrial function? Echocardiography. 2019, 36, 1806–1813. [Google Scholar] [CrossRef]
- Anqi, Y.; Yu, Z.; Mingjun, X.; Xiaoli, K.; Mengmeng, L.; Fangfang, L.; Mei, Z. Use of echocardiography to monitor myocardial damage during anthracycline chemotherapy. Echocardiography 2019, 36, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Laufer-Perl, M.; Arias, O.; Dorfman, S.S.; Baruch, G.; Rothschild, E.; Beer, G.; Hasson, S.P.; Arbel, Y.; Rozenbaum, Z.; Topilsky, Y.; et al. Left Atrial Strain changes in patients with breast cancer during anthracycline therapy. Int. J. Cardiol. 2021, 330, 238–244. [Google Scholar] [PubMed]
- Emerson, P.; Stefani, L.; Boyd, A.; Richards, D.; Hui, R.; Altman, M.; Thomas, L. Alterations in Left Atrial Strain in Breast Cancer Patients Immediately Post Anthracycline Exposure. Heart Lung Circ. 2023. [Google Scholar] [CrossRef] [PubMed]
- Upshaw, J.N.; Finkelman, B.; Hubbard, R.A.; Smith, A.M.; Narayan, H.K.; Arndt, L.; Domchek, S.; DeMichele, A.; Fox, K.; Shah, P. Comprehensive Assessment of Changes in Left Ventricular Diastolic Function with Contemporary Breast Cancer Therapy. JACC Cardiovasc. Imaging 2020, 13 (1 Pt 2), 198–210. [Google Scholar] [CrossRef] [PubMed]
- Henein, M.Y.; Gibson, D.G. Normal long axis function. Heart 1999, 81, 111–113. [Google Scholar] [CrossRef]
- Xu, L.; Jiang, C.Q.; Lam, T.H.; Yue, X.J.; Lin, J.M.; Cheng, K.K.; Liu, B.; Li Jin, Y.; Zhang, W.S.; Thomas, G.N. Arterial stiffness and left-ventricular diastolic dysfunction: Guangzhou Biobank Cohort Study-CVD. J. Hum. Hypertens 2011, 25, 152–158. [Google Scholar] [CrossRef][Green Version]
- Cameli, M.; Lisi, M.; Righini, F.M.; Mondillo, S. Novel echocardiographic techniques to assess left atrial size, anatomy and function. Cardiovasc. Ultrasound 2012, 10, 4. [Google Scholar] [CrossRef]
- Parkash, R.; Green, M.S.; Kerr, C.R.; Connolly, S.J.; Klein, G.J.; Sheldon, R.; Talajic, M.; Dorian, P.; Humphries, K.H. The association of left atrial size and occurrence of atrial fibrillation: A prospective cohort study from the Canadian Registry of Atrial Fibrillation. Am. Heart J. 2004, 148, 649–654. [Google Scholar] [CrossRef]
- Rusinaru, D.; Tribouilloy, C.; Grigioni, F.; Avierinos, J.F.; Suri, R.M.; Barbieri, A.; Szymanski, C.; Ferlito, M.; Michelena, H.; Tafanelli, L. Left atrial size is a potent predictor of mortality in mitral regurgitation due to flail leaflets: Results from a large international multicenter study. Circ. Cardiovasc. Imaging 2011, 4, 473–481. [Google Scholar] [CrossRef]
- Ratanasit, N.; Karaketklang, K.; Chirakarnjanakorn, S.; Krittayaphong, R.; Jakrapanichakul, D. Left atrial volume as an independent predictor of exercise capacity in patients with isolated diastolic dysfunction presented with exertional dyspnea. Cardiovasc. Ultrasound 2014, 12, 19. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).