Abstract
Background: The use of inert gas rebreathing for the non-invasive cardiac output measurement has produced measurements comparable to those obtained by various other methods. However, there are no guidelines for the inert gas rebreathing method during a cardiopulmonary exercise test (CPET). In addition, there is also a lack of specific standards for assessing the non-invasive measurement of cardiac output during CPET, both for healthy patients and those suffering from diseases and conditions. Aim: This systematic review aims to describe the use of IGR for a non-invasive assessment of cardiac output during cardiopulmonary exercise testing and, based on the information extracted, to identify a proposed CPET report that includes an assessment of the cardiac output using the IGR method. Methods: This systematic review was conducted by PRISMA (Preferred Reporting Items for Systematic Reviews and Meta Analyses) guidelines. PubMed, Web of Science, Scopus, and Cochrane Library databases were searched from inception until 29 December 2022. The primary search returned 261 articles, of which 47 studies met the inclusion criteria for this review. Results and Conclusions: This systematic review provides a comprehensive description of protocols, indications, technical details, and proposed reporting standards for a non-invasive cardiac output assessment using IGR during CPET. It highlights the need for standardized approaches to CPET and identifies gaps in the literature. The review critically analyzes the strengths and limitations of the studies included and offers recommendations for future research by proposing a combined report from CPET-IGR along with its clinical application.
1. Introduction
Cardiopulmonary exercise testing (CPET) offers the investigator the unique opportunity to study cellular, cardiovascular, and ventilatory system responses under controlled conditions of metabolic stress [1].
Cardiopulmonary exercise testing (CPET) is a versatile and comprehensive diagnostic tool that has gained popularity and is now utilized across various demographics and patient groups [2]. Previously, CPET was primarily performed on healthy individuals or those with specific cardiovascular conditions. However, its application has broadened significantly, and it is now employed in diverse populations. CPET is routinely conducted on both men and women, allowing for a gender-specific assessment of exercise capacity and functional limitations. It provides valuable insights into the physiological responses of different genders during exercise and helps identify any gender-specific exercise limitations [3].
Furthermore, CPET is not limited to specific age groups; it is now employed for children [4,5] and the elderly [6]. This allows for the evaluation of exercise performance and the identification of any age-related limitations.
In addition to studying healthy individuals and those with specific conditions, CPET is now applied to assess exercise capacity in both athletic and frail populations [7,8]. By comparing the performance of highly trained athletes with individuals who are frail or have reduced functional capacity, CPET helps determine the impact of physical fitness on exercise performance and identifies the limitations faced by the frail population during physical activity.
CPET has evolved beyond examining exercise performance and peak oxygen uptake alone. It now aims to answer broader questions about the underlying causes of exercise limitations. By analyzing various parameters during CPET, such as heart rate, blood pressure, gas exchange, and ventilatory responses, medical professionals can identify which organ or bodily function is responsible for the exercise limitation [9,10]. This information aids in the diagnosis and treatment of various conditions impacting exercise capacity. Moreover, CPET plays a crucial role in determining the prognosis of patients, being the strong and independent marker of risk for cardiovascular and all-cause mortality [11,12,13,14,15]. By assessing exercise performance and physiological responses, healthcare providers can predict a patient’s condition’s potential outcome, helping to make informed decisions regarding their treatment plans and interventions. What is more, CPET is considered an ideal pre-operative assessment tool, as it provides an objective measure of fitness or functional capacity [16]. It assesses the comprehensive function of the cardiac, circulatory, respiratory, and muscle metabolic systems during physiological stress. Further, it can pinpoint the underlying cause of exercise intolerance. The anaerobic threshold (AT) measured through CPET testing strongly predicts mortality resulting from cardiopulmonary causes during the postoperative period. By conducting preoperative screening with CPET testing, high-risk patients can be identified, enabling the selection of appropriate perioperative management strategies [17]. The current guidelines from the American Heart Association, American College of Cardiology [18], and the European Society of Cardiology [19] suggest that assessing exercise capacity, when feasible, can provide valuable insights in the evaluation of noncardiac surgery for high-risk patients with an unknown functional capacity.
Nevertheless, the European Society of Cardiology stated, “The full potential of CPET in the clinical and research setting remains underused” [10]. Despite its intrinsic advantages, CPET also has limitations due to how it assesses cardiac and pulmonary function, i.e., in the case of the heart, it only provides an indirect assessment of cardiac function as a pump. In contrast, in the case of the lungs, it gives intermediate AT values unless the method is extended to include arterial blood gasometry during the test. This review considers the use of the already-known IGR (Inert Gas Rebreathing) method to assess the direct cardiac function during CPET. This is presented in detail in this paper.
The inert gas rebreathing method is a non-invasive technique used to assess cardiac output (CO) and pulmonary blood flow (PBF) during exercise. It involves the rebreathing of a gas mixture containing inert gases, which are soluble and enter the bloodstream via pulmonary diffusion without binding to hemoglobin [20].
This method allows for the measurement of respiratory gas exchange and airflow, providing additional information on oxygen utilization and ventilation [21].
The inert gas rebreathing method has been shown to be a safe and reliable alternative to invasive and expensive techniques for measuring CO, such as thermodilution [22]. It has been used in numerous studies to assess cardiac function in patients with heart failure [22] and to evaluate the accuracy and precision of different rebreathing techniques [20,22,23]. The results of these studies have demonstrated that the inert gas rebreathing method can provide reasonable estimates of CO and pulmonary blood flow, with some variations in accuracy and precision depending on the specific gases used and the exercise intensity [20,23].
The use of CPET and IGR provides us with an even broader understanding of the clinical state of the patient under examination through lots of symptom-limited, incremental exercise, commonly in combination with the comprehensive breath-by-breath monitoring of cardiopulmonary variables (e.g., peak oxygen consumption/maximal oxygen consumption (peak VO2/VO2 max), pulmonary CO2 output (VCO2), minute ventilation (VE), heart rate (HR), cardiac output (CO), stroke volume (SV), and subjective responses (e.g., dyspnea and leg pain)) and, as needed, measurements such as exercise-related arterial oxygen desaturation and dynamic hyperinflation [3].
1.1. Cardiac Output Measurement
It is useful to add the measurement of CO, which is considered the best indicator of cardiac function during exercise, to the values described above [24]. The measurement of CO represents an added value to a standard CPET.
CO is defined as the volume of blood pumped by the heart per minute. It is an important parameter that reflects the ability of the heart to supply blood to the body’s tissues. CO is calculated as the product of the heart rate (HR) and stroke volume (SV), which is the volume of blood pumped by the heart with each beat.
CO can be expressed mathematically as CO = HR × SV. CO is a crucial determinant of oxygen delivery to the body’s tissues, and it can be affected by several factors, including heart rate, contractility, preload, and afterload [25]. Measuring CO is important for diagnosing and managing heart and circulatory conditions [26,27,28] and monitoring the response to therapy [29,30,31].
There are several methods of CO measurements. CO can be measured during pulmonary artery catheterization (PAC) using the thermodilution method described by W. T. McGee et al. [32]. The thermodilution method provides a relatively accurate and continuous CO measurement [33], and it is widely used in critical care settings. However, it is an invasive procedure and carries a risk of complications [34], including infection [35], bleeding [36], and arrhythmias [37,38]. Non-invasive methods for measuring CO include bioimpedance [39], bioreactance [40], pulse contour analysis [41], pulse wave velocity [42], photoplethysmography [43], partial CO2 rebreathing [44], and, finally, inert gas rebreathing (IGR) [45].
1.2. Inert Gas Rebreathing
IGR is a non-invasive technique used to measure CO and PBF. The use of IGR has been shown to produce pulmonary blood flow measurements that are comparable to those obtained by various other methods such as thermodilution, direct Fick, and modified Fick methods during rest as well as graded exercise [46,47,48,49]. Furthermore, this method offers high reproducibility for CO measurements during rest and exercise for both healthy individuals and those with cardiac indications [50,51].
The IGR method may use different neutral gases, i.e., nitrogen, helium, acetylene, carbon dioxide, and sulfur hexafluoride [52]. Nitrogen is an inert gas that is relatively insoluble in blood and tissue, making it an ideal tracer for measuring blood flow. Xenon gas is also used in inert gas rebreathing, as it has low solubility in blood and a high tissue-to-blood partition coefficient, which makes it a useful tracer for measuring pulmonary blood flow [53]. Due to its low solubility in blood, helium is often used as a diluent in the rebreathing mixture to increase the tracer gas concentration and improve measurement accuracy [53].
Acetylene is an inert gas used as a tracer gas in some IGR methods. However, compared to other commonly used tracer gases, acetylene is more soluble in blood and tissue and, therefore, is less ideal for measuring blood flow [54].
Another commonly used gas is carbon dioxide, as it is readily metabolized by the body and can provide information about gas exchange in the lungs [55].
Finally, sulfur hexafluoride (SF6) is also used as a tracer gas in IGR, which has a high molecular weight and is even less soluble in blood than nitrogen, making it an effective tracer for measuring CO [56].
1.3. IGR with the Use of SF6
The IGR technique involves a machine inflating a bag with an oxygen-enriched mixture of an inert soluble gas (0.5% nitrous oxide) and an inert insoluble gas (0.1% sulfur hexafluoride—SF6), which is an inert gas that does not alter the body’s metabolism [45,57,58,59,60,61]. Patients breathe into a respiratory valve via a mouthpiece and a bacterial filter with a nose clip, and at the end of expiration, the valve automatically activates so that patients rebreathe from the prefilled bag for 10 to 20 s. The CO measurement is taken by a photoacoustic analyzer over a three to five breath interval. The lung volume is determined by sulfur hexafluoride, while the nitrous oxide concentration decreases during rebreathing with a rate proportional to the PBF [45,57]. CO equals the PBF only if SpO2 is above 98% on the pulse oximeter, indicating no pulmonary shunt flow. If SpO2 is below 98%, CO equals the PBF plus the shunt flow [57].
This method can be successfully performed both at rest and during CPET [45,51].
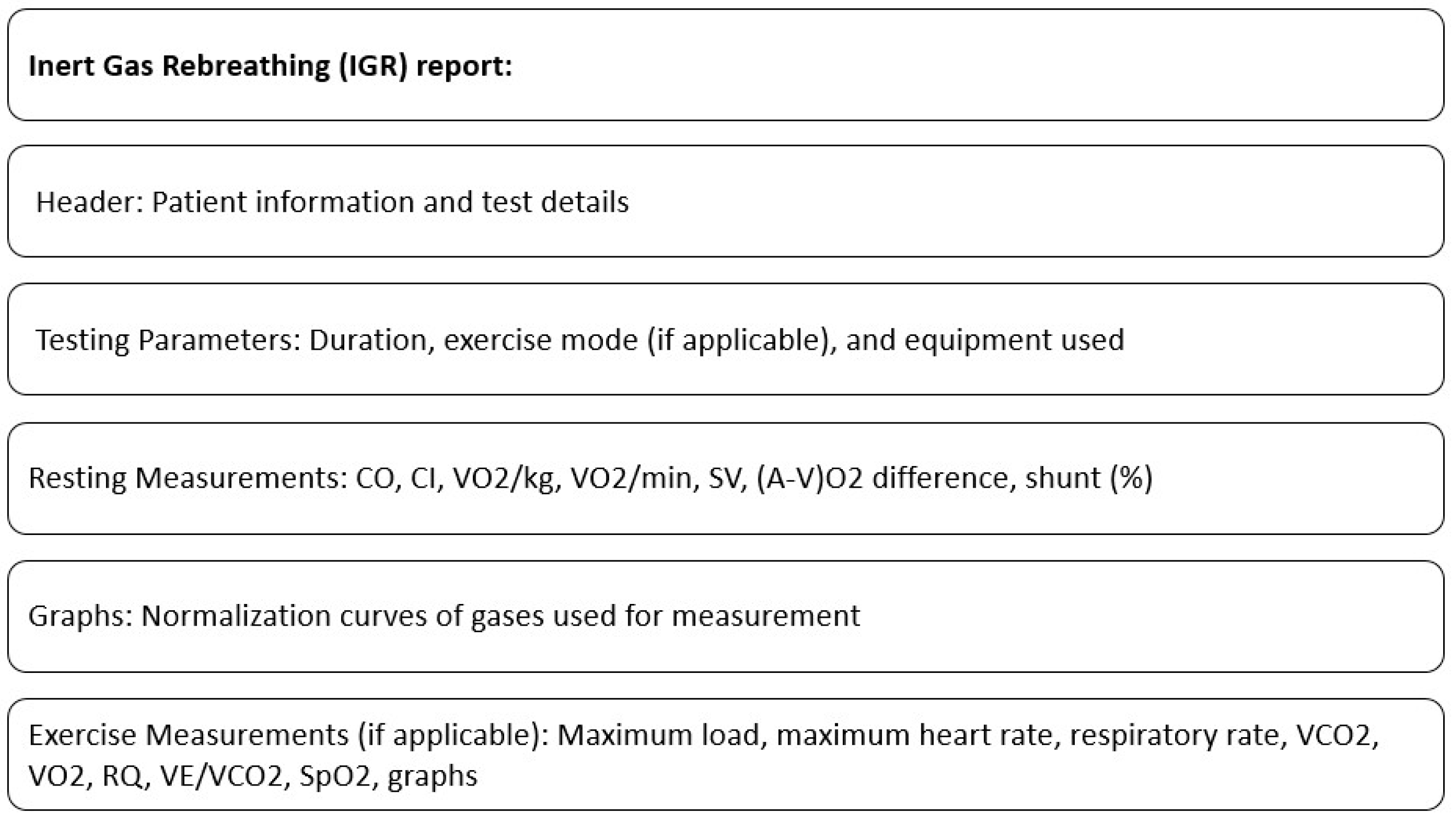
A standard IGR report typically includes resting measurements such as CO, CI (Cardiac Index), VO2/kg (Oxygen consumption per kilogram of body weight), VO2/min (Absolute oxygen consumption per minute), SV (Stroke Volume), (a-v)O2 difference (Oxygen content difference between arterial and venous blood), and shunt (%). The report may also feature graphs showing the normalization curves of the gases used for measurement.
If the IGR test is performed during an exercise protocol, the report includes additional results from the breath-by-breath exercise assessment. These results may consist of a maximum load (highest workload achieved), maximum heart rate, respiratory rate, VCO2 (Ventilatory Carbon Dioxide Output), VO2 (Oxygen Consumption), RQ (Respiratory Quotient), VE/VCO2 (Ratio of minute ventilation to carbon dioxide output), saturation levels, and other typical outcomes.
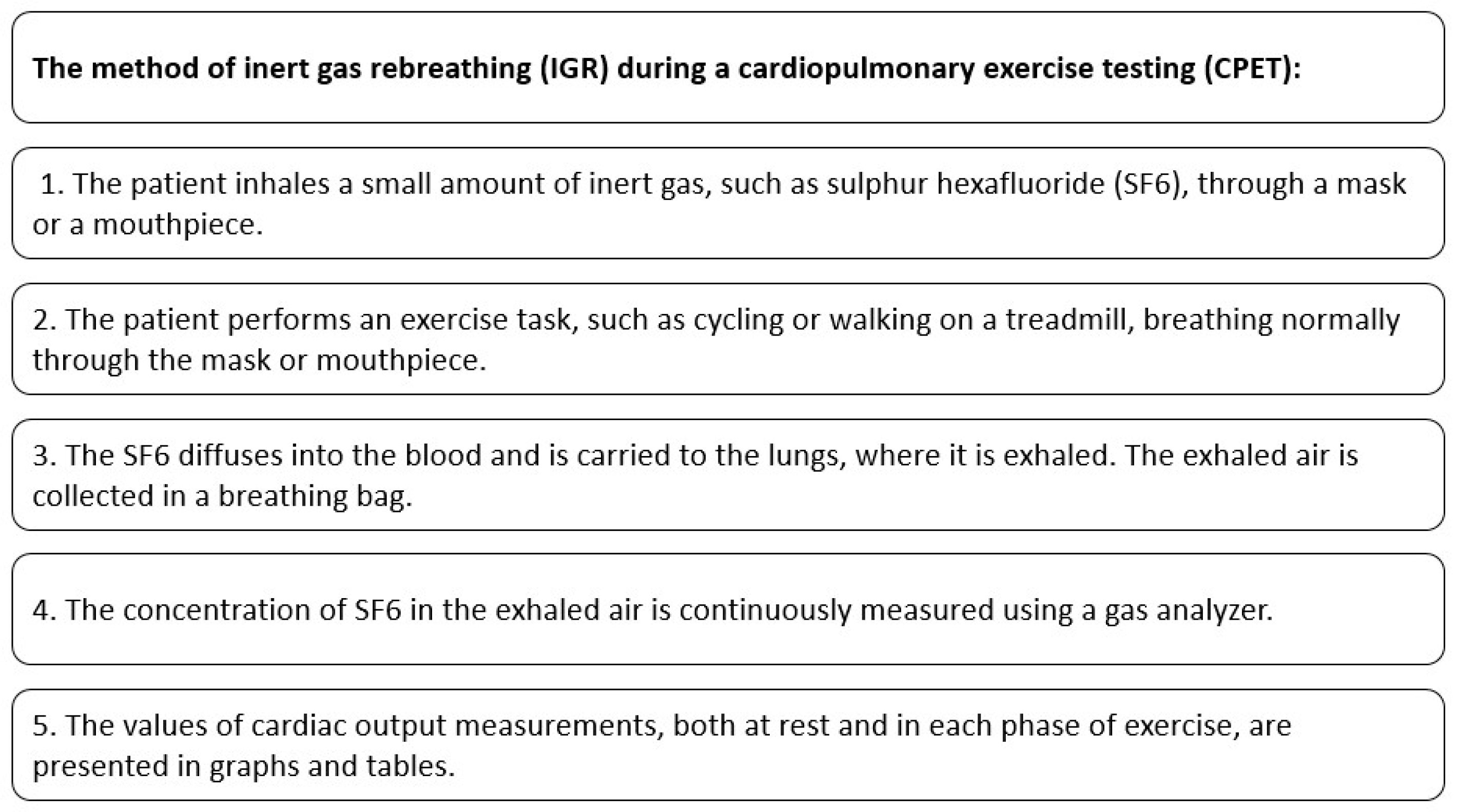
The process of performing an IGR during CPET is presented in Figure 1.
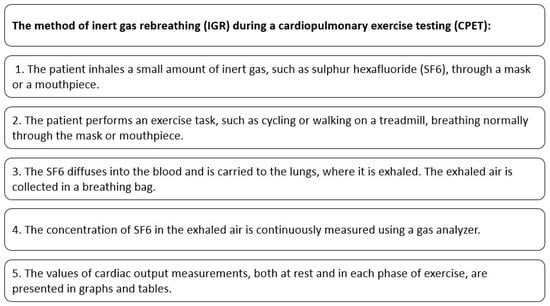
Figure 1.
Cardiac output measurement with the use of IGR method during CPET.
The contents of the standard IGR report are shown in Figure 2.
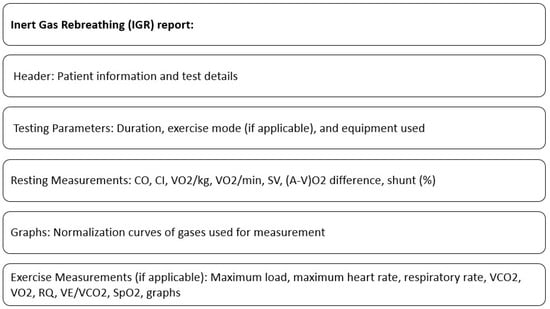
Figure 2.
Contents of standard IGR report.
The SF6 concentration measurement is repeated several times during the exercise challenge and the CO is calculated using a specialized computer software (Innocor™, Innovision, Odense, Denmark, ver. 8.10).
CO can be derived from the change in the SF6 concentration in the exhaled air, which reflects the amount of SF6 that has diffused into the blood and been transferred to the lungs. This method provides a continuous and relatively accurate measurement of CO [49,62,63] during CPET and can be used to monitor changes in CO over time. Currently, available IGR measurement systems using SF6 (i.e., Innocor® CO–Cardiac Output—COSMED) do not include a rhythmic assessment of the exercise ECG, which limits the possibility of monitoring patient safety during CPET-IGR. Of course, this difficulty can be overcome by performing simultaneous ECG recordings with an external system.
However, there are no guidelines for the inert gas rebreathing method during a cardiopulmonary exercise test (CPET). In addition, there is also a lack of specific standards for assessing the non-invasive measurement of CO during CPET, both for healthy patients and those suffering from diseases and conditions. Hence, there is the need to write a systematic review to bring together the available up-to-date knowledge on using IGR during CPET.
The purpose of this systematic review is to systematically describe the use of IGR for the non-invasive assessment of CO during cardiopulmonary exercise testing, present the extracted data, if applicable, and, based on the information extracted, to identify a proposed CPET report that includes the assessment of CO using the IGR method.
2. Methods
2.1. Literature Search
This systematic review was performed in accordance with the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) statement [64]. The PICO (Patients, Interventions, Comparisons, and Outcomes) approach was not used, as this systematic review was not to evaluate intervention effects. The following predetermined strategy was used to identify eligible articles. We searched PubMed, Web of Science, Scopus, and Cochrane.
2.2. Evidence Acquisition
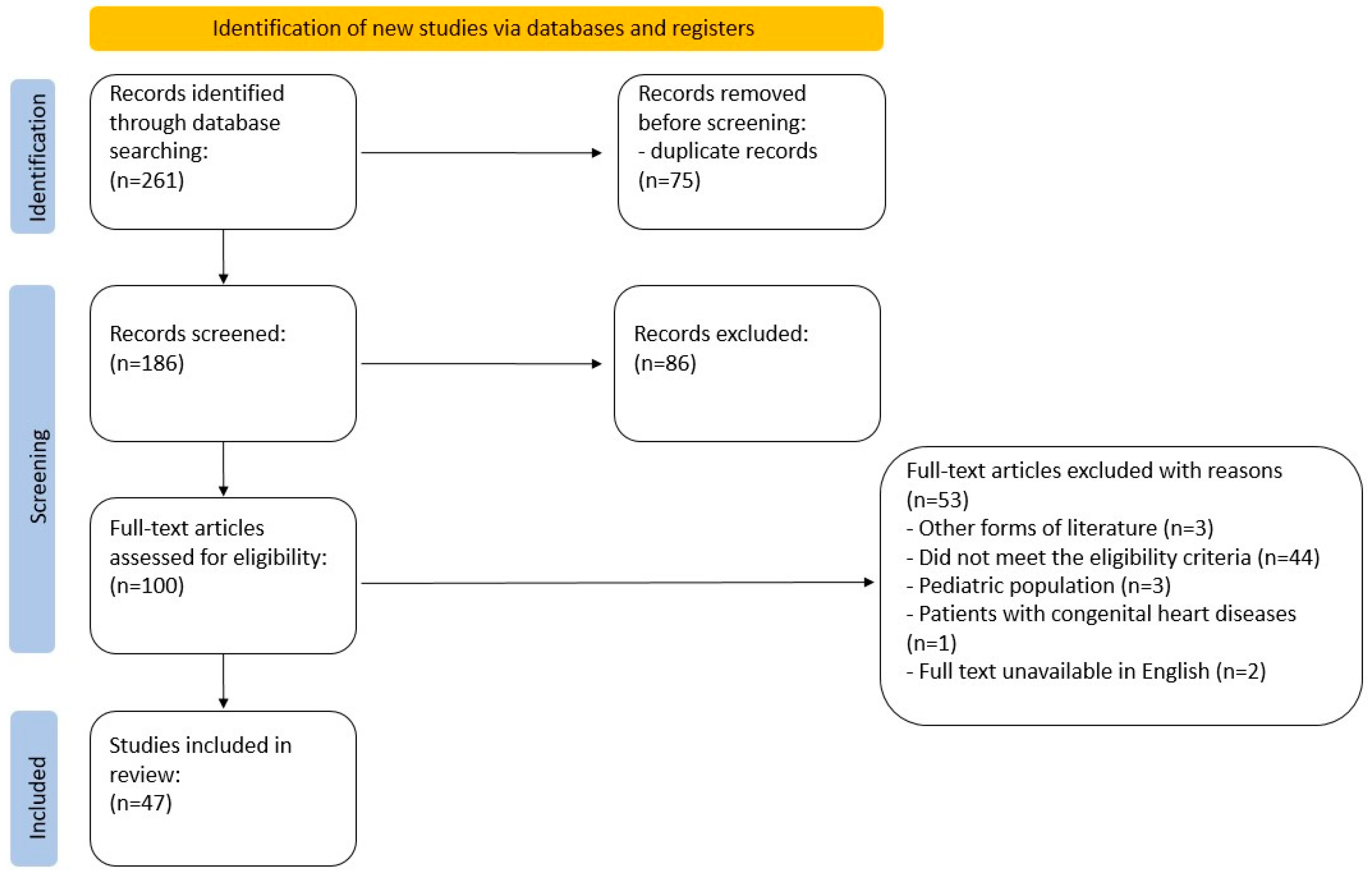
On 29 December 2022, the targeted online databases were searched. The search query was constructed with the use of MeSH structures: ((“inert gas rebreathing”) AND (“exercise test” OR “walk test” OR “ergometry” OR “stress test” OR “treadmill test” OR “bicycle ergometry test” OR “fitness testing” OR “cardiopulmonary exercise testing” OR “CPET”)). No search filters were applied. The search was completed with a manual search of references from key papers. The primary search returned 261 articles. The number of articles after duplicate removal was narrowed to 186. The initial screening process involved two researchers (AC, ŁM), independently reviewing the titles and abstracts of all the records. Any inconsistencies were discussed until a consensus was reached. Then, the researchers independently screened the full-text articles for inclusion. The data were collected using an Excel spreadsheet. In case of any disagreement, they discussed and reached a consensus on whether to include or exclude the article. The complete protocol is depicted in a PRISMA flowchart (Figure 3).
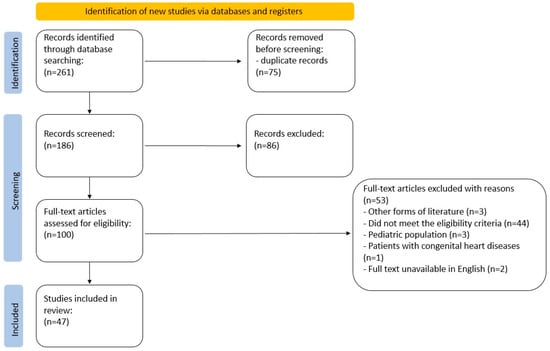
Figure 3.
PRISMA flowchart.
2.3. Inclusion and Exclusion Criteria
Studies were included in the current systematic review if they conformed with the following criteria: (1) a cardiopulmonary exercise test (CPET) had been performed, (2) the inert gas rebreathing method was used in the CO assessment, (3) it included an adult population, and (4) the CPET protocol was described in a precise and explicit manner, allowing the study to be reconstructed. If articles met the predefined criteria, they were included and categorized as eligible. The exclusion criteria included: (1) review articles, (2) studies including a pediatric population, (3) studies including patients with congenital heart diseases, or (4) the entire text was unavailable in English.
2.4. Evidence Synthesis and Quality Assessment
Given the experimental nature of the studies and the fact that, in most cases, the IGR method was not the focus of the research, it was not possible to use widely recognized tools for a quality assessment. Therefore, the quality assessment was performed manually by referring to a model study written by Agostoni et al. [57], the only author with defined reference values. The manual evaluation of the quality of the included studies consisted of assessing whether the study description contained an accurately described study protocol, allowing for reproduction. In addition, each study was evaluated against the inclusion criteria (described in Section 2.3) by two independent authors of the systematic review, and any disagreement was resolved by a third author. Reference data presented in the article by Agostoni and co-authors, titled: “Reference values for peak exercise cardiac output in healthy individuals” [57], are presented in Table 1. The study involved 500 normal subjects of varying ages and genders. They underwent a maximal cardiopulmonary exercise test with a peak CO measurement using a specific method. The results showed that peak CO was higher in males compared to females. Both peak CO and peak VO2 decreased with age. Moreover, the study provided the formula to predict peak CO from peak VO2 values. The equation shows that, in the general population, peak CO = 4.4 × peak VO2 + 4.3, while peak CO in males = 4.3 × peak VO2 + 4.5 and peak CO in females = 4.9 × peak VO2 + 3.6. The data reported in Agostoni’s study are clear, and the study model allows for the reproduction of protocol. Given the lack of established tools for assessing the quality, this approach seemed appropriate for the current systematic review. However, it is important to acknowledge the limitations of this method and the potential for bias in the quality assessment process. Therefore, the results of the quality assessment should be interpreted with caution.

Table 1.
Reference values for peak exercise cardiac output in healthy individuals by Agostoni et al. [57].
2.5. Study Registration
This systematic review has been registered with PROSPERO (International Prospective Register of Systematic Reviews) under registration number CRD42023456820. A detailed protocol for this systematic review is available in PROSPERO, where it can be accessed and evaluated.
2.6. Availability of Data
The data, code, and other materials used in this systematic review are available upon request from the corresponding author. We are committed to promoting transparency and reproducibility in research, and we encourage interested parties to reach out for access to the data and materials used in this study.
3. Results
3.1. Study Selection and Description
The search flowchart is presented in Figure 3.
In Table 2, all included studies were considered, and raw data related to measurements conducted using IGR were extracted wherever feasible (Table 3). The studies included in the systematic review where the extraction of raw data was unattainable are marked with “*” in Table 2. The reasons for the inability to obtain the raw data were data presented solely in graphical form, data presented as value differences, or data presented in units different from those sought in this systematic review. This comprehensive approach ensured a thorough assessment of the available data.

Table 2.
Results of the systematic review.

Table 3.
Results of the systematic review (with raw data extracted where feasible).
Table 2, presented herein, provides the most critical information, e.g., the protocols used, patients’ characteristics, the study design, and the primary and secondary points. Where feasible, there are comments and conclusions listed. This table serves to streamline and highlight key findings and relevant data points from the studies included, offering a succinct overview of the essential aspects of the research synthesized in this review.
This is the first systematic review to address the topic of a non-invasive CO assessment using IGR during CPET. And as such, we believe it is necessary to provide a comprehensive description of the protocols, indications for cardiopulmonary exercise testing with IGR (CPET-IGR), technical details of the testing, and proposed reporting standards. Due to the lack of a standardized approach to CPET-IGR, the inclusion criteria for this review were broad to capture as many relevant studies as possible.
3.2. Participants of the Studies Included
This systematic review resulted in the extraction of 48 articles that matched the search criteria. The total number of patients from all studies is 4465. The average age of the participants ranges from 21 to 72 years old. The populations are diverse in terms of health comorbidities (Section 3.4 and Table 4). For an accurate description of the population of each study, see Table 2.

Table 4.
Indications for CPET-IGR in included articles.
3.3. Protocols Used in CPET-IGR Studies
A cardiopulmonary exercise test typically requires a cycloergometer or a treadmill. The type of exercise equipment used depends on the patient’s preference, physical capabilities, and the test’s purpose. In the studies included in this systematic review, the authors used both cycle ergometers [58,86,88,90] and treadmills [84,87,89,91].
The standard CPET protocol involves the following steps. (1) Pre-test procedures: Before the test, the subject’s height, weight, age, and medical history are recorded. The subject is also instructed on using exercise equipment and wearing an interface for measuring respiratory gases. (2) Baseline measurements: the subject rests quietly for several minutes while measurements of their resting heart rate, blood pressure, and oxygen saturation are taken. (3) Incremental or constant load exercise: The subject begins exercising on a cycle ergometer or treadmill at a low intensity, gradually increasing every minute until exhaustion or a pre-determined endpoint is reached. The exercise protocol can vary based on the individual being tested and the test’s purpose. (4) Respiratory gas measurements: During exercise, the subject wears a mask connected to a metabolic cart that measures the volume of oxygen and carbon dioxide consumed and produced, respectively. These data allow for the various physiological parameters, such as the anaerobic threshold, peak oxygen uptake, and respiratory exchange ratio. (5) ECG monitoring: the subject’s heart rhythm is continuously monitored during exercise using an electrocardiogram (ECG) to detect any abnormalities or changes in the heart rate. (6) Termination of the test: the test is usually stopped when the subject reaches a pre-determined endpoint, such as the maximum heart rate, symptom limitation, or a predetermined workload. (7) Recovery: The subject rests for several minutes while their heart rate, blood pressure, and oxygen saturation measurements are taken. The subject may also be monitored for any post-exercise symptoms or complications. The protocol for CPET-IGR may vary depending on the patient’s specific medical history and condition, and may be adjusted accordingly by the healthcare provider performing the test (Table 1).
The researchers conducting the studies included in this systematic review conducted CPET-IGR using varied testing protocols. Most of these were tests involving a progressive work rate [31,59,60,61,63,65,66,67,68,69,70,72,73,74,75,76,77,78,79,80,82,83,85,93,94,95,96,97,98,101,102], while a few studies used constant load protocols in addition to progressive work rate tests [71,81,92,99,100].
The authors of the papers discussed do not strictly state how the measuring system is connected to the patient (mouthpiece or full-face mask). It should also be noted that there are no studies validating the different interfaces of CPET-IGR.
3.4. Health Conditions as Indication for IGR Study
As there are no separate recommendations available for the indications for CPET-IGR, general indications for CPET were used by the authors of the cited papers to perform CPET-IGR.
According to the American Thoracic Society and American College of Cardiology, there are several common indications for CPET: the diagnosis and assessment of an exercise intolerance in patients with cardiovascular or pulmonary diseases, such as heart failure, chronic obstructive pulmonary disease (COPD), or pulmonary hypertension; the assessment of preoperative risk in patients undergoing major surgery; the determination of exercise-induced bronchoconstriction in patients with suspected exercise-induced asthma; the evaluation of the fitness and training status in athletes or individuals participating in endurance sports; an unexplained dyspnea diagnosis; or the assessment of interstitial lung diseases [103].
Among the studies included in this systematic review, the predominant indications for CPET in patients were HF (53% of included articles) and a physical capacity assessment in healthy individuals or athletes (28%). Only 19% of the included articles concerned other patient populations. For instance, only two articles (4% of included articles) focused on COPD patients (Table 4).
3.5. Other
The IGR method has high repeatability, reproducibility, and its reliability is not compromised by atrial fibrillation or pulmonary diseases [45,51,55,56,63,76,84]. The IGR method is, therefore, ideally suitable for determining CO in LVAD (Left Ventricular Assist Device) patients at rest and during exercise. It allows the evaluation of the physical fitness of a particular population of patients. Since the method is non-invasive, it can be performed any number of times, allowing the long-term documentation of hemodynamics [104].
3.6. Proposed IGR Report Style
The proposed CPET-IGR report is presented in Table 5.

Table 5.
Proposed CPET-IGR report.
4. Discussion
The systematic review conducted on the topic of a non-invasive CO measurement using the IGR method during cardiopulmonary exercise testing (CPET) has revealed important insights into the current state of research in this field. While the application of IGR holds promise for assessing cardiac function in a non-invasive manner, the synthesis of findings across numerous studies has highlighted several key challenges that need to be addressed for this method to reach its full potential.
4.1. The Validation and Utility of CPET-IGR
This systematic review encompasses a diverse array of studies exploring the potential of IGR as a valuable tool for assessing CO during CPET. By synthesizing findings from studies across various clinical contexts, the discussion sheds light on the evolving landscape of CO measurement, with an emphasis on the insights provided by the IGR method.
A particularly important study conducted by Middlemiss et al. [56] reveals the potential of CPET-IGR. Normative data highlight age-related declines in CO and SV, particularly in males. Body size, assessed through BMI (Body Mass Index), significantly impacts CO and SV, especially in younger individuals. IGR proves sensitive to physiological changes, highlighting posture-induced CO shifts. Although limitations exist, this study paves the way for understanding age, body size, and posture effects on CO, offering insights for future investigations and clinical applications.
Several studies acknowledge the variability, validation, and limitations inherent in different CO assessment methods [56,59,70,81,86,91,93,94]. Articles compare the CO measurement methods during CPET, obtained by Innocor (IGR) and other methods including the Fick method, Physioflow (impedance cardiography), Nexfin (pulse contour analysis), finger photoplethysmography, cardiac magnetic resonance and bioreactance. The conclusions from the above studies suggest that the IGR method offers a straightforward, precise, and consistently replicable non-invasive approach for quantifying CO during CPET [56,59,70,91]. However, as stated by Siebenmann et al. [94] and Okwose et al. [70], the four extensively employed and valid techniques for assessing CO during exercise yield notably divergent measurements. Consequently, the choice of method significantly impacts the determination of CO during exercise, and those methods cannot be used interchangeably.
The systematic review includes, among others, studies focused on patients with heart failure (CHF), a population characterized by complex hemodynamic responses during exercise. The studies demonstrate the potential of IGR to provide valuable insights into the management of HF. A study conducted by Halbirk et al. [31] examines the effects of a 48 h infusion of glucagon-like peptide-1 in patients with compensated chronic heart failure and highlights the potential of IGR to elucidate cardiovascular and metabolic effects in this context. What is more, there are several articles investigating the management of patients with left ventricular assist devices (LVADs) [69,71,76,77,83,87]. Researchers highlight the usefulness of IGR in assessing CO changes and optimizing device settings. This application underscores the role of IGR as a non-invasive tool in the evolving landscape of LVAD care and optimization. Another notable theme in the discussion is the exploration of the predictive power of CO measurements, particularly in relation to exercise capacity and prognosis. Among the studies included in this systematic review, only a few articles addressing the predictive value of the IGR were identified [58,82,83,88,90]. In the Pastormerlo et al. [90] study, IGR was among other methods used in stratifying the risk of HF progression. Goda et al. [58] stated that CO at 25 W, measured non-invasively during submaximal exercise, may have potential value as a predictor of outcomes in patients with CHF. Additionally, Lang et al. [82] describe peak cardiac power, measured non-invasively, as an independent predictor of an outcome that can enhance the prognostic power of peak VO2 in the evaluation of patients with HF. However, Y. Shen et al. [88] concluded that peak CPO is not a predictor of cardiac death in Chinese CHF patients.
According to Jakovljevic et al. [83], powerful exercise-derived prognostic indicators, including peak VO2, AT, circulatory power, and the ventilatory efficiency slope, demonstrate limited capacity to reflect the cardiac organ function in patients treated with LVADs.
Hence, further research should focus on investigating the predictive value of the IGR method during CPET. The above-described studies on the prognostic and predictive value mainly focus on HF patients. Data are needed to provide the reliability, reproducibility, and predictive value of the IGR method in patients with conditions other than HF. Special attention should be given to patients suffering from lung diseases.
4.2. Lack of Uniform Result Presentation
One of the foremost observations arising from the systematic review is the lack of standardized results presentations across studies utilizing the IGR method. The absence of a consistent reporting format hampers the comparability of results, making it challenging to draw meaningful conclusions from the accumulated data. This inconsistency impedes one’s ability to discern trends, potential variations, or establish normative values for CO measurements.
4.3. Diverse Study Protocols
Another noteworthy finding is the substantial heterogeneity in the study protocols employed in investigations utilizing IGR for CO measurement. The diversity in experimental designs, including variations in the use of masks or mouthpieces, as well as interruptions in CPET for the transition between measurement devices, introduces confounding factors that could influence study outcomes. In particular, the practice of switching from a CPET mask to an IGR mouthpiece during peak exercise phases may introduce variability in the peak values, thereby impacting the reliability of the results obtained.
4.4. Inadequate Methodology Reporting
The systematic review also highlights the inconsistency in reporting the methodology of IGR measurements. Often, authors fail to detail whether a mask or a mouthpiece was used for IGR, which undermines the transparency and reproducibility of the studies. This lack of transparency poses challenges in interpreting the results and prevents the establishment of standardized best practices for IGR utilization.
4.5. Points for Clinical Practice
Considering these challenges, it is evident that there is an urgent need for the development of guidelines and standardized protocols for non-invasive CO measurement using the IGR method during CPET. By establishing clear guidelines on the instrumentation, experimental setup, and result reporting, researchers can enhance the consistency and reliability of their findings. This will enable more meaningful comparisons across studies, facilitating the accumulation of robust evidence for clinical applications.
4.6. Establishing Normative Ranges
Moreover, the systematic review underscores the necessity of generating comprehensive normative ranges for CO measurements using the IGR method. These normative values will serve as a valuable reference point, aiding in the interpretation of results obtained from different populations and under various conditions. Developing a wide range of normative values will contribute to the broader clinical utility of CPET-IGR. In conclusion, while the non-invasive measurement of CO using the IGR method during CPET holds significant potential, the systematic review illuminates the current challenges and limitations. Addressing the issues of result presentation, diverse study protocols, inadequate reporting, and the need for guidelines and normative ranges is imperative for the method’s advancement. By collaboratively addressing these challenges, researchers can unlock the true clinical and research potential of non-invasive CO assessment using IGR during CPET.
5. Limitations
The presented systematic review includes several limitations. Firstly, some of the studies included in this review were based on small patient groups [31,51,59,66,76,79], and most of the studies focused on patients with either healthy conditions or heart failure. There were few studies available that focused on other disease entities [85,89,95,96,97,98,99]. Additionally, not all the required values could be obtained from the studies because they were not described. In addition, in most studies, a non-invasive CO assessment using the IGR method was not the main subject under investigation and was only one of many measures used in the study. These limitations should be considered when interpreting the results of this systematic review. Further studies with larger and more diverse patient populations are needed to provide more comprehensive information on the topic.
6. Conclusions
Given that the investigators conducting the studies included in this systematic review identified heart failure or a physical capacity assessment in healthy patients as the main indications for CPET and a non-invasive CO assessment, it is reasonable to conclude that the IGR technique is not used in a wide range of patients. Therefore, there is a need to validate and expand research on the IGR technique in patients with various conditions.
In conclusion, this systematic review provides a comprehensive overview of the use of IGR for a non-invasive assessment of CO during CPET. The evidence supports the reliability and validity of IGR as a method for measuring CO during exercise. However, there is a need for standardized protocols, guidelines, and reporting standards to ensure the consistent and accurate use of IGR in CPET assessments. Future research should focus on establishing normative values, and validating quality assessment tools specific to IGR studies. By addressing these gaps, we can enhance the clinical utility of IGR and its contribution to our understanding of cardiovascular function during exercise.
Author Contributions
Conceptualization, A.C., Ł.M. and R.M.M.; methodology, A.C., Ł.M. and H.R.H.; software, A.C. and Ł.M; formal analysis, all authors; investigation, A.C. and Ł.M.; writing—original draft preparation, A.C. and Ł.M.; writing—review and editing, all authors; supervision, R.M.M. and H.R.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| AT | Anaerobic Threshold |
| a-vO2 diff | Arteriovenous Oxygen Difference |
| CI | Cardiac Index |
| CHF | Chronic Heart Failure |
| CO | Cardiac Output |
| COPD | Chronic Obstructive Pulmonary Disease |
| CPO | Cardiac Power Output |
| GLP-1 | Glucagon-like Peptide 1 |
| HF | Heart Failure |
| HR | Heart Rate |
| IGR | Inert Gas Rebreathing |
| LVAD | Left Ventricular Assist Device |
| PAC | Pulmonary Artery Catheterization |
| peak VO2 | Peak oxygen consumption |
| PH | Pulmonary Hypertension |
| PBF | Pulmonary Blood Flow |
| RQ | Respiratory Quotient |
| SF6 | Sulfur hexafluoride |
| SV | Stroke Volume |
| VCO2 | Ventilatory Carbon Dioxide Output |
| VE/VCO2 | Ratio of minute ventilation to carbon dioxide output |
| VO2 | Oxygen Consumption |
| VE | Minute Ventilation |
| VO2 max | Maximal oxygen consumption |
References
- Hansen, J.E.; Sue, D.Y.; Stringer, W.W.; Whipp, B.J. Principles of Exercise Testing and Interpretation, 4th ed.; Wasserman, K., Ed.; Lea & Febiger: Philadelphia, PA, USA, 2004. [Google Scholar]
- Schraufnagel, D.E.; Agostoni, P. Cardiopulmonary Exercise Testing. Ann. Am. Thorac. Soc. 2017, 14 (Suppl. S1), S1–S2. [Google Scholar] [CrossRef]
- Wasserman, B.W.K.; Hansen, J.; Sue, D.; Stringer, W. Principles of Exercise Testing and Interpretation. Can. J. Cardiol. 2007, 23, 274. [Google Scholar] [CrossRef]
- Phan, V.; Hershenson, J.; Caldarera, L.; Larkin, S.K.; Wheeler, K.; Cortez, A.L.; Dulman, R.; Briere, N.; Lewis, A.; Kuypers, F.A.; et al. Effect of voxelotor on cardiopulmonary testing in youths with sickle cell anemia in a pilot study. Pediatr. Blood Cancer 2023, 70, e30423. [Google Scholar] [CrossRef] [PubMed]
- Liem, R.I.; Reddy, M.; Pelligra, S.A.; Savant, A.P.; Fernhall, B.; Rodeghier, M.; Thompson, A.A. Reduced fitness and abnormal cardiopulmonary responses to maximal exercise testing in children and young adults with sickle cell anemia. Physiol. Rep. 2015, 3, e12338. [Google Scholar] [CrossRef]
- Palau, P.; Domínguez, E.; Seller, J.; Sastre, C.; Sanchis, J.; López, L.; Bodí, V.; Llàcer, P.; Miñana, G.; de la Espriella, R.; et al. Chronotropic index and long-term outcomes in heart failure with preserved ejection fraction. Rev. Esp. Cardiol. 2023, 76, 511–518. [Google Scholar] [CrossRef]
- Piepoli, M.F.; Corrà, U.; Agostoni, P. Cardiopulmonary exercise testing in patients with heart failure with specific comorbidities. Ann. Am. Thorac. Soc. 2017, 14, S110–S115. [Google Scholar] [CrossRef]
- Collins, S.; Phillips, D.B.; Brotto, A.R.; Rampuri, Z.H.; Stickland, M.K. Ventilatory efficiency in athletes, asthma and obesity. Eur. Respir. Rev. 2021, 30, 200206. [Google Scholar] [CrossRef] [PubMed]
- Mezzani, A. Cardiopulmonary Exercise Testing: Basics of Methodology and Measurements. Ann. Am. Thorac. Soc. 2017, 14, S3–S11. [Google Scholar] [CrossRef]
- Mezzani, A.; Agostoni, P.; Cohen-Solal, A.; Corra, U.; Jegier, A.; Kouidi, E.; Mazic, S.; Meurin, P.; Piepoli, M.; Simon, A.; et al. Standards for the use of cardiopulmonary exercise testing for the functional evaluation of cardiac patients: A report from the Exercise Physiology Section of the European Association for Cardiovascular Prevention and Rehabilitation. Eur. J. Prev. Cardiol. 2009, 16, 249–267. [Google Scholar] [CrossRef] [PubMed]
- Ross, R.; Blair, S.N.; Arena, R.; Church, T.S.; Després, J.P.; Franklin, B.A.; Haskell, W.L.; Kaminsky, L.A.; Levine, B.D.; Lavie, C.J.; et al. Importance of Assessing Cardiorespiratory Fitness in Clinical Practice: A Case for Fitness as a Clinical Vital Sign: A Scientific Statement from the American Heart Association. Circulation 2016, 134, e653–e699. [Google Scholar] [CrossRef]
- Myers, J.; McAuley, P.; Lavie, C.J.; Despres, J.P.; Arena, R.; Kokkinos, P. Physical activity and cardiorespiratory fitness as major markers of cardiovascular risk: Their independent and interwoven importance to health status. Prog. Cardiovasc. Dis. 2015, 57, 306–314. [Google Scholar] [CrossRef]
- Kokkinos, P.; Myers, J. Exercise and Physical Activity. Circulation 2010, 122, 1637–1648. [Google Scholar] [CrossRef]
- Kodama, S.; Saito, K.; Tanaka, S.; Maki, M.; Yachi, Y.; Asumi, M.; Sugawara, A.; Totsuka, K.; Shimano, H.; Ohashi, Y.; et al. Cardiorespiratory Fitness as a Quantitative Predictor of All-Cause Mortality and Cardiovascular Events in Healthy Men and Women: A Meta-analysis. JAMA 2009, 301, 2024–2035. [Google Scholar] [CrossRef]
- Faselis, C.; Doumas, M.; Pittaras, A.; Narayan, P.; Myers, J.; Tsimploulis, A.; Kokkinos, P. Exercise capacity and all-cause mortality in male veterans with hypertension aged ≥70 years. Hypertension 2014, 64, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Older, P.; Smith, R.; Courtney, P.; Hone, R. Preoperative evaluation of cardiac failure and ischemia in elderly patients by cardiopulmonary exercise testing. Chest 1993, 104, 701–704. [Google Scholar] [CrossRef] [PubMed]
- Older, P.; Hall, A.; Hader, R. Cardiopulmonary exercise testing as a screening test for perioperative management of major surgery in the elderly. Chest 1999, 116, 355–362. [Google Scholar] [CrossRef]
- Fleisher, L.A.; Fleischmann, K.E.; Auerbach, A.D.; Barnason, S.A.; Beckman, J.A.; Bozkurt, B.; Davila-Roman, V.G.; Gerhard-Herman, M.D.; Holly, T.A.; Kane, G.C.; et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: A report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. J. Am. Coll. Cardiol. 2014, 64, e77–e137. [Google Scholar] [CrossRef]
- Kristensen, S.D.; Knuuti, J.; Saraste, A.; Anker, S.; Bøtker, H.E.; Hert, S.D.; Ford, I.; Gonzalez-Juanatey, J.R.; Gorenek, B.; Heyndrickx, G.R.; et al. 2014 ESC/ESA Guidelines on non-cardiac surgery: Cardiovascular assessment and management: The Joint Task Force on non-cardiac surgery: Cardiovascular assessment and management of the European Society of Cardiology (ESC) and the European Society of Anaesthesiology (ESA). Eur. Heart J. 2014, 35, 2383–2431. [Google Scholar] [CrossRef]
- Jarvis, S.S.; Levine, B.D.; Prisk, G.K.; Shykoff, B.E.; Elliott, A.R.; Rosow, E.; Blomqvist, C.G.; Pawelczyk, J.A. Simultaneous determination of the accuracy and precision of closed-circuit cardiac output rebreathing techniques. J. Appl. Physiol. 2007, 103, 867–874. [Google Scholar] [CrossRef] [PubMed]
- Saur, J.; Fluechter, S.; Trinkmann, F.; Papavassiliu, T.; Schoenberg, S.; Weissmann, J.; Haghi, D.; Borggrefe, M.; Kaden, J.J. Noninvasive determination of cardiac output by the inert-gas-rebreathing method-comparison with cardiovascular magnetic resonance imaging. Cardiology 2009, 114, 247–254. [Google Scholar] [CrossRef]
- Gabrielsen, A.; Videbæk, R.; Schou, M.; Damgaard, M.; Kastrup, J.; Norsk, P. Non-invasive measurement of cardiac output in heart failure patients using a new foreign gas rebreathing technique. Clin. Sci. 2002, 102, 247–252. [Google Scholar] [CrossRef]
- Jakovljevic, D.G.; Nunan, D.; Donovan, G.; Hodges, L.D.; Sandercock, G.R.H.; Brodie, D.A. Comparison of cardiac output determined by different rebreathing methods at rest and at peak exercise. Eur. J. Appl. Physiol. 2008, 102, 593–599. [Google Scholar] [CrossRef] [PubMed]
- Straburzyńska-Migaj, E. Testy Spiroergometryczne w Praktyce Klinicznej; PZWL: Warsaw, Poland, 2022. [Google Scholar]
- Vincent, J.L. Understanding cardiac output. Crit Care 2008, 12, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Borlaug, B.A.; Reddy, Y.N.V. Determinants and Correlates of Exercise Capacity in Heart Failure. JACC Heart Fail. 2015, 3, 815–817. [Google Scholar] [CrossRef]
- Franciosa, J.A.; Ziesche, S.; Wilen, R.N.M. Functional capacity of patients with chronic left ventricular failure. Relationship of bicycle exercise performance to clinical and hemodynamic characterization. Am. J. Med. 1979, 67, 460–466. [Google Scholar] [CrossRef] [PubMed]
- Weber, K.T.; Janicki, J.S. Cardiopulmonary exercise testing for evaluation of chronic cardiac failure. Am. J. Cardiol. 1985, 55, A22–A31. [Google Scholar] [CrossRef] [PubMed]
- Giglio, M.; Dalfino, L.; Puntillo, F.; Rubino, G.; Marucci, M.; Brienza, N. Haemodynamic goal-directed therapy in cardiac and vascular surgery. A systematic review and meta-analysis. Interact. Cardiovasc. Thorac. Surg. 2012, 15, 878–887. [Google Scholar] [CrossRef]
- McGuinness, S.; Parke, R. Using cardiac output monitoring to guide perioperative haemodynamic therapy. Curr. Opin. Crit. Care 2015, 21, 364–368. [Google Scholar] [CrossRef]
- Halbirk, M.; Nørrelund, H.; Møller, N.; Holst, J.J.; Schmitz, O.; Nielsen, R.; Nielsen-Kudsk, J.E.; Nielsen, S.S.; Nielsen, T.T.; Eiskjaer, H.; et al. Cardiovascular and metabolic effects of 48-h glucagon-like peptide-1 infusion in compensated chronic patients with heart failure. Am. J. Physiol. Heart Circ. Physiol. 2010, 298, H1096–H1102. [Google Scholar] [CrossRef]
- McGee, W.T.; Mailloux, P.; Jodka, P.; Thomas, J. The pulmonary artery catheter in critical care. Semin. Dial. 2006, 19, 480–491. [Google Scholar] [CrossRef]
- Dobb, G.J.; Donovan, K.D. Non-invasive methods of measuring cardiac output. Intensive Care Med. 1987, 13, 304–309. [Google Scholar] [CrossRef]
- Leibowitz, A.B. Who benefits from pulmonary artery catheterization? Crit. Care Med. 2003, 31, 2805–2806. [Google Scholar] [CrossRef]
- Mermel, L.A.; Maki, D.G. Infectious complications of Swan-Ganz pulmonary artery catheters. Pathogenesis, epidemiology, prevention, and management. Am. J. Respir. Crit. Care Med. 2012, 149, 1020–1036. [Google Scholar] [CrossRef] [PubMed]
- Sirivella, S.; Gielchinsky, I.; Parsonnet, V. Management of catheter-induced pulmonary artery perforation: A rare complication in cardiovascular operations. Ann. Thorac. Surg. 2001, 72, 2056–2059. [Google Scholar] [CrossRef] [PubMed]
- Dracup, K. The Swan-Ganz pulmonary artery catheter. Incidence of complications, with particular reference to ventricular dysrhythmias, and their prevention. Anaesthesia 1979, 34, 125–128. [Google Scholar] [CrossRef]
- Gorenek, B. Arrhythmias in cardiac catheterization laboratories. Acta Cardiol. 2008, 63, 259–263. [Google Scholar] [CrossRef] [PubMed]
- Peyton, P.J.; Chong, S.W. Minimally invasive measurement of cardiac output during surgery and critical care: A meta-analysis of accuracy and precision. Anesthesiology 2010, 113, 1220–1235. [Google Scholar] [CrossRef] [PubMed]
- Squara, P. Bioreactance: A New Method for Non-invasive Cardiac Output Monitoring. In Yearbook of Intensive Care and Emergency Medicine; Springer: Berlin/Heidelberg, Germany, 2008; pp. 619–630. [Google Scholar] [CrossRef]
- Saugel, B.; Kouz, K.; Scheeren, T.W.L.; Greiwe, G.; Hoppe, P.; Romagnoli, S.; de Backer, D. Cardiac output estimation using pulse wave analysis-physiology, algorithms, and technologies: A narrative review. Br. J. Anaesth. 2021, 126, 67–76. [Google Scholar] [CrossRef]
- Yamada, T.; Tsutsui, M.; Sugo, Y.; Sato, T.; Akazawa, T.; Sato, N.; Yamashita, K.; Ishihara, H.; Takeda, J. Multicenter study verifying a method of noninvasive continuous cardiac output measurement using pulse wave transit time: A comparison with intermittent bolus thermodilution cardiac output. Anesth. Analg. 2012, 115, 82–86. [Google Scholar] [CrossRef]
- Dvir, A.; Goldstein, N.; Rapoport, A.; Balmor, R.G.; Nachman, D.; Merin, R.; Fons, M.; Ben Ishay, A.; Eisenkraft, A. Comparing Cardiac Output Measurements Using a Wearable, Wireless, Noninvasive Photoplethysmography-Based Device to Pulse Contour Cardiac Output in the General ICU: A Brief Report. Crit. Care Explor. 2022, 4, e0624. [Google Scholar] [CrossRef]
- Jaffe, M.B. Partial CO2 rebreathing cardiac output--operating principles of the NICO system. J. Clin. Monit. Comput. 1999, 15, 387–401. [Google Scholar] [CrossRef]
- Cattadori, G.; Schmid, J.P.; Agostoni, P. Noninvasive Measurement of Cardiac Output during Exercise by Inert Gas Rebreathing Technique. Heart Fail. Clin. 2009, 5, 209–215. [Google Scholar] [CrossRef]
- Agostoni, P.; Cattadori, G. Noninvasive cardiac output measurement: A new tool in heart failure. Cardiology 2009, 114, 244–246. [Google Scholar] [CrossRef]
- Dong, L.; Wang, J.A.; Jiang, C.Y. Validation of the use of foreign gas rebreathing method for non-invasive determination of cardiac output in heart disease patients. J. Zhejiang Univ. Sci. B 2005, 6, 1157–1162. [Google Scholar] [CrossRef]
- Christensen, P.; Clemensen, P.; Andersen, P.K.; Henneberg, S.W. Thermodilution versus inert gas rebreathing for estimation of effective pulmonary blood flow. Crit. Care Med. 2000, 28, 51–56. [Google Scholar] [CrossRef]
- Peyton, P.J.; Thompson, B. Agreement of an inert gas rebreathing device with thermodilution and the direct oxygen fick method in measurement of pulmonary blood flow. J. Clin. Monit. Comput. 2004, 8, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Peyton, P.J.; Bailey, M.; Thompson, B.R. Reproducibility of cardiac output measurement by the nitrous oxide rebreathing technique. J. Clin. Monit. Comput. 2009, 23, 233–236. [Google Scholar] [CrossRef] [PubMed]
- Okwose, N.C.; Zhang, J.; Chowdhury, S.; Houghton, D.; Ninkovic, S.; Jakovljević, S.; Jevtic, B.; Ropret, R.; Eggett, C.; Bates, M.; et al. Reproducibility of Inert Gas Rebreathing Method to Estimate Cardiac Output at Rest and during Cardiopulmonary Exercise Stress Testing. Int. J. Sports Med. 2019, 40, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Wagner, P.D.; Saltzman, H.A.; West, J.B. Measurement of continuous distributions of ventilation-perfusion ratios: Theory. J. Appl. Physiol. 1974, 36, 588–599. [Google Scholar] [CrossRef] [PubMed]
- Harris, P.D.; Barnes, R. The uses of helium and xenon in current clinical practice. Anaesthesia 2008, 63, 284–293. [Google Scholar] [CrossRef]
- Hansen, S.; Wendelboe, O.; Christensen, P. The non-invasive acetylene rebreathing method for estimation of cardiac output: Influence of breath-by-breath variation. Clin. Physiol. 1997, 17, 193–202. [Google Scholar] [CrossRef]
- Marks, C.; Katch, V.; Rocchini, A.; Beekman, R.; Rosenthal, A. Validity and reliability of cardiac output by CO2 rebreathing. Sports Med. 1985, 2, 432–446. [Google Scholar] [CrossRef] [PubMed]
- Middlemiss, J.E.; Cocks, A.; Paapstel, K.; Maki-Petaja, K.M.; Sunita Wilkinson, I.B.; McEniery, C.M.; ACCT Study Investigators. Evaluation of inert gas rebreathing for determination of cardiac output: Influence of age, gender and body size. Hypertens. Res. 2018, 42, 834–844. [Google Scholar] [CrossRef] [PubMed]
- Agostoni, P.; Vignati, C.; Gentile, P.; Boiti, C.; Farina, S.; Salvioni, E.; Mapelli, M.; Magrì, D.; Paolillo, S.; Corrieri, N.; et al. Reference Values for Peak Exercise Cardiac Output in Healthy Individuals. Chest 2017, 151, 1329–1337. [Google Scholar] [CrossRef] [PubMed]
- Goda, A.; Lang, C.C.; Williams, P.; Jones, M.; Farr, M.J.; Mancini, D.M. Usefulness of Non-Invasive Measurement of Cardiac Output during Sub-Maximal Exercise to Predict Outcome in Patients with Chronic Heart Failure. Am. J. Cardiol. 2009, 104, 1556–1560. [Google Scholar] [CrossRef]
- del Torto, A.; Skattebo, Ø.; Hallén, J.; Capelli, C. Cardiac output with modified cardio-impedance against inert gas rebreathing during sub-maximal and maximal cycling exercise in healthy and fit subjects. Eur. J. Appl. Physiol. 2019, 119, 163–170. [Google Scholar] [CrossRef]
- Lang, C.C.; Karlin, P.; Haythe, J.; Tsao, L.; Mancini, D.M. Ease of Noninvasive Measurement of Cardiac Output Coupled with Peak VO2 Determination at Rest and during Exercise in Patients with Heart Failure. Am. J. Cardiol. 2007, 99, 404–405. [Google Scholar] [CrossRef]
- Accalai, E.; Vignati, C.; Salvioni, E.; Pezzuto, B.; Contini, M.; Cadeddu, C.; Meloni, L.; Agostoni, P. Non-invasive estimation of stroke volume during exercise from oxygen in heart failure patients. Eur. J. Prev. Cardiol. 2021, 28, 280–286. [Google Scholar] [CrossRef]
- Agostoni, P.; Cattadori, G.; Apostolo, A.; Contini, M.; Palermo, P.; Marenzi, G.; Wasserman, K. Noninvasive measurement of cardiac output during exercise by inert gas rebreathing technique: A new tool for heart failure evaluation. J. Am. Coll. Cardiol. 2005, 46, 1779–1781. [Google Scholar] [CrossRef]
- Fontana, P.; Boutellier, U.; Toigo, M. Reliability of measurements with innocor(TM) during exercise. Int. J. Sports Med. 2009, 30, 747–753. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Syst. Rev. 2021, 10, 105906. [Google Scholar] [CrossRef]
- Koshy, A.; Okwose, N.C.; Nunan, D.; Toms, A.; Brodie, D.A.; Doherty, P.; Seferovic, P.; Ristic, A.; Velicki, L.; Filipovic, N.; et al. Association between heart rate variability and haemodynamic response to exercise in chronic heart failure. Scand. Cardiovasc. J. 2019, 53, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Fontana, P.; Betschon, K.; Boutellier, U.; Toigo, M. Cardiac output but not stroke volume is similar in a Wingate and O2peak test in young men. Eur. J. Appl. Physiol. 2011, 111, 155–158. [Google Scholar] [CrossRef] [PubMed]
- Corrieri, N.; Del Torto, A.; Vignati, C.; Maragna, R.; De Martino, F.; Cellamare, M.; Farina, S.; Salvioni, E.; Bonomi, A.; Agostoni, P. Cardiac output changes during exercise in heart failure patients: Focus on mid-exercise. ESC Heart Fail. 2021, 8, 55–62. [Google Scholar] [CrossRef]
- Shelton, R.J.; Ingle, L.; Rigby, A.S.; Witte, K.K.; Cleland, J.G.F.; Clark, A.L. Cardiac output does not limit submaximal exercise capacity in patients with chronic heart failure. Eur. J. Heart Fail. 2010, 12, 983–989. [Google Scholar] [CrossRef]
- Schmidt, T.; Bjarnason-Wehrens, B.; Mommertz, S.; Hannig, M.; Schulte-Eistrup, S.; Willemsen, D.; Reiss, N. Changes in Total Cardiac Output and Oxygen Extraction During Exercise in Patients Supported with an HVAD Left Ventricular Assist Device. Artif. Organs 2018, 42, 686–694. [Google Scholar] [CrossRef]
- Okwose, N.C.; Chowdhury, S.; Houghton, D.; Trenell, M.I.; Eggett, C.; Bates, M.; MacGowan, G.A.; Jakovljevic, D.G. Comparison of cardiac output estimates by bioreactance and inert gas rebreathing methods during cardiopulmonary exercise testing. Clin. Physiol. Funct. Imaging 2018, 38, 483–490. [Google Scholar] [CrossRef]
- Apostolo, A.; Paolillo, S.; Contini, M.; Vignati, C.; Tarzia, V.; Campodonico, J.; Mapelli, M.; Massetti, M.; Bejko, J.; Righini, F.; et al. Comprehensive effects of left ventricular assist device speed changes on alveolar gas exchange, sleep ventilatory pattern, and exercise performance. J. Heart Lung Transplant. 2018, 37, 1361–1371. [Google Scholar] [CrossRef] [PubMed]
- Del Torto, A.; Corrieri, N.; Vignati, C.; Gentile, P.; Cattadori, G.; Paolillo, S.; Agostoni, P. Contribution of central and peripheral factors at peak exercise in heart failure patients with progressive severity of exercise limitation. Int. J. Cardiol. 2017, 248, 252–256. [Google Scholar] [CrossRef]
- Vignati, C.; Morosin, M.; Fusini, L.; Pezzuto, B.; Spadafora, E.; De Martino, F.; Salvioni, E.; Rovai, S.; Filardi, P.P.; Sinagra, G.; et al. Do rebreathing manoeuvres for non-invasive measurement of cardiac output during maximum exercise test alter the main cardiopulmonary parameters? Eur. J. Prev. Cardiol. 2019, 26, 1616–1622. [Google Scholar] [CrossRef] [PubMed]
- Morosin, M.; Farina, S.; Vignati, C.; Spadafora, E.; Sciomer, S.; Salvioni, E.; Sinagra, G.; Agostoni, P. Exercise performance, haemodynamics, and respiratory pattern do not identify heart failure patients who end exercise with dyspnoea from those with fatigue. ESC Heart Fail. 2018, 5, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Cattadori, G.; Schmid, J.-P.; Brugger, N.; Gondoni, E.; Palermo, P.; Agostoni, P. Hemodynamic effects of exercise training in heart failure. J. Card. Fail. 2011, 17, 916–922. [Google Scholar] [CrossRef] [PubMed]
- Reiss, N.; Schmidt, T.; Mommertz, S.; Feldmann, C.; Schmitto, J.D. Inert gas rebreathing-helpful tool in the management of left ventricular assist device patients. Perfusion 2018, 33, 335–338. [Google Scholar] [CrossRef]
- Vignati, C.; Apostolo, A.; Cattadori, G.; Farina, S.; Del Torto, A.; Scuri, S.; Gerosa, G.; Bottio, T.; Tarzia, V.; Bejko, J.; et al. Lvad pump speed increase is associated with increased peak exercise cardiac output and vo2, postponed anaerobic threshold and improved ventilatory efficiency. Int. J. Cardiol. 2017, 230, 28–32. [Google Scholar] [CrossRef]
- Iellamo, F.; Manzi, V.; Caminiti, G.; Vitale, C.; Castagna, C.; Massaro, M.; Franchini, A.; Rosano, G.; Volterrani, M. Matched dose interval and continuous exercise training induce similar cardiorespiratory and metabolic adaptations in patients with heart failure. Int. J. Cardiol. 2013, 167, 2561–2565. [Google Scholar] [CrossRef]
- Pokan, R.; Ocenasek, H.; Hochgatterer, R.; Miehl, M.; Vonbank, K.; Von Duvillard, S.P.; Franklin, B.; Würth, S.; Volf, I.; Wonisch, M.; et al. Myocardial dimensions and hemodynamics during 24-h ultraendurance ergometry. Med. Sci. Sports Exerc. 2014, 46, 268–275. [Google Scholar] [CrossRef]
- Fontana, P.; Boutellier, U.; Toigo, M. Non-invasive haemodynamic assessments using InnocorTM during standard graded exercise tests. Eur. J. Appl. Physiol. 2010, 108, 573–580. [Google Scholar] [CrossRef] [PubMed]
- Prochnau, D.; Forberg, T.; Khnert, H.; Heinke, M.; Figulla, H.R.; Surber, R. Optimization of the atrioventricular delay during cardiac resynchronization therapy using a device for non-invasive measurement of cardiac index at rest and during exercise. Europace 2012, 14, 249–253. [Google Scholar] [CrossRef]
- Lang, C.C.; Karlin, P.; Haythe, J.; Lim, T.K.; Mancini, D.M. Peak cardiac power output, measured noninvasively, is a powerful predictor of outcome in chronic heart failure. Circ. Heart Fail. 2009, 2, 33–38. [Google Scholar] [CrossRef]
- Jakovljevic, D.G.; Birks, E.J.; George, R.S.; Trenell, M.I.; Seferovic, P.M.; Yacoub, M.H.; Brodie, D.A. Relationship between peak cardiac pumping capability and selected exercise-derived prognostic indicators in patients treated with left ventricular assist devices. Eur. J. Heart Fail. 2011, 13, 992–999. [Google Scholar] [CrossRef]
- Jakovljevic, D.G.; Seferovic, P.M.; Nunan, D.; Donovan, G.; Trenell, M.I.; Grocott-Mason, R.; Brodie, D.A. Reproducibility of cardiac power output and other cardiopulmonary exercise indices in patients with chronic heart failure. Clin. Sci. 2012, 122, 175–181. [Google Scholar] [CrossRef]
- Vignati, C.; De Martino, F.; Muratori, M.; Salvioni, E.; Tamborini, G.; Bartorelli, A.; Pepi, M.; Alamanni, F.; Farina, S.; Cattadori, G.; et al. Rest and exercise oxygen uptake and cardiac output changes 6 months after successful transcatheter mitral valve repair. ESC Heart Fail. 2021, 8, 4915–4924. [Google Scholar] [CrossRef]
- Bentley, R.F.; Jones, J.H.; Hirai, D.M.; Zelt, J.T.; Giles, M.D.; Raleigh, J.P.; Quadrilatero, J.; Gurd, B.J.; Neder, J.A.; Tschakovsky, M.E. Submaximal exercise cardiac output is increased by 4 weeks of sprint interval training in young healthy males with low initial -O2: Importance of cardiac response phenotype. PLoS ONE 2019, 14, e0195458. [Google Scholar] [CrossRef] [PubMed]
- Jakovljevic, D.G.; George, R.S.; Nunan, D.; Donovan, G.; Bougard, R.S.; Yacoub, M.H.; Birks, E.J.; Brodie, D.A. The impact of acute reduction of continuous-flow left ventricular assist device support on cardiac and exercise performance. Heart 2010, 96, 1390–1395. [Google Scholar] [CrossRef]
- Shen, Y.; Song, H.; Ma, W.; Gong, Z.; Ni, Y.; Zhang, X.; Xu, W.; Jiang, J.; Che, L.; Xu, J.; et al. The prognostic value of peak cardiac power output in Chinese patients with chronic heart failure. PLoS ONE 2016, 11, e0147423. [Google Scholar] [CrossRef] [PubMed]
- Rajani, R.; Rimington, H.; Chambers, J.B. Treadmill exercise in apparently asymptomatic patients with moderate or severe aortic stenosis: Relationship between cardiac index and revealed symptoms. Heart 2010, 96, 689–695. [Google Scholar] [CrossRef] [PubMed]
- Pastormerlo, L.E.; Agazio, A.; Benelli, E.; Gabutti, A.; Poletti, R.; Prontera, C.; Clerico, A.; Emdin, M.; Passino, C. Usefulness of high-sensitive troponin elevation after effort stress to unveil vulnerable myocardium in patients with heart failure. Am. J. Cardiol. 2015, 116, 567–572. [Google Scholar] [CrossRef]
- Hassan, M.; Wagdy, K.; Kharabish, A.; Selwanos, P.P.; Nabil, A.; Elguindy, A.; ElFaramawy, A.; Elmahdy, M.F.; Mahmoud, H.; Yacoub, M.H. Validation of noninvasive measurement of cardiac output using inert gas rebreathing in a cohort of patients with heart failure and reduced ejection fraction. Circ. Heart Fail. 2017, 10, e003592. [Google Scholar] [CrossRef]
- Lee, W.-T.N.; Brown, A.; Peacock, A.J.; Johnson, M.K. Use of non-invasive haemodynamic measurements to detect treatment response in precapillary pulmonary hypertension. Thorax 2011, 66, 810–814. [Google Scholar] [CrossRef]
- Bentley, R.F.; Jones, J.H.; Hirai, D.M.; Zelt, J.T.; Giles, M.D.; Raleigh, J.P.; Quadrilatero, J.; Gurd, B.J.; Neder, J.A.; Tschakovsky, M.E. Do interindividual differences in cardiac output during submaximal exercise explain differences in exercising muscle oxygenation and ratings of perceived exertion? Physiol. Rep. 2018, 6, e13570. [Google Scholar] [CrossRef]
- Siebenmann, C.; Rasmussen, P.; Sørensen, H.; Zaar, M.; Hvidtfeldt, M.; Pichon, A.; Secher, N.H.; Lundby, C. Cardiac output during exercise: A comparison of four methods. Scand. J. Med. Sci. Sports 2015, 25, e20–e27. [Google Scholar] [CrossRef]
- Ananey, O.M.; Malone, J.; Warmington, S.; O’Shea, D.; Green, S.; Egaña, M. Cardiac output is not related to the slowed O2 uptake kinetics in type 2 diabetes. Med. Sci. Sports Exerc. 2011, 43, 935–942. [Google Scholar] [CrossRef] [PubMed]
- Le, V.D.T. Cardiopulmonary Exercise Testing in Aortic Stenosis. Dan. Med. J. 2017, 64, B5352. [Google Scholar] [PubMed]
- O’Connor, E.; Green, S.; Kiely, C.; O’Shea, D.; Egaña, M. Differential effects of age and type 2 diabetes on dynamic vs. peak response of pulmonary oxygen uptake during exercise. J. Appl. Physiol. 2015, 118, 1031–1039. [Google Scholar] [CrossRef] [PubMed]
- Galera, R.; Casitas, R.; Martínez-Cerón, E.; Rodríguez-Fraga, O.; Utrilla, C.; Torres, I.; Cubillos-Zapata, C.; García-Río, F. Effect of Dynamic Hyperinflation on Cardiac Response to Exercise of Patients with Chronic Obstructive Pulmonary Disease|Efecto de la hiperinsuflación dinámica en la respuesta cardíaca al ejercicio de pacientes con enfermedad pulmonar obstructiva crónica. Arch. Bronconeumol. 2021, 57, 406–414. [Google Scholar] [CrossRef]
- Jones, J.H.; Zelt, J.T.; Hirai, D.M.; Diniz, C.V.; Zaza, A.; O’Donnell, D.E.; Neder, J.A. Emphysema on Thoracic CT and Exercise Ventilatory Inefficiency in Mild-to-Moderate COPD. Copd-J. Chronic Obstr. Pulm. Dis. 2017, 14, 210–218. [Google Scholar] [CrossRef]
- Kiely, C.; Rocha, J.; O’Connor, E.; O’Shea, D.; Green, S.; Egaña, M. Influence of menopause and Type 2 diabetes on pulmonary oxygen uptake kinetics and peak exercise performance during cycling. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015, 309, R875–R883. [Google Scholar] [CrossRef]
- Laroche, D.; Joussain, C.; Espagnac, C.; Morisset, C.; Tordi, N.; Gremeaux, V.; Casillas, J.M. Is it possible to individualize intensity of eccentric cycling exercise from perceived exertion on concentric test? Arch. Phys. Med. Rehabil. 2013, 94, 1621–1627. [Google Scholar] [CrossRef]
- Schmid, J.P.; Noveanu, M.; Morger, C.; Gaillet, R.; Capoferri, M.; Anderegg, M.; Saner, H. Influence of water immersion, water gymnastics and swimming on cardiac output in patients with heart failure. Heart 2007, 93, 722–727. [Google Scholar] [CrossRef]
- American Thoracic Society; American College of Chest Physicians. ATS/ACCP Statement on cardiopulmonary exercise testing. Am. J. Respir. Crit. Care Med. 2003, 167, 211–277, Erratum in Am. J. Respir. Crit. Care Med. 2003, 1451–1452. [Google Scholar] [CrossRef]
- Reiss, N.; Schmidt, T.; Workowski, A.; Willemsen, D.; Schmitto, J.D.; Haverich, A.; Bjarnason-Wehrens, B. Physical capacity in LVAD patients: Hemodynamic principles, diagnostic tools and training control. Int. J. Artif. Organs 2016, 39, 451–459. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).