Free-Field Hearing Test in Noise with Free Head Rotation for Evaluation of Monaural Hearing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Population
2.2. Study Materials
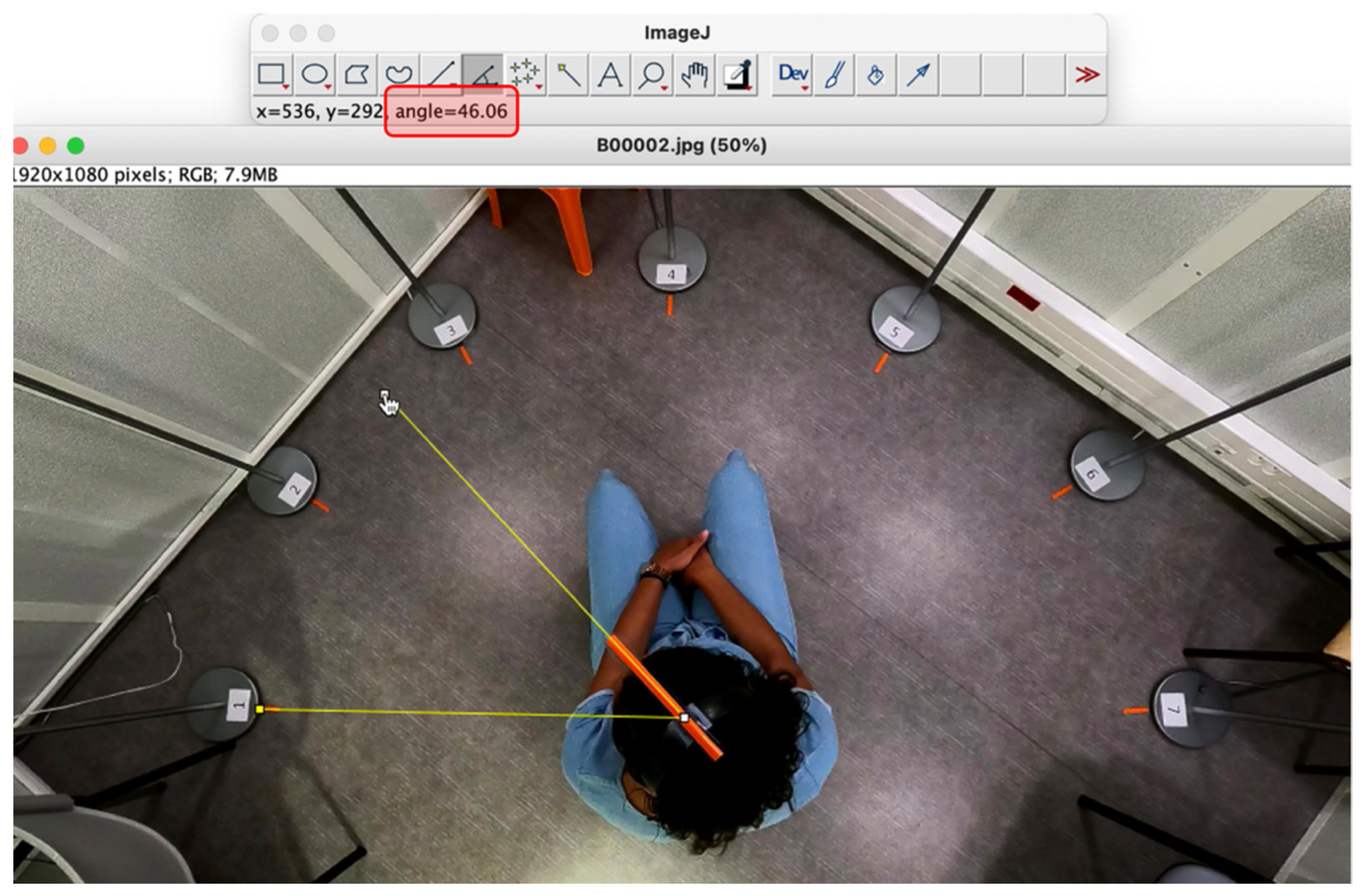
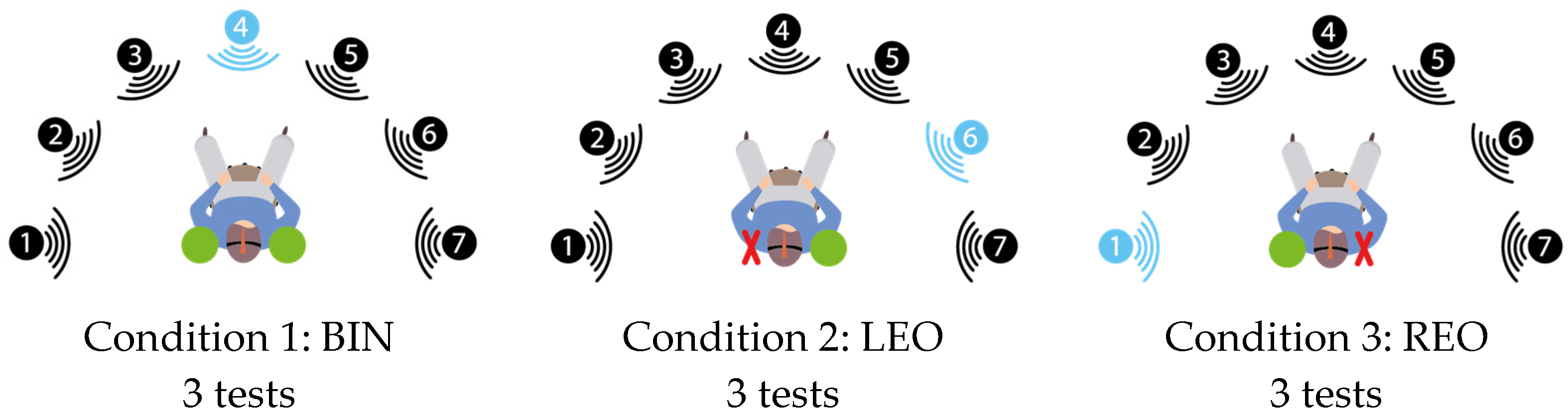
2.3. Study Process
- The subject started facing the central speaker (no. 4) and the noise was activated.
- The signal was activated, and head position recording began;
- The subject was then asked to rotate into the most comfortable position for optimal discrimination. In this search phase, as many sentences as necessary were presented, with a 5 s delay between each presentation;
- The subject then maintained his head position until the end of the series and repeated the next 10 sentences;
- At the end of the series, the subject was asked to give the number of the loudspeaker identified as the source of the signal.
- -
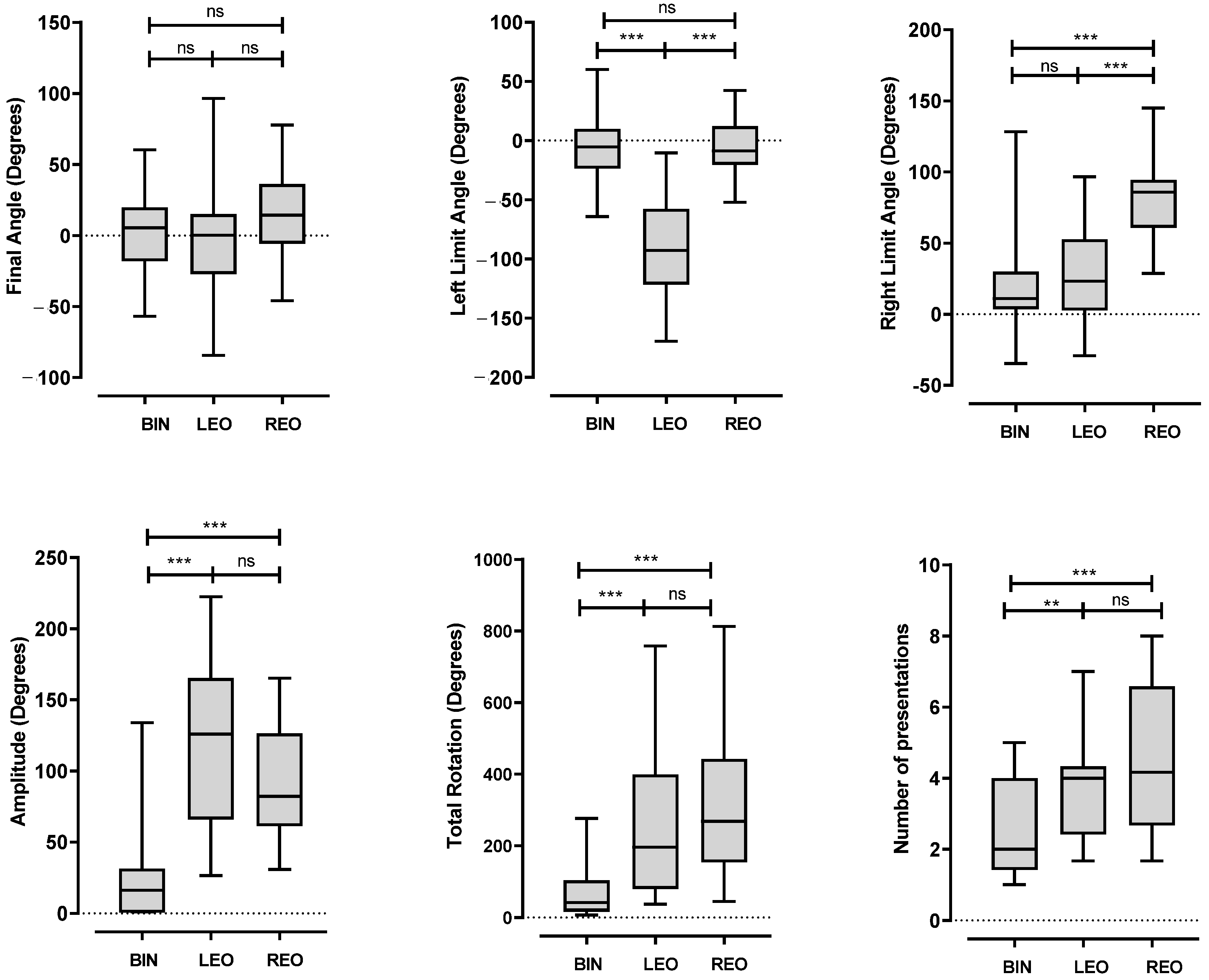
- During the exploration phase: the sum of rotations (in degrees), right and left boundary angles (relative to the signal azimuth, in degrees), amplitude of rotations (between right and left boundary angles, in degrees), final angle (relative to the signal azimuth, in degrees), and number of sentences required for the subject to find a comfortable listening position (number of presentations).
- -
- After the exploration phase: the number of correctly repeated sentences (discrimination score out of 10) and the offset between the speaker identified by the subject as the source of the signal and the signal speaker.
2.4. Assessment Criteria
2.5. Statistics
3. Results
3.1. Population
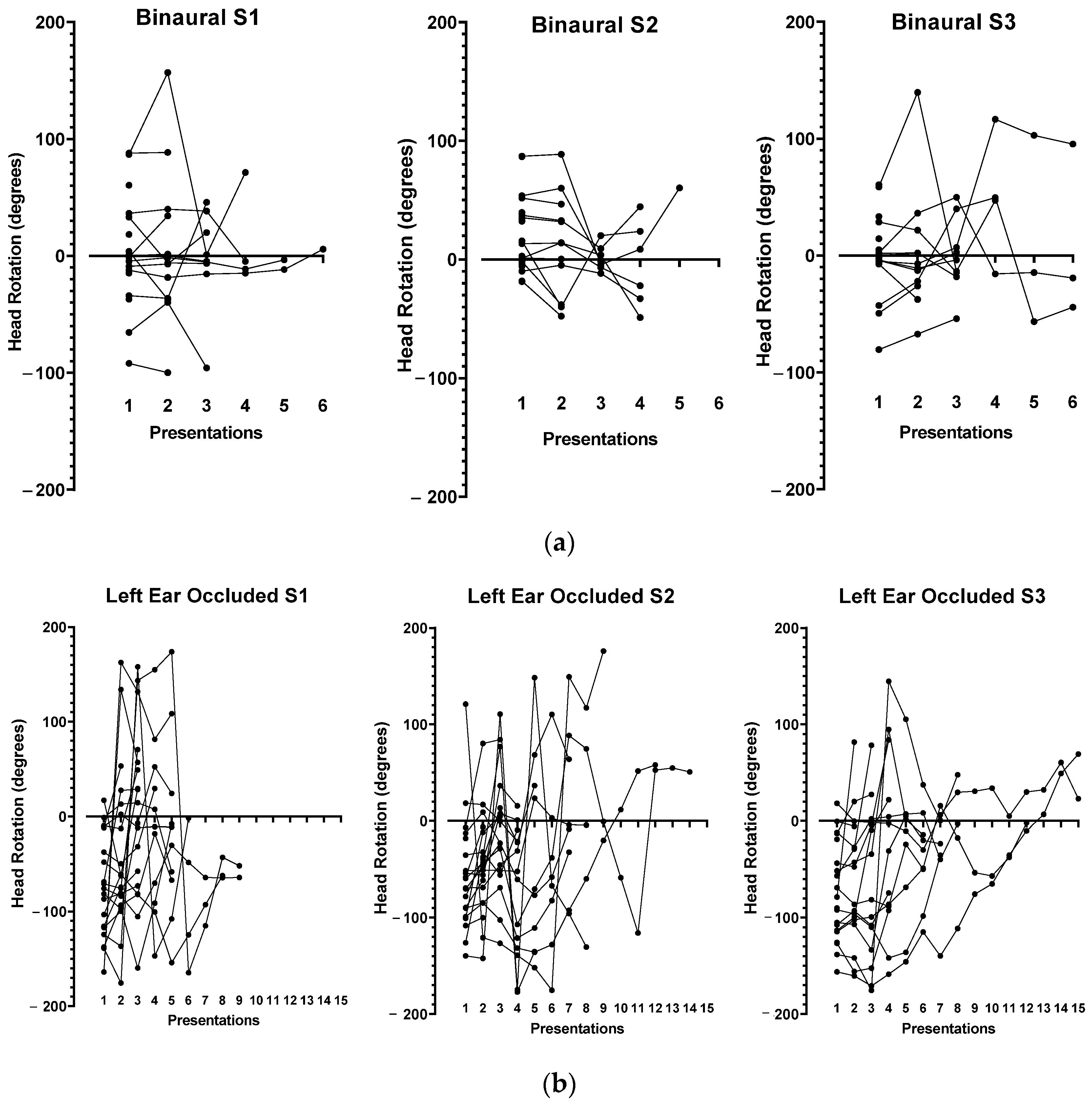
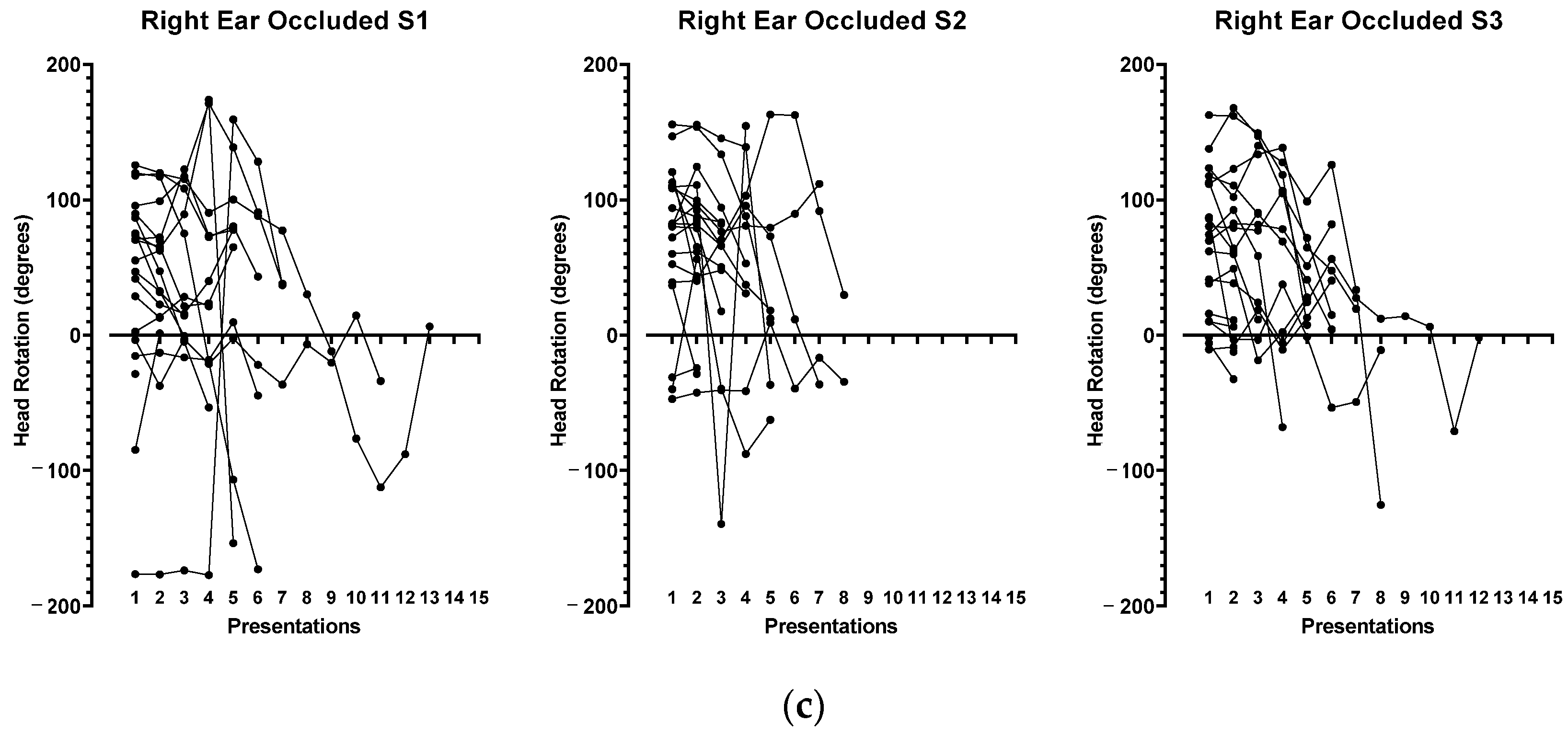
3.2. Head Position
3.3. Hearing Performances
3.4. Localization
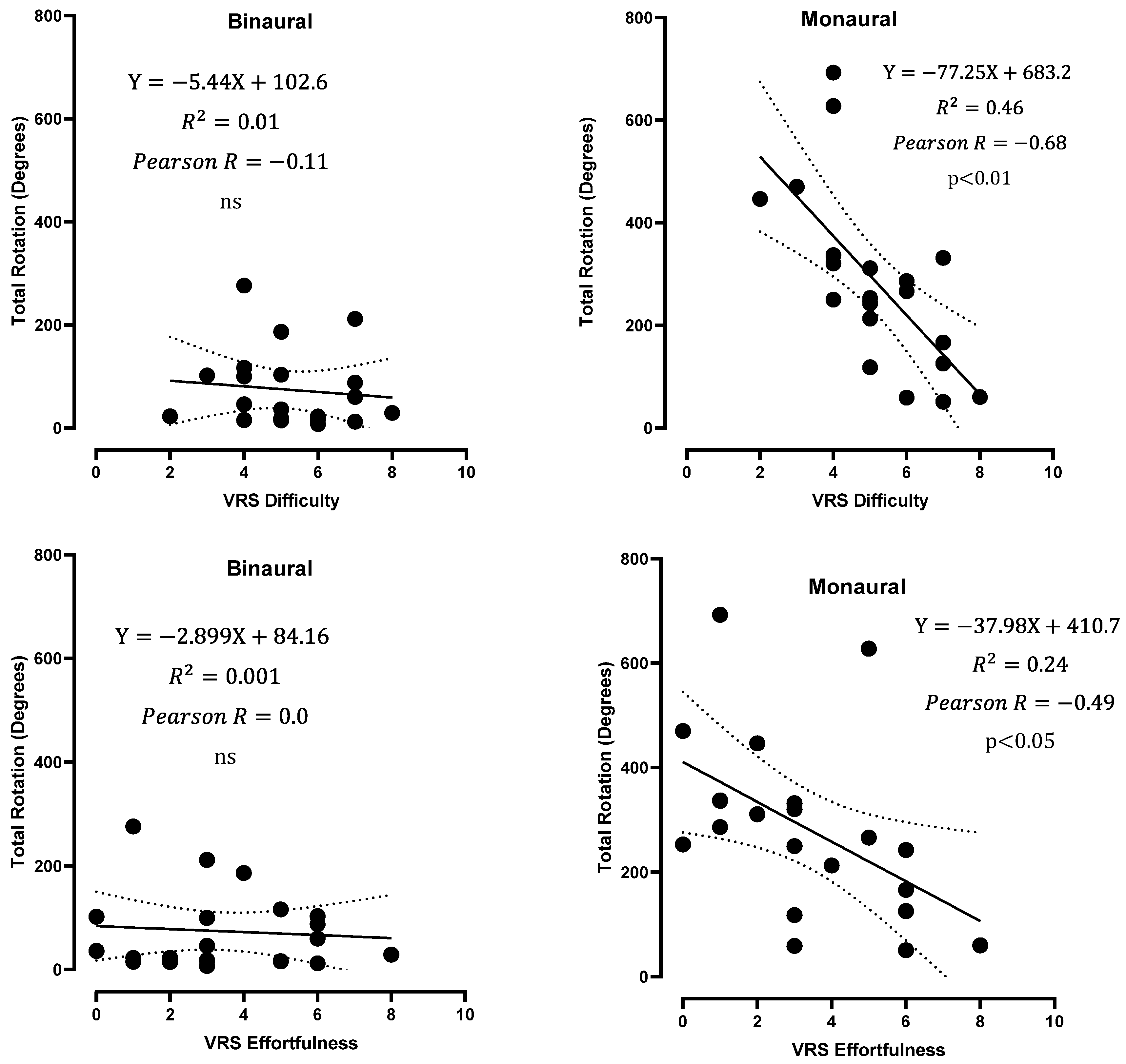
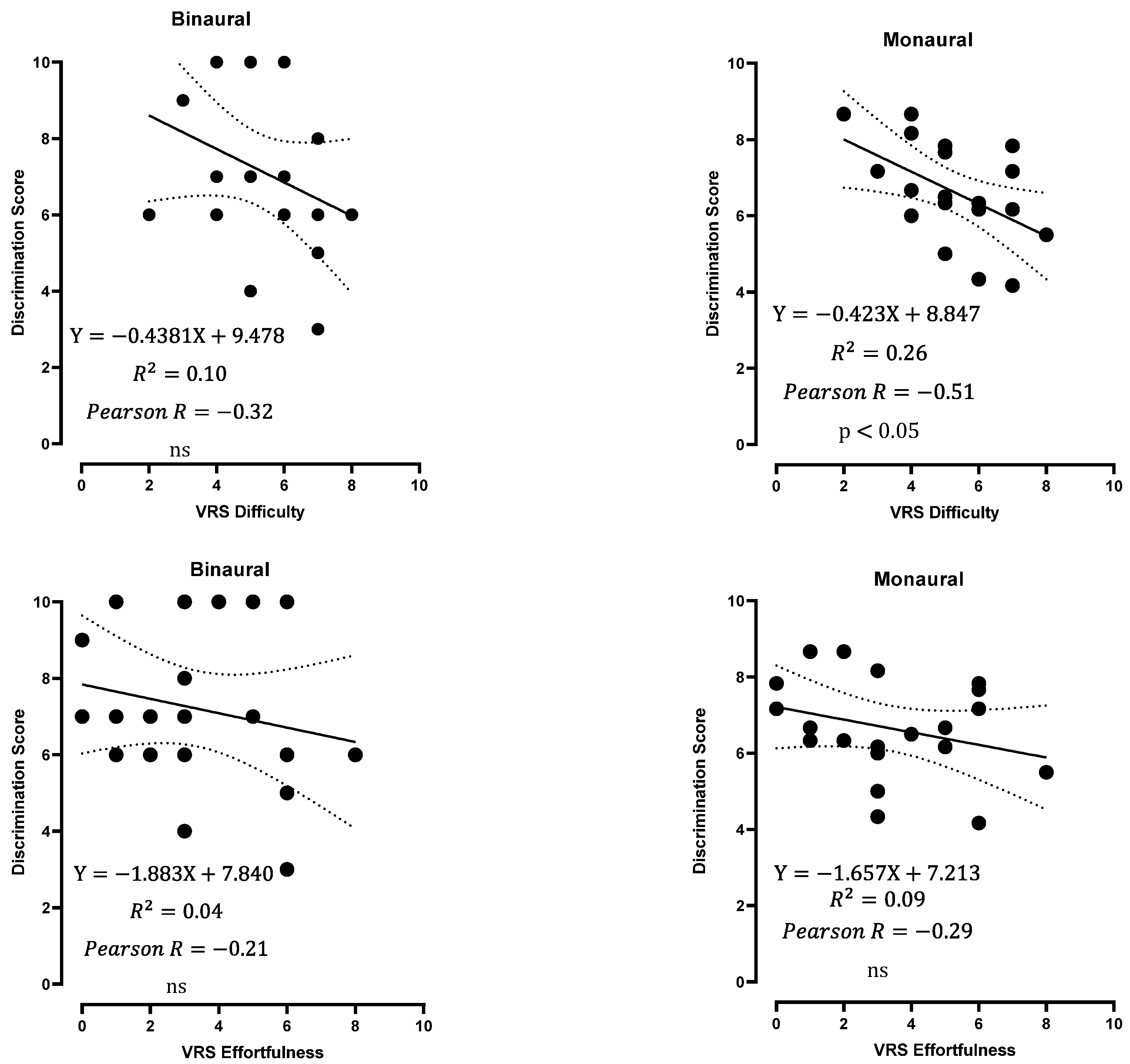
3.5. Subjective Evaluation of the Test
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chadha, S.; Kamenov, K.; Cieza, A. The world report on hearing, 2021. Bull. World Health Organ. 2021, 99, 242A. [Google Scholar] [CrossRef] [PubMed]
- Kay-Rivest, E.; Irace, A.L.; Golub, J.S.; Svirsky, M.A. Prevalence of Single-Sided Deafness in the United States. Laryngoscope 2022, 132, 1652–1656. [Google Scholar] [CrossRef] [PubMed]
- Golub, J.S.; Brickman, A.M.; Ciarleglio, A.J. Association of subclinical hearing loss with cognitive performance. JAMA Otolaryngol. Head Neck Surg. 2020, 146, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Avan, P.; Giraudet, F.; Büki, B. Importance of binaural hearing. Audiol. Neurotol. 2015, 20 (Suppl. S1), 3–6. [Google Scholar] [CrossRef]
- Steven Colburn, H.; Shinn-Cunningham, B.; Kidd, G., Jr. The perceptual consequences of binaural hearing. Int. J. Audiol. 2006, 45 (Suppl. S1), 34–44. [Google Scholar] [CrossRef]
- Abbagnaro, L.A.; Bauer, B.B.; Torick, E.L. Measurements of diffraction and interaural delay of a progressive sound wave caused by the human head. J. Acoust. Soc. Am. 1975, 58, 693–700. [Google Scholar] [CrossRef]
- Mills, A.W. On the minimum audible angle. J. Acoust. Soc. Am. 1958, 30, 237–246. [Google Scholar] [CrossRef]
- Vincent, C.; Arndt, S.; Marx, M. Identification and evaluation of cochlear implant candidates with asymmetrical hearing loss. Audiol. Neurotol. 2015, 20 (Suppl. S1), 87–89. [Google Scholar] [CrossRef]
- Snapp, H.A.; Ausili, S.A. Hearing with one ear: Consequences and treatments for profound unilateral hearing loss. J. Clin. Med. 2020, 9, 1010. [Google Scholar] [CrossRef]
- Vannson, N.; Fraysse, B.; Marx, M. Quality of life and auditory performance in adults with asymmetric hearing loss. Audiol. Neurotol. 2015, 20 (Suppl. S1), 38–43. [Google Scholar] [CrossRef]
- Lieu, J.E.C. Permanent Unilateral Hearing Loss (UHL) and Childhood Development. Curr. Otorhinolaryngol. Rep. 2018, 6, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Arndt, S.; Aschendorff, A.; Laszig, R. Comparison of pseudobinaural hearing to real binaural hearing rehabilitation after cochlear implantation in patients with unilateral deafness and tinnitus. Otol. Neurotol. 2011, 32, 39–47. [Google Scholar] [CrossRef]
- Desmet, J.; Bouzegta, R.; Hofkens, A. Clinical need for a BAHA trial in patients with single-sided sensorineural deafness. Analysis of a BAHA database of 196 patients. Eur. Arch. Otorhinolaryngol. 2012, 269, 799–805. [Google Scholar] [CrossRef] [PubMed]
- Joly, C.-A.; Reynard, P.; Mezzi, K. Guidelines of the French Society of Otorhinolaryngology-Head and Neck Surgery (SFORL) and the French Society of Audiology (SFA) for Speech-in-Noise Testing in Adults. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2022, 139, 21–27. [Google Scholar] [CrossRef]
- Theunissen, M.; Swanepoel, D.W.; Hanekom, J. Sentence recognition in noise: Variables in compilation and interpretation of tests. Int. J. Audiol. 2009, 48, 743–757. [Google Scholar] [CrossRef] [PubMed]
- Schrøder, S.A.; Ravn, T.; Bonding, P. BAHA in single-sided deafness: Patient compliance and subjective benefit. Otol. Neurotol. 2010, 31, 404–408. [Google Scholar] [CrossRef]
- Niclou, A. Etablissement du Rapport D’évaluation Clinique du Logiciel Amplinext. Mémoire pour l’obtention du Diplôme d’Etat d’Audioprothésiste. Bachelor’s Thesis, Faculté de Pharmacie, Université de Loraine, Lorraine, France, 2018. [Google Scholar]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.-G. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- García, J.M.; Urquijo, D.P.; Puerta, M. Cochlear implant in patients with single sided deafness: Hearing results and communicative benefits. Cochlear Implant. Int. 2020, 21, 136–144. [Google Scholar] [CrossRef]
- Cicchetti, D.V. Guidelines, criteria, and rules of thumb for evaluating normed and standardized assessment instruments in psychology. Psychol. Assess. 1994, 6, 284–290. [Google Scholar] [CrossRef]
- Kompis, M.; Pfiffner, F.; Krebs, M. Factors influencing the decision for BAHA in unilateral deafness: The Bern benefit in single-sided deafness questionnaire. Adv. Otorhinolaryngol. 2011, 71, 103–111. [Google Scholar] [PubMed]
- Grange, J.A.; Culling, J.F. The benefit of head orientation to speech intelligibility in noise. J. Acoust. Soc. Am. 2016, 139, 703–712. [Google Scholar] [CrossRef] [PubMed]
- Pastore, M.T.; Natale, S.J.; Yost, W.A. Head movements allow listeners bilaterally implanted with cochlear implants to resolve front-back confusions. Ear. Hear. 2018, 39, 1224–1231. [Google Scholar] [CrossRef]
- Kumpik, D.P.; King, A.J. A review of the effects of unilateral hearing loss on spatial hearing. Hear. Res. 2019, 372, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Alzaher, M.; Valzolgher, C.; Verdelet, G.; Pavani, F.; Farnè, A.; Barone, P.; Marx, M. Audiovisual Training in Virtual Reality Improves Auditory Spatial Adaptation in Unilateral Hearing Loss Patients. J. Clin. Med. 2023, 12, 2357. [Google Scholar] [CrossRef]
- Sanchez Jimenez, A.; Willard, K.J.; Bajo, V.M.; King, A.J.; Nodal, F.R. Persistence and generalization of adaptive changes in auditory localization behavior following unilateral conductive hearing loss. Front. Neurosci. 2023, 17, 1067937. [Google Scholar] [CrossRef]
- Gao, X.; Yan, T.; Huang, T.; Li, X.; Zhang, Y.X. Speech in noise perception improved by training fine auditory discrimination: Far and applicable transfer of perceptual learning. Sci. Rep. 2020, 10, 19320. [Google Scholar] [CrossRef]
- Hennessy, S.; Wood, A.; Wilcox, R.; Habibi, A. Neurophysiological improvements in speech-in-noise task after short-term choir training in older adults. Aging 2021, 13, 9468–9495. [Google Scholar] [CrossRef]
- Renard, C.; Hanson, J.-N.; Vincent, C. Stéréo-Audiométrie. Rapport 2014 de la SFORL: Audiométrie; Elsevier Masson: Gainesville, FL, USA, 2015; Chapitre 5. [Google Scholar]
- Marx, M.; Mosnier, I.; Vincent, C. Treatment choice in single-sided deafness and asymmetric hearing loss: A prospective, multi-center cohort study on 155 patients. Clin. Otolaryngol. 2021, 46, 736–743. [Google Scholar] [CrossRef]
- Bernstein, J.G.W.; Phatak, S.A.; Schuchman, G.I. Single-sided deafness cochlear implant sound-localization behavior with multiple concurrent sources. Ear. Hear. 2022, 43, 206–219. [Google Scholar] [CrossRef]
- Agterberg, M.J.H.; Snik, A.F.M.; Van de Goor, R.M.G. Sound-localization performance of patients with single-sided deafness is not improved when listening with a bone-conduction device. Hear. Res. 2019, 372, 62–68. [Google Scholar] [CrossRef]
- Ortiz, A.L.P.; Orduña-Bustamante, F. Improving speech intelligibility for binaural voice transmission under disturbing noise and reverberation using virtual speaker lateralization. J. Appl. Res. Technol. 2015, 13, 351–358. [Google Scholar] [CrossRef]
- Brimijoin, W.O.; McShefferty, D.; Akeroyd, M.A. Auditory and visual orienting responses in listeners with and without hearing-impairment. J. Acoust. Soc. Am. 2010, 127, 3678–3688. [Google Scholar] [CrossRef] [PubMed]
- Carlile, S.; Delaney, S.; Corderoy, A. The localisation of spectrally restricted sounds by human listeners. Hear. Res. 1999, 128, 175–189. [Google Scholar] [CrossRef] [PubMed]
- Fischer, M.E.; Cruickshanks, K.J.; Pinto, A. Hearing impairment and retirement. J. Am. Acad. Audiol. 2014, 25, 164–170. [Google Scholar] [CrossRef]
- de Araújo, P.G.V.; Mondelli, M.F.C.G.; Lauris, J.R.P. Assessment of the auditory handicap in adults with unilateral hearing loss. Braz. J. Otorhinolaryngol. 2010, 76, 378–383. [Google Scholar]
- Cox, R.M.; Alexander, G.C. The Abbreviated Profile of Hearing Aid Benefit. Ear Hear. 1995, 16, 176–186. [Google Scholar] [CrossRef] [PubMed]
- Gatehouse, S.; Noble, W. The Speech, Spatial and Qualities of Hearing Scale (SSQ). Int. J. Audiol. 2004, 43, 85–99. [Google Scholar] [CrossRef]

| S1 | S2 | S3 | Pearson R, p-Value | ||
|---|---|---|---|---|---|
| BINAURAL | Final angle | 1.8 ± 47.47 [−99.9; 88.50] | 9.1 ± 37.70 [−49.0; 86.7] | 1.5 ± 35.78 [−54.0; 95.4] | S1–S2: 0.383 ns S1–S3: 0.37 ns S2–S3: 0.23 ns |
| Left limit | −10.7 ± 42.00 [−99.9; 87.9] | 0.9 ± 35.66 [−49.0; 86.7] | −10.9 ± 30.50 [−80.4; 60.7] | S1–S2: 0.5 ** S1–S3: 0.43 ns S2–S3: 0.05 ns | |
| Right limit | 15.9 ± 50.99 [−91.9; 156.8] | 23.7 ± 31.63 [−18.5; 88.8] | 20.0 ±45.69 [−54.0; 139.6] | S1–S2: 0.002 ns S1–S3: 0.05 ns S2–S3: 0.20 ns | |
| Amplitude | 26.6 ± 38.41 [0; 155.7] | 22.8 ± 28.71 [0; 93.1] | 30.9 ± 46.10 [0; 153.2] | S1–S2: 0.76 *** S1–S3: 0.68 *** S2–S3: 0.67 ** | |
| Total rotation | 76.3 ± 85.30 [1.0; 316.0] | 65.6 ± 59.04 [3.7; 254.2] | 81.0 ± 109.9 [1.0; 388.1] | S1–S2: 0.78 *** S1–S3: 0.58 ** S2–S3: 0.75 *** | |
| LEFT EAR OCCLUDED | Final angle | −3.2 ± 55.89 [−72.9; 108.5] | 0.2 ± 71.68 [−135.2; 176.1] | −5.0 ± 61.15 [−125.7; 84.0] | S1–S2: 0.22 ns S1–S3: −0.16 ns S2–S3: 0.38 ns |
| Left limit | −97.0 ± 54.25 [−175.5; −11.6] | −85.6 ± 52.71 [−177.0; −6.7] | −87.9 ± 53.45 [−175.4; −5.4] | S1–S2: 0.48 * S1–S3: 0.80 *** S2–S3: 0.29 ns | |
| Right limit | 36.4 ± 73.6 [−72.9; 174.0] | 35.1 ± 64.91 [−99.9; 176.1] | 11.57 ± 71.58 [−125.7; 144.7] | S1–S2: −0.21 ns S1–S3: −0.29 ns S2–S3: 0.06 ns | |
| Amplitude | 133.4 ± 108.40 [13.9; 338.6] | 120.6 ± 99.52 [0; 351.4] | 99.4 ± 83.40 [0; 320.2] | S1–S2: 0.05 ns S1–S3: 0.135 ns S2–S3: 0.06 ns | |
| Total rotation | 341.5 ± 297.30 [46.0; 1107.0] | 380.4 ± 373.02 [17.9; 1277.0] | 355.3 ± 351.90 [5.9; 1468.0] | S1–S2: 0.28 ns S1–S3: 0.73 *** S2–S3: 0.26 ns | |
| RIGHT EAR OCCLUDED | Final angle | 2.2 ± 69.29 [−172.8; 78.1] | 30.0 ± 56.6 [−62.6; 154.5] | 7.7 ± 47.9 [−125.3; 90.5] | S1–S2: −0.18 ns S1–S3: 0.30 ns S2–S3: −0.24 ns |
| Left limit | −26.0 ± 78.00 [−177.2; 71.0] | 5.6 ± 60.01 [−139.4; 83.4] | −5.7 ± 46.7 [−125.3; 64.1] | S1–S2: 0.23 ns S1–S3: 0.21 ns S2–S3: −0.09 ns | |
| Right limit | 76.9 ± 58.22 [−28.7; 173.9] | 88.5 ± 51.03 [−24.4; 162.9] | 78.1 ± 52.92 [−5.8; 167.8] | S1–S2: 0.57 ** S1–S3: 0.56 ** S2–S3: 0.49 * | |
| Amplitude | 102.9 ± 107.90 [0; 336.5] | 82.9 ± 79.19 [1.0; 294.0] | 83.8 ± 67.02 [0; 265.1] | S1–S2: 0.30 ns S1–S3: 0.27 ns S2–S3: 0.12. ns | |
| Total rotation | 330.1 ± 319.80 [28.7; 1034] | 318.6 ± 212.0 [55.4; 781.4] | 288.0 ± 249.1 [1.5; 876.4] | S1–S2: 0.29 ns S1–S3: 0.12 ns S2–S3: 0.01 ns |
| Condition | S1 | S2 | S3 | Correlation |
|---|---|---|---|---|
| BIN | 7.2 ± 2.09 [3; 10] | 8.35 ± 1.73 [5; 10] | 7.6 ± 1.85 [5; 10] | S1–S2: 0.17 ns S1–S3: 0.46 * S2–S3: 0.53 * |
| LEO | 6.4 ± 2.23 [1; 10] | 6.8 ± 2.15 [3; 10] | 6.3 ± 2.73 [0; 10] | S1–S2: 0.07 ns S1–S3: 0.47 * S2–S3: 0.24 ns |
| REO | 7.1 ± 2.06 [4; 10] | 7.1 ± 1.83 [4; 10] | 6.4 ± 2.18 [2; 10] | S1–S2: 0.11 ns S1–S3: 0.15 ns S2–S3: 0.39 ns |
| Condition | S1 | S2 | S3 | Correlation |
|---|---|---|---|---|
| BIN | 0.05 ± 0.22 [0; 1] | 0.0 ± 0.0 [0; 0] | 0.05 ± 0.22 [0; 1] | S1–S2: 0.17, ns S1–S3: 0.46, * S2–S3: 0.53, * |
| LEO | 0.40 ± 0.60 [0; 2] | 0.95 ± 0.76 [0; 3] | 0.60 ± 0.50 [0; 1] | S1–S2: 0.07, ns S1–S3: 0.47, * S2–S3: 0.24, ns |
| REO | 0.65 ± 0.93 [0; 3] | 0.65 ± 0.59 [0; 2] | 0.60 ± 0.82 [0; 2] | S1–S2: 0.11, ns S1–S3: 0.15, ns S2–S3: 0.39, ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tetard, S.; Guigou, C.; Sonnet, C.-E.; Al Burshaid, D.; Charlery-Adèle, A.; Bozorg Grayeli, A. Free-Field Hearing Test in Noise with Free Head Rotation for Evaluation of Monaural Hearing. J. Clin. Med. 2023, 12, 7143. https://doi.org/10.3390/jcm12227143
Tetard S, Guigou C, Sonnet C-E, Al Burshaid D, Charlery-Adèle A, Bozorg Grayeli A. Free-Field Hearing Test in Noise with Free Head Rotation for Evaluation of Monaural Hearing. Journal of Clinical Medicine. 2023; 12(22):7143. https://doi.org/10.3390/jcm12227143
Chicago/Turabian StyleTetard, Stanley, Caroline Guigou, Charles-Edouard Sonnet, Dhari Al Burshaid, Ambre Charlery-Adèle, and Alexis Bozorg Grayeli. 2023. "Free-Field Hearing Test in Noise with Free Head Rotation for Evaluation of Monaural Hearing" Journal of Clinical Medicine 12, no. 22: 7143. https://doi.org/10.3390/jcm12227143
APA StyleTetard, S., Guigou, C., Sonnet, C.-E., Al Burshaid, D., Charlery-Adèle, A., & Bozorg Grayeli, A. (2023). Free-Field Hearing Test in Noise with Free Head Rotation for Evaluation of Monaural Hearing. Journal of Clinical Medicine, 12(22), 7143. https://doi.org/10.3390/jcm12227143






