Efficacy of Percutaneous Treatment of Primary Aneurysmal Bone Cysts (ABCs): A Systematic Review and Meta-Analysis
Abstract
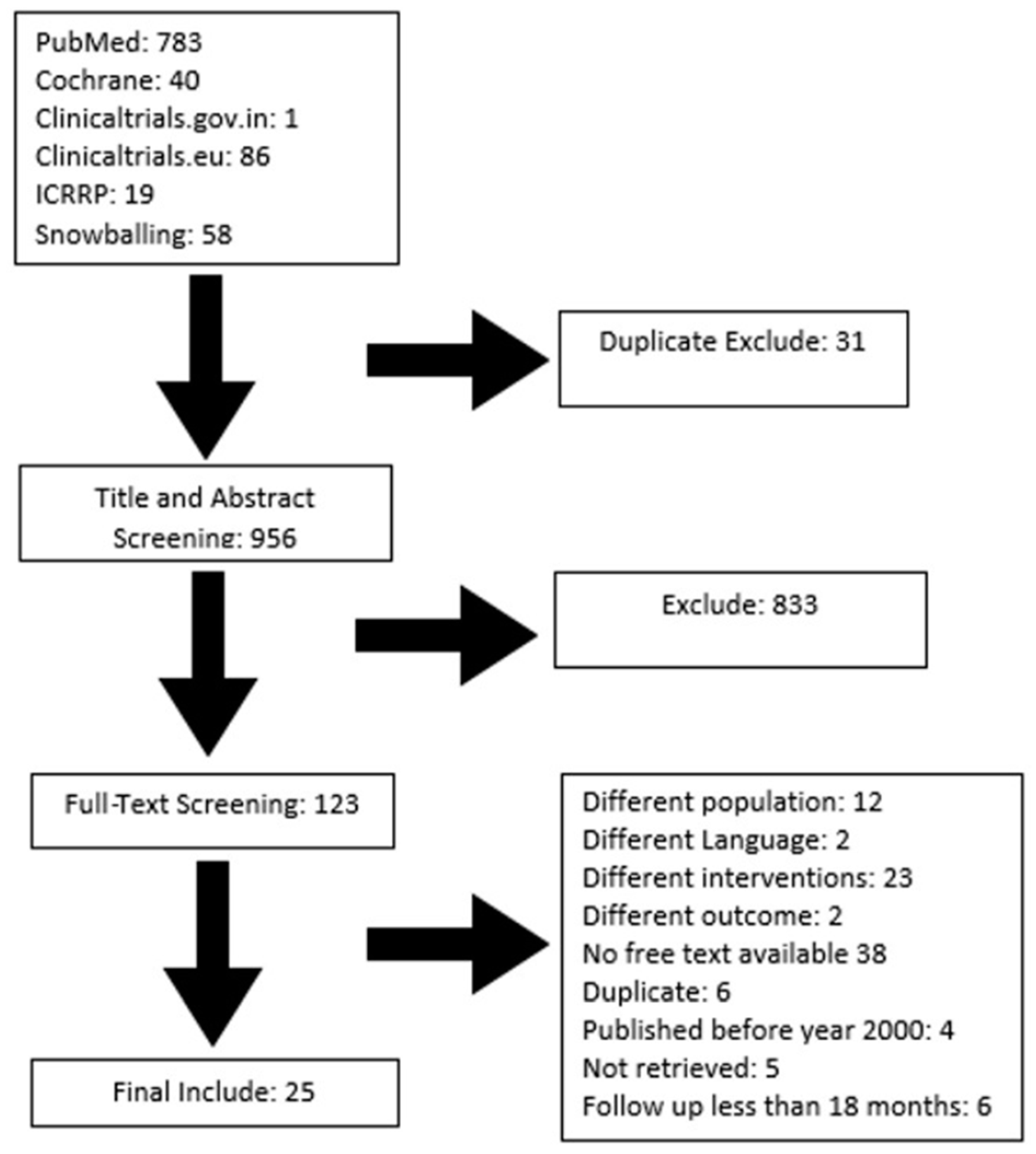
:1. Introduction
2. Materials and Methods
2.1. Searching
2.2. Selection and Screening
- Population: Studies that had primary percutaneous ABC treatments in which interventions were evaluated with a mean follow-up period of at least 18 months.
- Intervention: Studies that used embolization, doxycycline/albumin, calcitonin with methylprednisolone, alcohol, Ethibloc, or polidocanol for treating primary ABCs.
- Comparator: Studies comparing any sclerotherapy agents, either using surgical or no intervention.
- Outcome: Complete healing, partial healing, failure, recurrence, number of injections administered for healing, and complications.
- Studies: Retrospective or prospective studies, RCTs, clinical trials. In studies with multiple intervention groups, only patients administered any sclerotherapy agents or embolization would be selected.
- Language: English.
- Time: Published from 2000 to 2021.
- Population: Studies that had primary percutaneous ABC treatments and evaluations of interventions with a mean follow-up period less than 18 months. Studies assessing secondary ABCs.
- Intervention: Studies that used any sclerotherapy as secondary treatment for primary ABCs, surgical therapy, non-injection therapy, or only mixed treatments.
- Comparator: Studies comparing other modalities apart from our interventions of interest.
- Outcome: Other outcomes apart from our interventions of interest.
- Studies: Case reports, letters, narrative reviews, or systematic reviews.
- Language: Non-English.
- Time: Studies published before 2000.
2.3. Data Extraction and Quality Assessment
2.4. Narrative Synthesis and Meta-Analysis
3. Results
3.1. Study Characteristics
3.2. Study Outcomes
3.3. Narrative Synthesis
3.4. Meta-Analysis
3.5. Subgroup Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Deventer, N.; Schulze, M.; Gosheger, G.; de Vaal, M.; Deventer, N. Primary Aneurysmal Bone Cyst and Its Recent Treatment Options: A Comparative Review of 74 Cases. Cancers 2021, 13, 2362. [Google Scholar] [CrossRef]
- Amendola, L.; Simonetti, L.; Simoes, C.E.; Bandiera, S.; De Iure, F.; Boriani, S. Aneurysmal Bone Cyst of the Mobile Spine: The Therapeutic Role of Embolization. Eur. Spine J. 2013, 22, 533–541. [Google Scholar] [CrossRef]
- Bavan, L.; Wijendra, A.; Kothari, A. Efficacy of Treatment Interventions for Primary Aneurysmal Bone Cysts: A Systematic Review. Bone Jt Open 2021, 2, 125–133. [Google Scholar] [CrossRef]
- Cottalorda, J.; Bourelle, S. Modern Concepts of Primary Aneurysmal Bone Cyst. Arch. Orthop. Trauma Surg. 2007, 127, 105–114. [Google Scholar] [CrossRef]
- Gutierrez, L.B.; Link, T.M.; Horvai, A.E.; Joseph, G.B.; O’Donnell, R.J.; Motamedi, D. Secondary Aneurysmal Bone Cysts and Associated Primary Lesions: Imaging Features of 49 Cases. Clin. Imaging 2020, 62, 23–32. [Google Scholar] [CrossRef]
- Park, H.Y.; Yang, S.K.; Sheppard, W.L.; Hegde, V.; Zoller, S.D.; Nelson, S.D.; Federman, N.; Bernthal, N.M. Current Management of Aneurysmal Bone Cysts. Curr. Rev. Musculoskelet. Med. 2016, 9, 435–444. [Google Scholar] [CrossRef]
- Muratori, F.; Mondanelli, N.; Rizzo, A.R.; Beltrami, G.; Giannotti, S.; Capanna, R.; Campanacci, D.A. Aneurysmal Bone Cyst: A Review of Management. Surg. Technol. Int. 2019, 35, 325–335. [Google Scholar]
- Restrepo, R.; Zahrah, D.; Pelaez, L.; Temple, H.T.; Murakami, J.W. Update on Aneurysmal Bone Cyst: Pathophysiology, Histology, Imaging and Treatment. Pediatr. Radiol. 2022, 52, 1601–1614. [Google Scholar] [CrossRef]
- Varshney, M.K.; Rastogi, S.; Khan, S.A.; Trikha, V. Is Sclerotherapy Better than Intralesional Excision for Treating Aneurysmal Bone Cysts? Clin. Orthop. Relat. Res. 2010, 468, 1649–1659. [Google Scholar] [CrossRef]
- Puthoor, D.; Francis, L.; Ismail, R. Is Sclerotherapy with Polidocanol a Better Treatment Option for Aneurysmal Bone Cyst Compared to Conventional Curettage and Bone Grafting? J. Orthop. 2021, 25, 265–270. [Google Scholar] [CrossRef]
- Cruz, G.S.; Cuevas-Suárez, C.E.; Saavedra, J.P.A.; Giorgis, R.; Teixeira, M.R.K.; Muniz, F.W.M.G. Percutaneous Treatments of Primary Aneurysmal Bone Cysts: Systematic Review and Meta-Analysis. Eur. J. Orthop. Surg. Traumatol. 2021, 31, 1287–1295. [Google Scholar] [CrossRef]
- Rossi, G.; Mavrogenis, A.F.; Papagelopoulos, P.J.; Rimondi, E.; Ruggieri, P. Successful Treatment of Aggressive Aneurysmal Bone Cyst of the Pelvis With Serial Embolization. Orthopedics 2012, 35, e963–e968. [Google Scholar] [CrossRef]
- Protas, M.; Jones, L.W.; Sardi, J.P.; Fisahn, C.; Iwanaga, J.; Oskouian, R.J.; Tubbs, R.S. Cervical Spine Aneurysmal Bone Cysts in the Pediatric Population: A Systematic Review of the Literature. Pediatr. Neurosurg. 2017, 52, 219–224. [Google Scholar] [CrossRef]
- Ohashi, M.; Ito, T.; Hirano, T.; Endo, N. Percutaneous Intralesional Injection of Calcitonin and Methylprednisolone for Treatment of an Aneurysmal Bone Cyst at C-2: Case Report. J. Neurosurg. Pediatr. 2008, 2, 365–369. [Google Scholar] [CrossRef]
- Shiels, W.E.; Mayerson, J.L. Percutaneous Doxycycline Treatment of Aneurysmal Bone Cysts With Low Recurrence Rate: A Preliminary Report. Clin. Orthop. Relat. Res. 2013, 471, 2675–2683. [Google Scholar] [CrossRef]
- Wong, M.N.; Braswell, L.E.; Murakami, J.W. Doxycycline Sclerotherapy of Cervical Spine Aneurysmal Bone Cysts: Single-Institution 13-Year Experience. Pediatr. Radiol. 2022, 52, 1528–1538. [Google Scholar] [CrossRef]
- Study Quality Assessment Tools | NHLBI, NIH. Available online: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools (accessed on 8 September 2023).
- Adamsbaum, C.; Mascard, E.; Guinebretière, J.M.; Kalifa, G.; Dubousset, J. Intralesional Ethibloc Injections in Primary Aneurysmal Bone Cysts: An Efficient and Safe Treatment. Skelet. Radiol. 2003, 32, 559–566. [Google Scholar] [CrossRef]
- Batisse, F.; Schmitt, A.; Vendeuvre, T.; Herbreteau, D.; Bonnard, C. Aneurysmal Bone Cyst: A 19-Case Series Managed by Percutaneous Sclerotherapy. Orthop. Traumatol. Surg. Res. 2016, 102, 213–216. [Google Scholar] [CrossRef]
- Dubois, J.; Chigot, V.; Grimard, G.; Isler, M.; Garel, L. Sclerotherapy in Aneurysmal Bone Cysts in Children: A Review of 17 Cases. Pediatr. Radiol. 2003, 33, 365–372. [Google Scholar] [CrossRef]
- Falappa, P.; Fassari, F.M.; Fanelli, A.; Genovese, E.; Ascani, E.; Crostelli, M.; Salsano, V.; Montanaro, A.; Di Lazzaro, A.; Serra, F. Aneurysmal Bone Cysts: Treatment with Direct Percutaneous Ethibloc Injection: Long-Term Results. Cardiovasc. Interv. Radiol. 2002, 25, 282–290. [Google Scholar] [CrossRef]
- Topouchian, V.; Mazda, K.; Hamze, B.; Laredo, J.-D.; Penneçot, G.-F. Aneurysmal Bone Cysts in Children: Complications of Fibrosing Agent Injection. Radiology 2004, 232, 522–526. [Google Scholar] [CrossRef]
- Garg, N.K.; Carty, H.; Walsh, H.P.; Dorgan, J.C.; Bruce, C.E. Percutaneous Ethibloc Injection in Aneurysmal Bone Cysts. Skelet. Radiol. 2000, 29, 211–216. [Google Scholar] [CrossRef]
- George, H.L.; Unnikrishnan, P.N.; Garg, N.K.; Sampath, J.S.; Bass, A.; Bruce, C.E. Long-Term Follow-up of Ethibloc Injection in Aneurysmal Bone Cysts. J. Pediatr. Orthop. B 2009, 18, 375–380. [Google Scholar] [CrossRef]
- de Gauzy, J.S.; Abid, A.; Accadbled, F.; Knorr, G.; Darodes, P.; Cahuzac, J.P. Percutaneous Ethibloc Injection in the Treatment of Primary Aneurysmal Bone Cysts. J. Pediatr. Orthop. B 2005, 14, 367–370. [Google Scholar] [CrossRef]
- Shiels, W.E.; Beebe, A.C.; Mayerson, J.L. Percutaneous Doxycycline Treatment of Juxtaphyseal Aneurysmal Bone Cysts. J. Pediatr. Orthop. 2016, 36, 205–212. [Google Scholar] [CrossRef]
- Woon, J.T.K.; Hoon, D.; Graydon, A.; Flint, M.; Doyle, A.J. Aneurysmal Bone Cyst Treated with Percutaneous Doxycycline: Is a Single Treatment Sufficient? Skelet. Radiol. 2019, 48, 765–771. [Google Scholar] [CrossRef]
- Liu, X.; Han, S.B.; Si, G.; Yang, S.M.; Wang, C.M.; Jiang, L.; Wei, F.; Wu, F.L.; Liu, X.G.; Liu, Z.J. Percutaneous Albumin/Doxycycline Injection versus Open Surgery for Aneurysmal Bone Cysts in the Mobile Spine. Eur. Spine J. 2019, 28, 1529–1536. [Google Scholar] [CrossRef]
- Rossi, G.; Mavrogenis, A.F.; Facchini, G.; Bartalena, T.; Rimondi, E.; Renzulli, M.; Andreone, A.; Durante, S.; Angelini, A.; Errani, C. How Effective Is Embolization with N-2-Butyl-Cyanoacrylate for Aneurysmal Bone Cysts? Int. Orthop. (SICOT) 2017, 41, 1685–1692. [Google Scholar] [CrossRef]
- Cheng, Z.; Peng, X.; He, W. Arterial Embolization of Primary Sacral Aneurysmal Bone Cyst. Chin. Med. J. 2014, 127, 1785–1787. [Google Scholar]
- Henrichs, M.P.; Beck, L.; Gosheger, G.; Streitbuerger, A.; Koehler, M.; Heindel, W.; Hardes, J.; Vieth, V. Selective Arterial Embolisation of Aneurysmal Bone Cysts of the Sacrum: A Promising Alternative to Surgery. Rofo 2016, 188, 53–59. [Google Scholar] [CrossRef]
- Lambot-Juhan, K.; Pannier, S.; Grévent, D.; Péjin, Z.; Breton, S.; Berteloot, L.; Emond-Gonsard, S.; Boddaert, N.; Glorion, C.; Brunelle, F. Primary Aneurysmal Bone Cysts in Children: Percutaneous Sclerotherapy with Absolute Alcohol and Proposal of a Vascular Classification. Pediatr. Radiol. 2012, 42, 599–605. [Google Scholar] [CrossRef]
- Marie-Hardy, L.; El Sayed, L.; Alves, A.; Brunelle, F.; Ouchrif, Y.; Naggara, O.; Breton, S.; Mascard, E.; Glorion, C.; Pannier, S. Percutaneous Alcohol-Based Sclerotherapy in Aneurysmal Bone Cyst in Children and Adolescents. Orthop. Traumatol. Surg. Res. 2020, 106, 1313–1318. [Google Scholar] [CrossRef]
- Oliveira, M.B.D.R.; Meohas, W.; Silva, R.R.; de Carvalho, G.S.; Mello, F.C.D.Q.; Paschoal, M.E.M. Percutaneous Treatment of Aneurysmal Bone Cyst with Calcitonin and Methylprednisolone. Acta Ortop. Bras. 2018, 26, 314–319. [Google Scholar] [CrossRef]
- Rastogi, S.; Varshney, M.K.; Trikha, V.; Khan, S.A.; Choudhury, B.; Safaya, R. Treatment of Aneurysmal Bone Cysts with Percutaneous Sclerotherapy Using Polidocanol. A Review of 72 Cases with Long-Term Follow-Up. J. Bone Jt. Surg. Br. 2006, 88, 1212–1216. [Google Scholar] [CrossRef]
- Puri, A.; Hegde, P.; Gulia, A.; Parikh, M. Primary Aneurysmal Bone Cysts. Bone Jt. J. 2020, 102-B, 186–190. [Google Scholar] [CrossRef]
- Jasper, J.; van der Heijden, L.; van Rijswijk, C.S.P.; van de Sande, M.A.J. Efficacy of Sclerotherapy With Polidocanol (Ethoxysclerol) in Primary Aneurysmal Bone Cysts in Children and Adolescents. J. Pediatr. Orthop. 2021, 41, e555–e562. [Google Scholar] [CrossRef]
- Cottalorda, J.; Louahem Sabah, D.; Joly Monrigal, P.; Jeandel, C.; Delpont, M. Minimally Invasive Treatment of Aneurysmal Bone Cysts: Systematic Literature Review. Orthop. Traumatol. Surg. Res. 2022, 108, 103272. [Google Scholar] [CrossRef]
- van Geloven, T.P.G.; van de Sande, M.A.J.; van der Heijden, L. The Treatment of Aneurysmal Bone Cysts. Curr. Opin. Pediatr. 2023, 35, 131–137. [Google Scholar] [CrossRef]
- Strohm, J.A.; Strohm, P.C.; Kühle, J.; Schmal, H.; Zwingmann, J. Management of Juvenile and Aneurysmal Bone Cysts: A Systematic Literature Review with Meta-Analysis. Eur. J. Trauma Emerg. Surg. 2023, 49, 361–372. [Google Scholar] [CrossRef]









| Study Name | Country | Study Design | Sample Size | Treatments/Interventions | Quality of Study |
|---|---|---|---|---|---|
| Adamsbaum 2003 [18] | France | Retrospective study | 17 | Inj. Ethibloc | Fair |
| Batisse 2016* [19] | France | Retrospective study | 6 | Inj. Ethibloc | Poor * |
| Dubois 2003 [20] | Canada | Retrospective study | 14 | Inj. Ethibloc | Fair |
| Falappa 2002 [21] | Italy | Retrospective study | 13 | Inj. Ethibloc | Fair |
| Topouchian 2004 [22] | France | Retrospective study | 15 | Inj. Ethibloc | Good |
| Garg 2000 [23] | UK | Retrospective study | 10 | Inj. Ethibloc | Poor |
| George 2009 [24] | UK | Retrospective study | 31 | Inj. Ethibloc | Good |
| De Gauzy 2005 [25] | France | Retrospective study | 12 | Inj. Ethibloc | Poor |
| Shiels 2013 [15] | USA | Retrospective study | 20 | Inj. doxycycline/albumin | Fair |
| Shiels 2016 [26] | USA | Retrospective study | 16 | Inj. doxycycline/albumin | Fair |
| Woon 2019 [27] | New Zealand | Retrospective study | 7 | Inj. doxycycline/albumin | Poor |
| Liu 2019 [28] | China | Retrospective study | 14 | Inj. doxycycline/albumin | Fair |
| Rossi 2016 [29] | Italy | Retrospective study | 88 | Embolization | Fair |
| Cheng 2014 [30] | China | Retrospective study | 9 | Embolization | Poor |
| Henrichs 2015 [31] | Germany | Retrospective study | 6 | Embolization | Poor |
| Amendola 2013 [2] | Italy | Retrospective study | 7 | Embolization | Poor |
| Lambot-Juhan 2012 [32] | France | Retrospective study | 29 | Inj. alcohol | Good |
| Marie-Hardy 2020 [33] | France | Retrospective study | 54 | Inj. alcohol | Fair |
| Oliveira 2018 [34] | Brazil | Retrospective study | 47 | Inj. calcitonin with methylprednisolone | Fair |
| Batisse 2016 * [19] | France | Retrospective study | 9 | Inj. polidocanol | Poor * |
| Rastogi 2006 [35] | India | Prospective study | 72 | Inj. polidocanol | Fair |
| Deventer 2021 [1] | Germany | Retrospective study | 32 | Inj. polidocanol | Good |
| Varshney 2010 [9] | India | RCT | 45 | Inj. polidocanol | Good |
| Puri 2020 [36] | India | Retrospective study | 55 | Inj. polidocanol | Fair |
| Puthoor 2021 [10] | India | Retrospective study | 31 | Inj. polidocanol | Good |
| Jasper 2021 [37] | Netherlands | Retrospective study | 70 | Inj. polidocanol | Good |
| Study Name | Mean Age (Year) | ABC Site | Mean Follow-Up Time (Months) |
|---|---|---|---|
| Adamsbaum 2003 [18] | 8 [2–18] | Pelvis, femur, humerus, fibula, clavicle, ulna, metacarpal | 60 [18–132] |
| Batisse 2016 [19] | 12 [3–17] | Femur, humerus, foot and ankle, pelvis, spine, fibula | 35 [3–96] |
| Dubois 2003 [20] | 10.71 [2.5–15] | Metatarsal bone, humerus, paranasal sinus, mandible, humerus, acetabulum, fibula, ribs, sacrum | 57.3 [24–108] |
| Falappa 2002 [21] | 12 [5–23] | Femur, tibia, humerus, pelvis, ankle, foot | 25.5 [6–67] |
| Topouchian 2004 [22] | 10.3 [3–15] | Femur, tibia, humerus, pelvis | 80 [47–116] |
| Garg 2000 [23] | 11.8 [4–16] | Ilium, tibia, humerus, femur | 29.8 [6–60] |
| George 2009 [24] | [3–16] | Humerus, femur, tibia, fibula, pelvis | 54 [22–90] |
| De Gauzy 2005 [25] | 9.6 [5–13.5] | Tibia, femur, fibula, femur, metatarsal bone, clavicle, humerus | 64 [24–81] |
| Shiels 2013 [15] | 10 [3–18] | Humerus, spine, clavicle, fibula, femur, ulna, tibia, scapula | 38 [24–63] |
| Shiels 2016 [26] | 7.1 [2–15] | Tibia, humerus, fibula, femur, ulna | 42 [24–67] |
| Woon 2019 [27] | 12.42 [8–18] | Spine, sacrum, pelvis, femur | 26 [14–60] |
| Liu 2019 [28] | 18.4 [6–36] | Spine | 30.7 [24–50] |
| Rossi 2016 [29] | 16 [3–60] | Pelvis, sacrum, spine, femur, tibia, fibula, humerus, scapula, clavicle | 84 [30–156] |
| Cheng 2014 [30] | 24 [11–43] | Sacrum | 32 [24–47] |
| Henrichs 2015 [31] | 13.7 [8–18] | Sacrum | 36.5 [14–56] |
| Amendola 2013 [2] | 17 [6–41] | Spine | 46 [24–64] |
| Lambot-Juhan 2012 [32] | [2–16] | Ulna, pelvis, femur, humerus, tibia, phalanx, spine, radius, clavicle, scapula | 30 [3–90] |
| Marie-Hardy 2020 [33] | 9.6 | Pelvis, calcaneum, clavicle, cuboid, femur, fibula, humerus, metacarpal bone, cervical spine, radius, tibia | 51 [24–117] |
| Oliveira 2018 [34] | 17.5 [4–54] | Scapula, clavicle, humerus, hand, femur, tibia, foot, fibula, pelvis | 45.5 [24–59.5] |
| Rastogi 2006 [35] | 15.6 [3–38] | Upper limb, lower limb, axial skeleton | 34 [25.5–80] |
| Deventer 2021 [1] | 16.88 | Ankle, foot, clavicle, scapula, pelvis | 40.5 [13.1–104] |
| Varshney 2010 [9] | 21.3 | Humerus, forearm, femur, tibia, fibula, pelvis, clavicle, hand, spine | 52.8 [38.4–73.2] |
| Puri 2020 [36] | 20 [1–54] | Clavicle, humerus, femur, tibia | 62 [20–111] |
| Puthoor 2021 [10] | 17.67 [4–45] | Clavicle, femur, fibula, humerus, pelvis, metacarpal bones, metatarsal bones, phalanx, radius, sacrum, scapula, talus, tibia | 24 |
| Jasper 2021 [37] | 11 [3–17] | Humerus, tibia, pelvis, femur, fibula, scapula, foot, hand, clavicle, scapula, ulna | 40 [18–144] |
| Study Name | Sample Size Receiving the Treatment | Mean No. of Injections | CO * or >75% Reduction in Cyst Size | PO ** or 25–75% Reduction in Cyst Size | No-Ossification or Failure | Recurrence | Post-Therapy Surgery |
|---|---|---|---|---|---|---|---|
| Adamsbaum et al., 2003 [18] | 17 | 1 [1–3] | 14 | 1 | 2 | ||
| Batisse et al., 2016 [19] (Ethibloc group) | 6 | 1.3 [1–2] | 5 | 1 | |||
| Batisse et al., 2016 [19] (polidocanol group) | 9 | 1.2 [1–2] | 5 | 4 | |||
| Dubois et al., 2003 [20] | 14 | 1.8 [1–4] | 13 | 1 | |||
| Falappa et al., 2002 [21] | 13 | 2.4 [1–4] | 12 | 1 | |||
| Topouchian et al., 2004 [22] | 15 | 1.1 [1–3] | 9 | 2 | 4 | ||
| Garg et al., 2000 [23] | 10 | 1.3 [1–2] | 7 | 3 | |||
| George et al., 2009 [24] | 31 | 1.3 [1–2] | 18 | 11 | 2 | ||
| De Gauzy et al., 2005 [25] | 12 | 1.1 [1–2] | 6 | 3 | 3 | 3 | |
| Shiels et al., 2013 [15] | 20 | 5.9 [1–14] | 19 | 1 | |||
| Shiels et al., 2016 [26] | 16 | 5.9 [2–14] | 16 | ||||
| Woon et al., 2019 [27] | 7 | 1.3 [1–2] | 6 | 1 | |||
| Liu et al., 2019 [28] | 14 | 3 [2–4] | 13 | 1 | |||
| Amendola et al., 2013 [2] | 7 | 2.1 [1–5] | 7 | ||||
| Rossi et al., 2016 [29] | 88 | 1.3 [1–3] | 72 | 16 | |||
| Cheng et al., 2014 [30] | 9 | 4.1 [3–7] | 4 | 5 | |||
| Henrichs et al., 2015 [31] | 6 | 1.5 [1–3] | 4 | 2 | |||
| Lambot-Juhan et al., 2012 [32] | 29 | 1.7 [1–4] | 17 | 9 | 3 | 4 | |
| Marie-Hardy et al., 2020 [33] | 54 | 1.7 [1–4] | 45 | 9 | 5 | ||
| Oliveira et al., 2018 [34] | 47 | 2.8 [1–7] | 34 | 12 | 1 | ||
| Rastogi et al., 2006 [35] | 72 | 3 [1–5] | 48 | 24 | |||
| Deventer et al., 2021 [1] | 32 | 5.7 [1–12] | 3 | 19 | 10 | 10 | |
| Varshney et al., 2010 [9] | 45 | 2.3 [1–5] | 42 | 2 | 1 | ||
| Puri et al., 2020 [36] | 55 | 2 [1–5] | 46 | 9 | 4 | 9 | |
| Puthoor et al., 2021 [10] | 31 | 1.1 [1–2] | 19 | 12 | |||
| Jasper et al., 2021 [37] | 70 | 1.5 [1–5] | 58 | 12 | 1 | 12 |
| Interventions | Sample Size | CH */≥75% Reduction in Cyst Size (%(n)) | Failure/No-Ossification (%(n)) | Complication Rate (%(n)) | Types of Complications | Recurrence Rate (%(n)) |
|---|---|---|---|---|---|---|
| Ethibloc Inj. | 118 | 71% (84) | 7.6% (9) | 52.5% (62) | Fever + local inflammation: 16 Cutaneous fistula: 7 Intercostal arterial reflux: 1 Post-operative pain: 1 Local inflammation: 4 Small blister leakage: 1 Fever + pain: 6 Fever + pain + leakage: 2 Pulmonary embolization: 1 Fever: 10 Sterile abscess: 1 Leakage of contrast: 3 Skin rashes and edema: 6 Sterile abscess: 1 Sustained fracture following trauma: 2 | 1.7% (2) |
| Doxycycline/albumin Inj. | 57 | 94.7% (54) | 0 | 3.5% (2) | Focal skin necrosis: 2 | 1.7% (1) |
| Embolization | 110 | 79% (87) | 0 | 4.5% (5) | Skin necrosis: 2 Permanent paresthesia: 1 Pseudoaneurysm of femoral artery: 1 Hip pain and paralysis: 1 | 14.5% (16) |
| Alcohol Inj. | 83 | 74.6% (62) | 5% (4) | 2.4% (2) | Nerve palsy: 1 Bradycardia during Inj.: 1 | 0 |
| Polidocanol Inj. | 314 | 70.6% (221) | 10% (31) | 25% (78) | Inflammatory nodule: 1 Cutaneous erythema: 1 Induration at Inj. site: 18 Hypopigmentation: 13 Local inflammatory reaction: 1 Dizziness: 1 Local induration: 37 Dizziness: 1 Local complications: 2 Other: 2 | 2.1% (7) |
| Calcitonin with methylprednisolone Inj. | 47 | 72.3% (34) | 2.1% (1) | 0 | None | 0 |
| Interventions | Sample Size | Mean No. of Inj. | Single Inj. (%(n)) | Two Inj. (%(n)) | Three Inj. (%(n)) | Four Inj. (%(n)) | Five Inj. (%(n)) | Six or More Than Six Inj. (%(n)) |
|---|---|---|---|---|---|---|---|---|
| Ethibloc Inj. | 118 | 1.35 | 66% (77) | 23% (27) | 2% (8) | 3% (4) | 0 | 0 |
| Doxycycline/albumin Inj. | 57 | 3.95 | 5% (3) | 20% (11) | 20% (11) | 16% (9) | 14% (8) | 25% (14) |
| Embolization | 110 | 1.6 | 72% (79) | 16% (18) | 9% (10) | 5% (5) | 2% (2) | 1% (1) |
| Alcohol Inj. | 83 | 1.7 | 46% (38) | 37% (31) | 14% (12) | 2.4% (2) | 0 | 0 |
| Polidocanol Inj. | 314 | 2.8 | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear |
| Calcitonin with methylprednisolone Inj. | 47 | 2.8 | 19% (9) | 30% (14) | 21% (10) | 15% (7) | 10% (5) | 4% (2) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samargandi, R.; Alkameshki, M.; Barnawi, M.; Alzahrani, K.; Iskander, O.; Nicolas, Q.; Hetaimish, B.; Berhouet, J.; Le Nail, L.-R. Efficacy of Percutaneous Treatment of Primary Aneurysmal Bone Cysts (ABCs): A Systematic Review and Meta-Analysis. J. Clin. Med. 2023, 12, 7213. https://doi.org/10.3390/jcm12237213
Samargandi R, Alkameshki M, Barnawi M, Alzahrani K, Iskander O, Nicolas Q, Hetaimish B, Berhouet J, Le Nail L-R. Efficacy of Percutaneous Treatment of Primary Aneurysmal Bone Cysts (ABCs): A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2023; 12(23):7213. https://doi.org/10.3390/jcm12237213
Chicago/Turabian StyleSamargandi, Ramy, Muhand Alkameshki, Mohammed Barnawi, Khalid Alzahrani, Othman Iskander, Quentin Nicolas, Bandar Hetaimish, Julien Berhouet, and Louis-Romée Le Nail. 2023. "Efficacy of Percutaneous Treatment of Primary Aneurysmal Bone Cysts (ABCs): A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 12, no. 23: 7213. https://doi.org/10.3390/jcm12237213
APA StyleSamargandi, R., Alkameshki, M., Barnawi, M., Alzahrani, K., Iskander, O., Nicolas, Q., Hetaimish, B., Berhouet, J., & Le Nail, L.-R. (2023). Efficacy of Percutaneous Treatment of Primary Aneurysmal Bone Cysts (ABCs): A Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 12(23), 7213. https://doi.org/10.3390/jcm12237213





