Biomarkers in Acute Myocarditis and Chronic Inflammatory Cardiomyopathy: An Updated Review of the Literature
Abstract
1. Introduction
2. Acute Myocarditis and Chronic Inflammatory Cardiomyopathy: A General Overview
2.1. Definition, Diagnostic Approach, and Treatment
2.2. Biomarkers
3. Specific Forms of Myocarditis
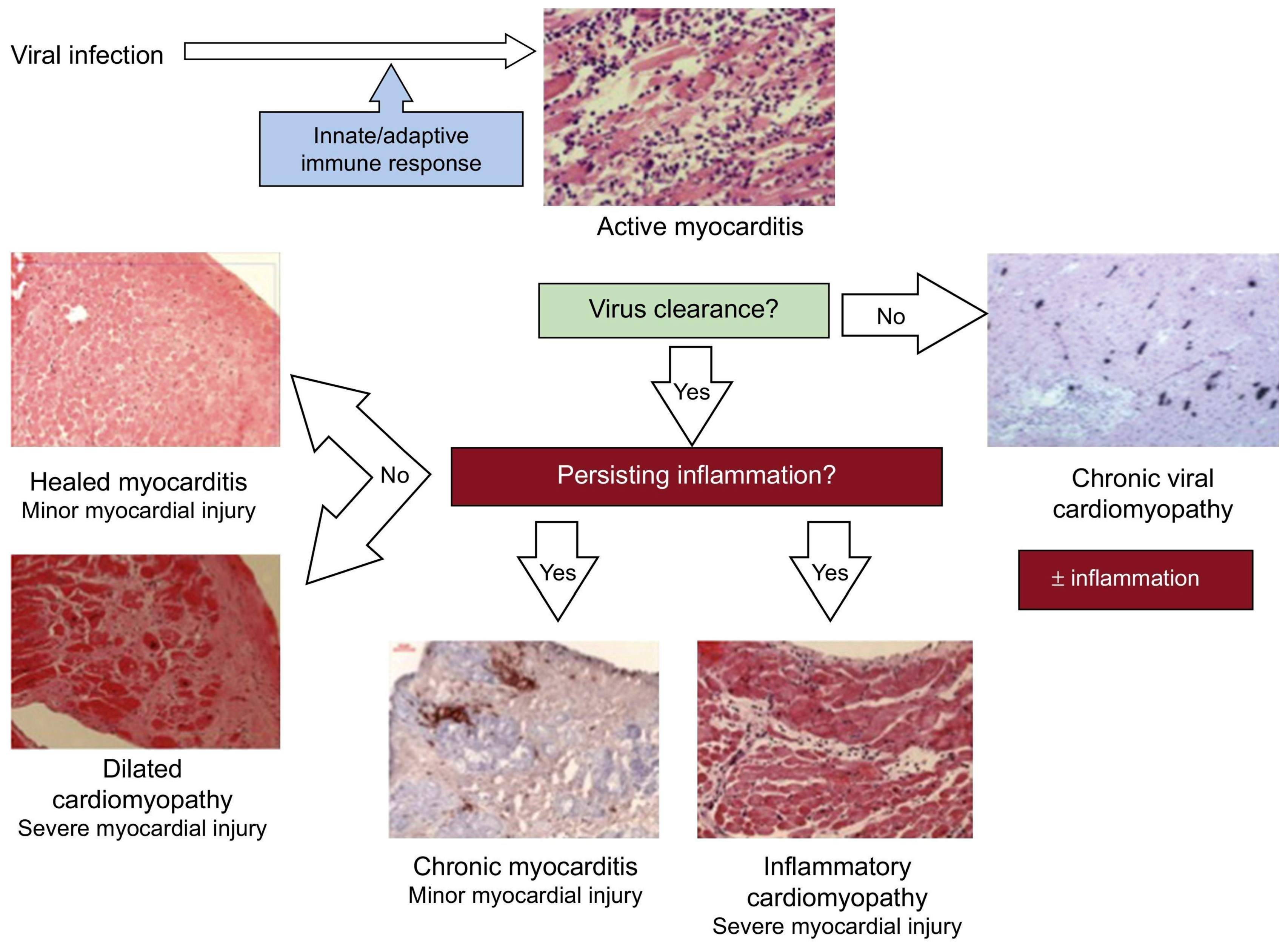
3.1. Infectious Myocarditis
3.1.1. Definition, Diagnostic Approach, and Treatment
3.1.2. Biomarkers
Circulating Biomarkers
Imaging Biomarkers
3.2. COVID-19 and Post-Vaccination Associated Myocarditis
3.2.1. Definition, Diagnostic Approach, and Treatment
3.2.2. Biomarkers
3.3. Sarcoidotic Myocarditis
3.3.1. Definition, Diagnostic Approach, and Treatment
3.3.2. Biomarkers
Circulating Biomarkers
Imaging Biomarkers
3.4. Giant Cell Myocarditis
3.4.1. Definition, Diagnostic Approach, and Treatment
3.4.2. Biomarkers
Circulating Biomarkers
Imaging Biomarkers
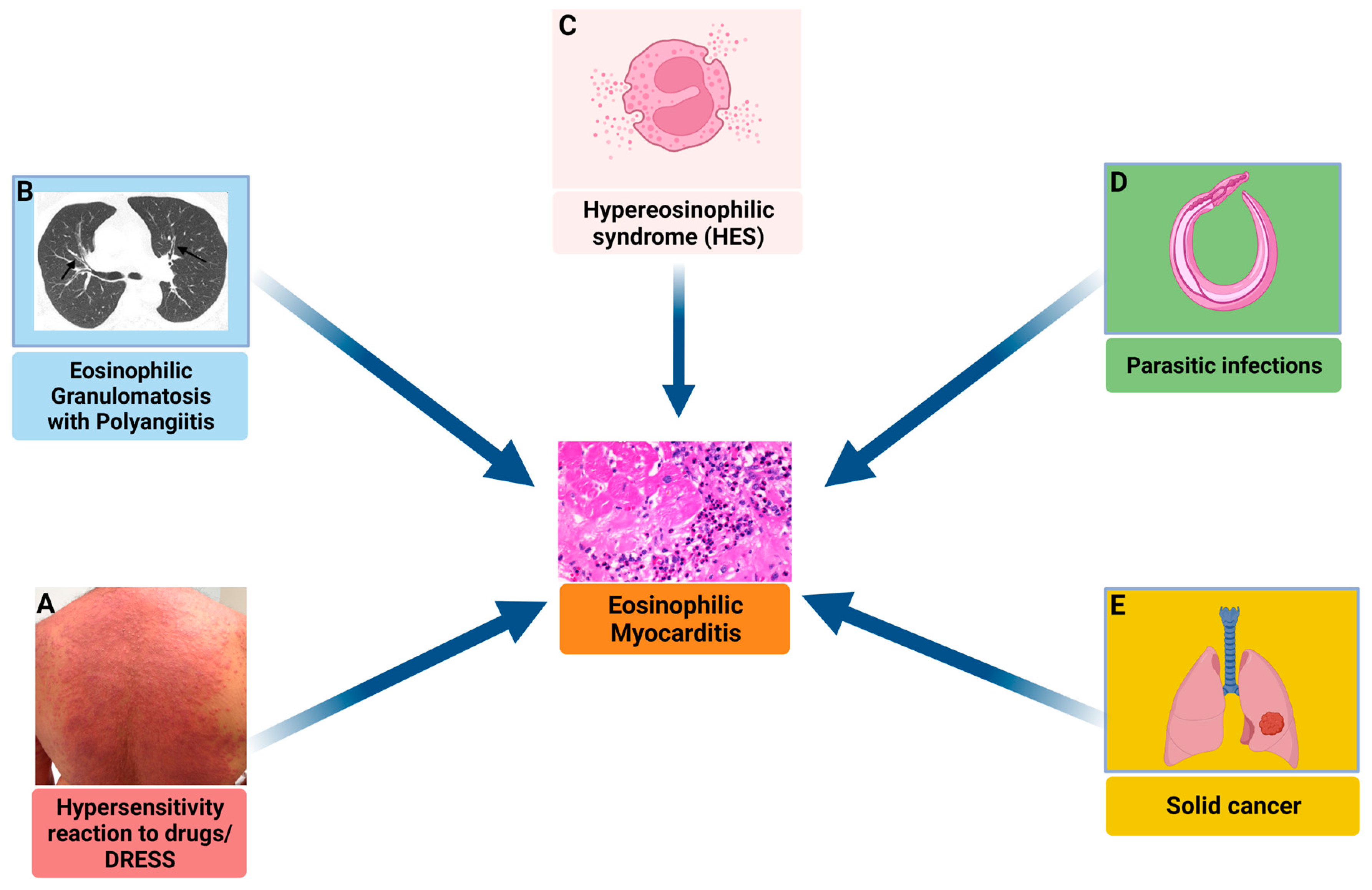
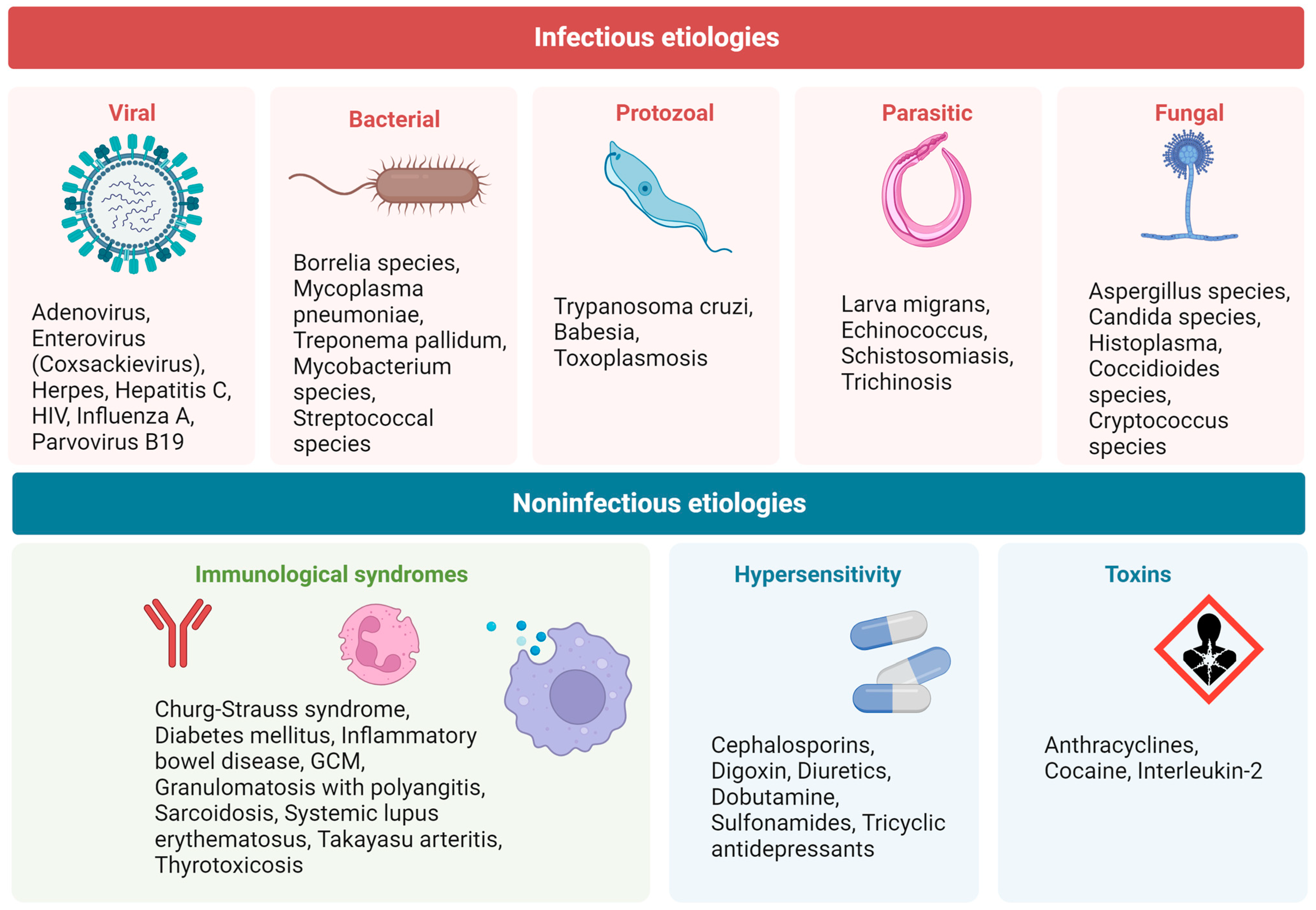
3.5. Eosinophilic Myocarditis
3.5.1. Definition, Diagnostic Approach, and Treatment
3.5.2. Biomarkers
Circulating Biomarkers
Imaging Biomarkers
3.6. Check Point Inhibitors Myocarditis
3.6.1. Definition, Diagnostic Approach, and Treatment
3.6.2. Biomarkers
Circulating Biomarkers
Imaging Biomarkers
4. Future Perspectives and Research
4.1. Liquid Biopsy
4.1.1. Micro-RNA
4.1.2. Circulating Cell-Free DNA
4.2. Soluble ST2 Receptors
4.3. Galectin-3
4.4. Molecular Inflammation Imaging Using PET
4.5. Cardiac Autoantibodies (aabs)
- Muscle-specific anti-sarcolemmal (ASA; i.e., AFA, anti-fibrillary, IFA, anti-interfibrillary aabs, and AMLA, anti-myolemmal aabs), index of myocytolysis and with a prevalence ranging 28–59% in myocarditis and 9–41% in DCM;
- Cardiac-specific (AHA, organ-specific and partially organ-specific anti-heart aabs [4]; AIDA, anti-intercalated disks-aabs, and anti-alpha-myosin heavy chain, MHC) [145,146], early predictors of disease, and able to predict DMC development in relatives, with a prevalence ranging 17–56% in myocarditis and 16–30% in DCM [145,146];
- Anti-beta 1- adrenergic receptors (33–96% and 27–95% respectively in myocarditis and DCM), associated with a negative prognosis and in vitro pro-apoptotic effects [4];
- Anti-muscarin acetylcholine receptor-2 (11% and 30–83% respectively), with negative inotropic, muscarin effects and associated with atrial arrhythmia [4];
- Anti-lamin (73% and 78%) [147];
- Anti-ANT, adenine nucleotide translocator, with negative inotropic effects (91% and 57%) [148];
- Anti-M7, against mitochondria (13% and 31%) [149];
- Anti-BCKD-E2, branched chain alpha-ketoacid dehydrogenase dihydrolipoyl transacylase (100% and 60%) [150].
5. Gaps in Evidence and Conclusions
Funding
Conflicts of Interest
References
- Cooper, L.T., Jr. Myocarditis. N. Engl. J. Med. 2009, 360, 1526–1538. [Google Scholar] [CrossRef]
- Trachtenberg, B.H.; Hare, J.M. Inflammatory Cardiomyopathic Syndromes. Circ. Res. 2017, 121, 803–818. [Google Scholar] [CrossRef]
- Ammirati, E.; Frigerio, M.; Adler, E.D.; Basso, C.; Birnie, D.H.; Brambatti, M.; Friedrich, M.G.; Klingel, K.; Lehtonen, J.; Moslehi, J.J.; et al. Management of Acute Myocarditis and Chronic Inflammatory Cardiomyopathy: An Expert Consensus Document. Circ. Heart Fail. 2020, 13, e007405. [Google Scholar] [CrossRef]
- Caforio, A.L.; Pankuweit, S.; Arbustini, E.; Basso, C.; Gimeno-Blanes, J.; Felix, S.B.; Fu, M.; Helio, T.; Heymans, S.; Jahns, R.; et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: A position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur. Heart J. 2013, 34, 2636–2648. [Google Scholar] [CrossRef]
- Tschope, C.; Ammirati, E.; Bozkurt, B.; Caforio, A.L.P.; Cooper, L.T.; Felix, S.B.; Hare, J.M.; Heidecker, B.; Heymans, S.; Hubner, N.; et al. Myocarditis and inflammatory cardiomyopathy: Current evidence and future directions. Nat. Rev. Cardiol. 2021, 18, 169–193. [Google Scholar] [CrossRef]
- Fung, G.; Luo, H.; Qiu, Y.; Yang, D.; McManus, B. Myocarditis. Circ. Res. 2016, 118, 496–514. [Google Scholar] [CrossRef]
- Fu, M.; Kontogeorgos, S.; Thunstrom, E.; Zverkova Sandstrom, T.; Kroon, C.; Bollano, E.; Schaufelberger, M.; Rosengren, A. Trends in myocarditis incidence, complications and mortality in Sweden from 2000 to 2014. Sci. Rep. 2022, 12, 1810. [Google Scholar] [CrossRef]
- Dominguez, F.; Kühl, U.; Pieske, B.; Garcia-Pavia, P.; Tschöpe, C. Update on Myocarditis and Inflammatory Cardiomyopathy: Reemergence of Endomyocardial Biopsy. Rev. Española Cardiol. 2016, 69, 178–187. [Google Scholar] [CrossRef]
- Bobbio, E.; Karason, K. Myocarditis and Inflammatory Cardiomyopathy. In Cardiomyopathy; Mattsson, G., Magnusson, P., Eds.; IntechOpen: Rijeka, Croatia, 2021; p. Ch. 17. [Google Scholar]
- Krejci, J.; Mlejnek, D.; Sochorova, D.; Nemec, P. Inflammatory Cardiomyopathy: A Current View on the Pathophysiology, Diagnosis, and Treatment. Biomed. Res. Int. 2016, 2016, 4087632. [Google Scholar] [CrossRef]
- Veronese, G.; Ammirati, E.; Brambatti, M.; Merlo, M.; Cipriani, M.; Potena, L.; Sormani, P.; Aoki, T.; Sugimura, K.; Sawamura, A.; et al. Viral genome search in myocardium of patients with fulminant myocarditis. Eur. J. Heart Fail. 2020, 22, 1277–1280. [Google Scholar] [CrossRef]
- Lopez-Ayala, J.M.; Pastor-Quirante, F.; Gonzalez-Carrillo, J.; Lopez-Cuenca, D.; Sanchez-Munoz, J.J.; Oliva-Sandoval, M.J.; Gimeno, J.R. Genetics of myocarditis in arrhythmogenic right ventricular dysplasia. Heart Rhythm. 2015, 12, 766–773. [Google Scholar] [CrossRef]
- Belkaya, S.; Kontorovich, A.R.; Byun, M.; Mulero-Navarro, S.; Bajolle, F.; Cobat, A.; Josowitz, R.; Itan, Y.; Quint, R.; Lorenzo, L.; et al. Autosomal Recessive Cardiomyopathy Presenting as Acute Myocarditis. J. Am. Coll. Cardiol. 2017, 69, 1653–1665. [Google Scholar] [CrossRef]
- Richardson, P.; McKenna, W.; Bristow, M.; Maisch, B.; Mautner, B.; O’Connell, J.; Olsen, E.; Thiene, G.; Goodwin, J.; Gyarfas, I.; et al. Report of the 1995 World Health Organization/International Society and Federation of Cardiology Task Force on the Definition and Classification of cardiomyopathies. Circulation 1996, 93, 841–842. [Google Scholar] [CrossRef]
- Suresh, A.; Martens, P.; Tang, W.H.W. Biomarkers for Myocarditis and Inflammatory Cardiomyopathy. Curr. Heart Fail. Rep. 2022, 19, 346–355. [Google Scholar] [CrossRef]
- Salzano, A.; D’Assante, R.; Israr, M.Z.; Eltayeb, M.; D’Agostino, A.; Bernieh, D.; De Luca, M.; Rega, S.; Ranieri, B.; Mauro, C.; et al. Biomarkers in Heart Failure: Clinical Insights. Heart Fail. Clin. 2021, 17, 223–243. [Google Scholar] [CrossRef]
- Salzano, A.; Marra, A.M.; D’Assante, R.; Arcopinto, M.; Bossone, E.; Suzuki, T.; Cittadini, A. Biomarkers and Imaging: Complementary or Subtractive? Heart Fail. Clin. 2019, 15, 321–331. [Google Scholar] [CrossRef]
- Marra, A.M.; Arcopinto, M.; Bobbio, E.; Salzano, A.; Saccà, L.; Cittadini, A. An unusual case of dilated cardiomyopathy associated with partial hypopituitarism. Intern. Emerg. Med. 2012, 7 (Suppl. 2), S85–S87. [Google Scholar] [CrossRef]
- Biomarkers Definitions Working, G. Biomarkers and surrogate endpoints: Preferred definitions and conceptual framework. Clin. Pharmacol. Ther. 2001, 69, 89–95. [Google Scholar] [CrossRef]
- Braunwald, E. Biomarkers in heart failure. N. Engl. J. Med. 2008, 358, 2148–2159. [Google Scholar] [CrossRef]
- Ammirati, E.; Cipriani, M.; Moro, C.; Raineri, C.; Pini, D.; Sormani, P.; Mantovani, R.; Varrenti, M.; Pedrotti, P.; Conca, C.; et al. Clinical Presentation and Outcome in a Contemporary Cohort of Patients with Acute Myocarditis: Multicenter Lombardy Registry. Circulation 2018, 138, 1088–1099. [Google Scholar] [CrossRef]
- Younis, A.; Matetzky, S.; Mulla, W.; Masalha, E.; Afel, Y.; Chernomordik, F.; Fardman, A.; Goitein, O.; Ben-Zekry, S.; Peled, Y.; et al. Epidemiology Characteristics and Outcome of Patients with Clinically Diagnosed Acute Myocarditis. Am. J. Med. 2020, 133, 492–499. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Bohm, M.; Burri, H.; Butler, J.; Celutkiene, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) with the special contribution of the Heart Failure Association (HFA) of the ESC. Rev. Esp. Cardiol. 2022, 75, 523. [Google Scholar] [CrossRef]
- Ammirati, E.; Veronese, G.; Cipriani, M.; Moroni, F.; Garascia, A.; Brambatti, M.; Adler, E.D.; Frigerio, M. Acute and Fulminant Myocarditis: A Pragmatic Clinical Approach to Diagnosis and Treatment. Curr. Cardiol. Rep. 2018, 20, 114. [Google Scholar] [CrossRef]
- Veronese, G.; Ammirati, E.; Chen, C.; Klingel, K.; Suzuki, M.; Okumura, T.; Maisch, B.; Zuo, H.; Ni, L.; Jiang, J.; et al. Management perspectives from the 2019 Wuhan international workshop on fulminant myocarditis. Int. J. Cardiol. 2021, 324, 131–138. [Google Scholar] [CrossRef]
- Ferreira, V.M.; Schulz-Menger, J.; Holmvang, G.; Kramer, C.M.; Carbone, I.; Sechtem, U.; Kindermann, I.; Gutberlet, M.; Cooper, L.T.; Liu, P.; et al. Cardiovascular Magnetic Resonance in Nonischemic Myocardial Inflammation: Expert Recommendations. J. Am. Coll. Cardiol. 2018, 72, 3158–3176. [Google Scholar] [CrossRef]
- Polte, C.L.; Bobbio, E.; Bollano, E.; Bergh, N.; Polte, C.; Himmelman, J.; Lagerstrand, K.M.; Gao, S.A. Cardiovascular Magnetic Resonance in Myocarditis. Diagnostics 2022, 12, 399. [Google Scholar] [CrossRef]
- Cannata, A.; Bhatti, P.; Roy, R.; Al-Agil, M.; Daniel, A.; Ferone, E.; Jordan, A.; Cassimon, B.; Bradwell, S.; Khawaja, A.; et al. Prognostic relevance of demographic factors in cardiac magnetic resonance-proven acute myocarditis: A cohort study. Front. Cardiovasc. Med. 2022, 9, 1037837. [Google Scholar] [CrossRef]
- Friedrich, M.G.; Sechtem, U.; Schulz-Menger, J.; Holmvang, G.; Alakija, P.; Cooper, L.T.; White, J.A.; Abdel-Aty, H.; Gutberlet, M.; Prasad, S.; et al. Cardiovascular magnetic resonance in myocarditis: A JACC White Paper. J. Am. Coll. Cardiol. 2009, 53, 1475–1487. [Google Scholar] [CrossRef]
- Aquaro, G.D.; Perfetti, M.; Camastra, G.; Monti, L.; Dellegrottaglie, S.; Moro, C.; Pepe, A.; Todiere, G.; Lanzillo, C.; Scatteia, A.; et al. Cardiac MR With Late Gadolinium Enhancement in Acute Myocarditis with Preserved Systolic Function: ITAMY Study. J. Am. Coll. Cardiol. 2017, 70, 1977–1987. [Google Scholar] [CrossRef]
- Grani, C.; Eichhorn, C.; Biere, L.; Murthy, V.L.; Agarwal, V.; Kaneko, K.; Cuddy, S.; Aghayev, A.; Steigner, M.; Blankstein, R.; et al. Prognostic Value of Cardiac Magnetic Resonance Tissue Characterization in Risk Stratifying Patients with Suspected Myocarditis. J. Am. Coll. Cardiol. 2017, 70, 1964–1976. [Google Scholar] [CrossRef]
- Birnie, D.H.; Sauer, W.H.; Bogun, F.; Cooper, J.M.; Culver, D.A.; Duvernoy, C.S.; Judson, M.A.; Kron, J.; Mehta, D.; Cosedis Nielsen, J.; et al. HRS expert consensus statement on the diagnosis and management of arrhythmias associated with cardiac sarcoidosis. Heart Rhythm. 2014, 11, 1305–1323. [Google Scholar] [CrossRef]
- Kadkhodayan, A.; Chareonthaitawee, P.; Raman, S.V.; Cooper, L.T. Imaging of Inflammation in Unexplained Cardiomyopathy. JACC Cardiovasc. Imaging 2016, 9, 603–617. [Google Scholar] [CrossRef]
- Kruse, M.J.; Kovell, L.; Kasper, E.K.; Pomper, M.G.; Moller, D.R.; Solnes, L.; Chen, E.S.; Schindler, T.H. Myocardial Blood Flow and Inflammatory Cardiac Sarcoidosis. JACC Cardiovasc. Imaging 2017, 10, 157–167. [Google Scholar] [CrossRef]
- Vita, T.; Okada, D.R.; Veillet-Chowdhury, M.; Bravo, P.E.; Mullins, E.; Hulten, E.; Agrawal, M.; Madan, R.; Taqueti, V.R.; Steigner, M.; et al. Complementary Value of Cardiac Magnetic Resonance Imaging and Positron Emission Tomography/Computed Tomography in the Assessment of Cardiac Sarcoidosis. Circ. Cardiovasc. Imaging 2018, 11, e007030. [Google Scholar] [CrossRef]
- Cooper, L.T.; Baughman, K.L.; Feldman, A.M.; Frustaci, A.; Jessup, M.; Kuhl, U.; Levine, G.N.; Narula, J.; Starling, R.C.; Towbin, J.; et al. The role of endomyocardial biopsy in the management of cardiovascular disease: A scientific statement from the American Heart Association, the American College of Cardiology, and the European Society of Cardiology. Circulation 2007, 116, 2216–2233. [Google Scholar] [CrossRef]
- Singh, V.; Mendirichaga, R.; Savani, G.T.; Rodriguez, A.; Blumer, V.; Elmariah, S.; Inglessis-Azuaje, I.; Palacios, I. Comparison of Utilization Trends, Indications, and Complications of Endomyocardial Biopsy in Native Versus Donor Hearts (from the Nationwide Inpatient Sample 2002 to 2014). Am. J. Cardiol. 2018, 121, 356–363. [Google Scholar] [CrossRef]
- Bennett, M.K.; Gilotra, N.A.; Harrington, C.; Rao, S.; Dunn, J.M.; Freitag, T.B.; Halushka, M.K.; Russell, S.D. Evaluation of the role of endomyocardial biopsy in 851 patients with unexplained heart failure from 2000–2009. Circ. Heart Fail. 2013, 6, 676–684. [Google Scholar] [CrossRef]
- Chow, L.H.; Radio, S.J.; Sears, T.D.; McManus, B.M. Insensitivity of right ventricular endomyocardial biopsy in the diagnosis of myocarditis. J. Am. Coll. Cardiol. 1989, 14, 915–920. [Google Scholar] [CrossRef]
- Veronese, G.; Cipriani, M.; Bottiroli, M.; Garascia, A.; Mondino, M.; Pedrotti, P.; Pini, D.; Cozzi, O.; Messina, A.; Droandi, G.; et al. Fulminant myocarditis triggered by OC43 subtype coronavirus: A disease deserving evidence-based care bundles. J. Cardiovasc. Med. 2020, 21, 529–531. [Google Scholar] [CrossRef]
- Bock, C.T.; Klingel, K.; Kandolf, R. Human parvovirus B19-associated myocarditis. N. Engl. J. Med. 2010, 362, 1248–1249. [Google Scholar] [CrossRef]
- Law, Y.M.; Lal, A.K.; Chen, S.; Cihakova, D.; Cooper, L.T., Jr.; Deshpande, S.; Godown, J.; Grosse-Wortmann, L.; Robinson, J.D.; Towbin, J.A.; et al. Diagnosis and Management of Myocarditis in Children: A Scientific Statement from the American Heart Association. Circulation 2021, 144, e123–e135. [Google Scholar] [CrossRef]
- Tschope, C.; Elsanhoury, A.; Schlieker, S.; Van Linthout, S.; Kuhl, U. Immunosuppression in inflammatory cardiomyopathy and parvovirus B19 persistence. Eur. J. Heart Fail. 2019, 21, 1468–1469. [Google Scholar] [CrossRef]
- Rosa, V.E.E.; Lopes, M.P.; Spina, G.S.; Soares Junior, J.; Salazar, D.; Romero, C.E.; Lottenberg, M.P.; de Santis, A.; Pires, L.J.N.T.; Gonçalves, L.F.T.; et al. Rheumatic Myocarditis: A Poorly Recognized Etiology of Left Ventricular Dysfunction in Valvular Heart Disease Patients. Front. Cardiovasc. Med. 2021, 8, 676694. [Google Scholar] [CrossRef]
- Rosa, V.E.; Lopes, A.S.; Accorsi, T.A.; Fernandes, J.R.; Spina, G.S.; Sampaio, R.O.; Bacal, F.; Tarasoutchi, F. Heart Transplant in Patients with Predominantly Rheumatic Valvular Heart Disease. J. Heart Valve Dis. 2015, 24, 629–634. [Google Scholar]
- Lauer, B.; Niederau, C.; Kühl, U.; Schannwell, M.; Pauschinger, M.; Strauer, B.E.; Schultheiss, H.P. Cardiac troponin T in patients with clinically suspected myocarditis. J. Am. Coll. Cardiol. 1997, 30, 1354–1359. [Google Scholar] [CrossRef]
- Mahfoud, F.; Gärtner, B.; Kindermann, M.; Ukena, C.; Gadomski, K.; Klingel, K.; Kandolf, R.; Böhm, M.; Kindermann, I. Virus serology in patients with suspected myocarditis: Utility or futility? Eur. Heart J. 2011, 32, 897–903. [Google Scholar] [CrossRef]
- Rodrigues, A.B.; da Gama Torres, H.O.; Nunes, M.D.C.P.; de Assis Silva Gomes, J.; Pinho, L.L.N.; Rocha, M.O.; Botoni, F.A. Biomakers in Chronic Chagas Cardiomyopathy. Microorganisms 2022, 10, 1602. [Google Scholar] [CrossRef]
- Marra, A.M.; Arcopinto, M.; Salzano, A.; Bobbio, E.; Milano, S.; Misiano, G.; Ferrara, F.; Vriz, O.; Napoli, R.; Triggiani, V.; et al. Detectable interleukin-9 plasma levels are associated with impaired cardiopulmonary functional capacity and all-cause mortality in patients with chronic heart failure. Int. J. Cardiol. 2016, 209, 114–117. [Google Scholar] [CrossRef]
- Gómez-Ochoa, S.A.; Bautista-Niño, P.K.; Rojas, L.Z.; Hunziker, L.; Muka, T.; Echeverría, L.E. Circulating MicroRNAs and myocardial involvement severity in chronic Chagas cardiomyopathy. Front. Cell Infect. Microbiol. 2022, 12, 922189. [Google Scholar] [CrossRef]
- Cannata, A.; Bromage, D.I.; McDonagh, T.A. COVID-19 and heart failure: The dark side of the moon. Eur. J. Heart Fail. 2022, 24, 1129–1131. [Google Scholar] [CrossRef]
- Ammirati, E.; Lupi, L.; Palazzini, M.; Hendren, N.S.; Grodin, J.L.; Cannistraci, C.V.; Schmidt, M.; Hekimian, G.; Peretto, G.; Bochaton, T.; et al. Prevalence, Characteristics, and Outcomes of COVID-19-Associated Acute Myocarditis. Circulation 2022, 145, 1123–1139. [Google Scholar] [CrossRef]
- Bozkurt, B.; Kamat, I.; Hotez, P.J. Myocarditis With COVID-19 mRNA Vaccines. Circulation 2021, 144, 471–484. [Google Scholar] [CrossRef]
- Rosner, C.M.; Genovese, L.; Tehrani, B.N.; Atkins, M.; Bakhshi, H.; Chaudhri, S.; Damluji, A.A.; de Lemos, J.A.; Desai, S.S.; Emaminia, A.; et al. Myocarditis Temporally Associated with COVID-19 Vaccination. Circulation 2021, 144, 502–505. [Google Scholar] [CrossRef]
- Inciardi, R.M.; Lupi, L.; Zaccone, G.; Italia, L.; Raffo, M.; Tomasoni, D.; Cani, D.S.; Cerini, M.; Farina, D.; Gavazzi, E.; et al. Cardiac Involvement in a Patient with Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020, 5, 819–824. [Google Scholar] [CrossRef]
- Usui, E.; Nagaoka, E.; Ikeda, H.; Ohmori, M.; Tao, S.; Yonetsu, T.; Maejima, Y.; Arai, H.; Amemiya, K.; Ikeda, Y.; et al. Fulminant myocarditis with COVID-19 infection having normal C-reactive protein and serial magnetic resonance follow-up. ESC Heart Fail. 2022. [Google Scholar] [CrossRef]
- Siripanthong, B.; Nazarian, S.; Muser, D.; Deo, R.; Santangeli, P.; Khanji, M.Y.; Cooper, L.T.; Chahal, C.A.A. Recognizing COVID-19-related myocarditis: The possible pathophysiology and proposed guideline for diagnosis and management. Heart Rhythm. 2020, 17, 1463–1471. [Google Scholar] [CrossRef]
- Bollano, E.; Bergh, N.; Dudas, A.; Bobbio, E.; Polte, C.L. Somatostatin receptor positron emission tomography/computed tomography in myocarditis following mRNA COVID-19 vaccination. Eur. Heart J. Case Rep. 2022, 6, ytac117. [Google Scholar] [CrossRef]
- Birnie, D.H.; Nery, P.B.; Ha, A.C.; Beanlands, R.S. Cardiac Sarcoidosis. J. Am. Coll. Cardiol. 2016, 68, 411–421. [Google Scholar] [CrossRef]
- Kandolin, R.; Lehtonen, J.; Airaksinen, J.; Vihinen, T.; Miettinen, H.; Ylitalo, K.; Kaikkonen, K.; Tuohinen, S.; Haataja, P.; Kerola, T.; et al. Cardiac sarcoidosis: Epidemiology, characteristics, and outcome over 25 years in a nationwide study. Circulation 2015, 131, 624–632. [Google Scholar] [CrossRef]
- Bobbio, E.; Hjalmarsson, C.; Bjorkenstam, M.; Polte, C.L.; Oldfors, A.; Lindstrom, U.; Dahlberg, P.; Bartfay, S.E.; Szamlewski, P.; Taha, A.; et al. Diagnosis, management, and outcome of cardiac sarcoidosis and giant cell myocarditis: A Swedish single center experience. BMC Cardiovasc. Disord. 2022, 22, 192. [Google Scholar] [CrossRef]
- Kouranos, V.; Tzelepis, G.E.; Rapti, A.; Mavrogeni, S.; Aggeli, K.; Douskou, M.; Prasad, S.; Koulouris, N.; Sfikakis, P.; Wells, A.; et al. Complementary Role of CMR to Conventional Screening in the Diagnosis and Prognosis of Cardiac Sarcoidosis. JACC Cardiovasc. Imaging 2017, 10, 1437–1447. [Google Scholar] [CrossRef]
- Komoriyama, H.; Omote, K.; Nagai, T.; Kato, Y.; Nagano, N.; Koyanagawa, K.; Kamiya, K.; Konishi, T.; Sato, T.; Kobayashi, Y.; et al. Lower left ventricular ejection fraction and higher serum angiotensin-converting enzyme activity are associated with histopathological diagnosis by endomyocardial biopsy in patients with cardiac sarcoidosis. Int. J. Cardiol. 2020, 321, 113–117. [Google Scholar] [CrossRef]
- Yodogawa, K.; Fukushima, Y.; Ando, T.; Iwasaki, Y.K.; Akiyama, K.; Kumita, S.I.; Azuma, A.; Seino, Y.; Shimizu, W. Prevalence of atrial FDG uptake and association with atrial arrhythmias in patients with cardiac sarcoidosis. Int. J. Cardiol. 2020, 313, 55–59. [Google Scholar] [CrossRef]
- Kandolin, R.; Lehtonen, J.; Airaksinen, J.; Vihinen, T.; Miettinen, H.; Kaikkonen, K.; Haataja, P.; Kerola, T.; Kupari, M. Usefulness of Cardiac Troponins as Markers of Early Treatment Response in Cardiac Sarcoidosis. Am. J. Cardiol. 2015, 116, 960–964. [Google Scholar] [CrossRef]
- Baba, Y.; Kubo, T.; Kitaoka, H.; Okawa, M.; Yamanaka, S.; Kawada, Y.; Yamasaki, N.; Matsumura, Y.; Furuno, T.; Sugiura, T.; et al. Usefulness of high-sensitive cardiac troponin T for evaluating the activity of cardiac sarcoidosis. Int. Heart J. 2012, 53, 287–292. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Sato, T.; Nagai, T.; Hirata, K.; Tsuneta, S.; Kato, Y.; Komoriyama, H.; Kamiya, K.; Konishi, T.; Omote, K.; et al. Association of high serum soluble interleukin 2 receptor levels with risk of adverse events in cardiac sarcoidosis. ESC Heart Fail. 2021, 8, 5282–5292. [Google Scholar] [CrossRef]
- Odawara, K.; Inoue, T.; Hirooka, Y. Effective steroid therapy in an elderly patient with cardiac sarcoidosis and severe left ventricular dysfunction. J. Cardiol. Cases 2019, 19, 165–168. [Google Scholar] [CrossRef]
- Kim, J.S.; Judson, M.A.; Donnino, R.; Gold, M.; Cooper, L.T.; Prystowsky, E.N.; Prystowsky, S. Cardiac sarcoidosis. Am. Heart J. 2009, 157, 9–21. [Google Scholar] [CrossRef]
- Sekhri, V.; Sanal, S.; Delorenzo, L.J.; Aronow, W.S.; Maguire, G.P. Cardiac sarcoidosis: A comprehensive review. Arch. Med. Sci. 2011, 7, 546–554. [Google Scholar] [CrossRef]
- Habib, G.; Bucciarelli-Ducci, C.; Caforio, A.L.P.; Cardim, N.; Charron, P.; Cosyns, B.; Dehaene, A.; Derumeaux, G.; Donal, E.; Dweck, M.R.; et al. Multimodality Imaging in Restrictive Cardiomyopathies: An EACVI expert consensus document in collaboration with the “Working Group on myocardial and pericardial diseases” of the European Society of Cardiology Endorsed by The Indian Academy of Echocardiography. Eur. Heart J. Cardiovasc. Imaging 2017, 18, 1090–1121. [Google Scholar] [CrossRef]
- Judson, M.A.; Costabel, U.; Drent, M.; Wells, A.; Maier, L.; Koth, L.; Shigemitsu, H.; Culver, D.A.; Gelfand, J.; Valeyre, D.; et al. The WASOG Sarcoidosis Organ Assessment Instrument: An update of a previous clinical tool. Sarcoidosis Vasc. Diffuse Lung Dis. 2014, 31, 19–27. [Google Scholar]
- Patel, A.R.; Klein, M.R.; Chandra, S.; Spencer, K.T.; Decara, J.M.; Lang, R.M.; Burke, M.C.; Garrity, E.R.; Hogarth, D.K.; Archer, S.L.; et al. Myocardial damage in patients with sarcoidosis and preserved left ventricular systolic function: An observational study. Eur. J. Heart Fail. 2011, 13, 1231–1237. [Google Scholar] [CrossRef]
- Patel, M.R.; Cawley, P.J.; Heitner, J.F.; Klem, I.; Parker, M.A.; Jaroudi, W.A.; Meine, T.J.; White, J.B.; Elliott, M.D.; Kim, H.W.; et al. Detection of myocardial damage in patients with sarcoidosis. Circulation 2009, 120, 1969–1977. [Google Scholar] [CrossRef]
- Nadel, J.; Lancefield, T.; Voskoboinik, A.; Taylor, A.J. Late gadolinium enhancement identified with cardiac magnetic resonance imaging in sarcoidosis patients is associated with long-term ventricular arrhythmia and sudden cardiac death. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 634–641. [Google Scholar] [CrossRef]
- Greulich, S.; Deluigi, C.C.; Gloekler, S.; Wahl, A.; Zürn, C.; Kramer, U.; Nothnagel, D.; Bültel, H.; Schumm, J.; Grün, S.; et al. CMR imaging predicts death and other adverse events in suspected cardiac sarcoidosis. JACC Cardiovasc. Imaging 2013, 6, 501–511. [Google Scholar] [CrossRef]
- Youssef, G.; Leung, E.; Mylonas, I.; Nery, P.; Williams, K.; Wisenberg, G.; Gulenchyn, K.Y.; Dekemp, R.A.; Dasilva, J.; Birnie, D.; et al. The use of 18F-FDG PET in the diagnosis of cardiac sarcoidosis: A systematic review and metaanalysis including the Ontario experience. J. Nucl. Med. 2012, 53, 241–248. [Google Scholar] [CrossRef]
- Yamagishi, H.; Shirai, N.; Takagi, M.; Yoshiyama, M.; Akioka, K.; Takeuchi, K.; Yoshikawa, J. Identification of cardiac sarcoidosis with (13)N-NH(3)/(18)F-FDG PET. J. Nucl. Med. 2003, 44, 1030–1036. [Google Scholar]
- Osborne, M.T.; Hulten, E.A.; Singh, A.; Waller, A.H.; Bittencourt, M.S.; Stewart, G.C.; Hainer, J.; Murthy, V.L.; Skali, H.; Dorbala, S.; et al. Reduction in ¹⁸F-fluorodeoxyglucose uptake on serial cardiac positron emission tomography is associated with improved left ventricular ejection fraction in patients with cardiac sarcoidosis. J. Nucl. Cardiol. 2014, 21, 166–174. [Google Scholar] [CrossRef]
- Erthal, F.; Juneau, D.; Lim, S.P.; Dwivedi, G.; Nery, P.B.; Birnie, D.; Beanlands, R.S. Imaging of cardiac sarcoidosis. Q. J. Nucl. Med. Mol. Imaging 2016, 60, 252–263. [Google Scholar]
- Lurz, P.; Eitel, I.; Adam, J.; Steiner, J.; Grothoff, M.; Desch, S.; Fuernau, G.; de Waha, S.; Sareban, M.; Luecke, C.; et al. Diagnostic performance of CMR imaging compared with EMB in patients with suspected myocarditis. JACC Cardiovasc. Imaging 2012, 5, 513–524. [Google Scholar] [CrossRef]
- Cooper, L.T., Jr.; Berry, G.J.; Shabetai, R. Idiopathic giant-cell myocarditis--natural history and treatment. Multicenter Giant Cell Myocarditis Study Group Investigators. N. Engl. J. Med. 1997, 336, 1860–1866. [Google Scholar] [CrossRef]
- Kandolin, R.; Lehtonen, J.; Salmenkivi, K.; Raisanen-Sokolowski, A.; Lommi, J.; Kupari, M. Diagnosis, treatment, and outcome of giant-cell myocarditis in the era of combined immunosuppression. Circ. Heart Fail. 2013, 6, 15–22. [Google Scholar] [CrossRef]
- Ekstrom, K.; Lehtonen, J.; Kandolin, R.; Raisanen-Sokolowski, A.; Salmenkivi, K.; Kupari, M. Long-term outcome and its predictors in giant cell myocarditis. Eur. J. Heart Fail. 2016, 18, 1452–1458. [Google Scholar] [CrossRef]
- Bobbio, E.; Bjorkenstam, M.; Nwaru, B.I.; Giallauria, F.; Hessman, E.; Bergh, N.; Polte, C.L.; Lehtonen, J.; Karason, K.; Bollano, E. Short- and long-term outcomes after heart transplantation in cardiac sarcoidosis and giant-cell myocarditis: A systematic review and meta-analysis. Clin. Res. Cardiol. 2022, 111, 125–140. [Google Scholar] [CrossRef]
- Gilotra, N.A.; Minkove, N.; Bennett, M.K.; Tedford, R.J.; Steenbergen, C.; Judge, D.P.; Halushka, M.K.; Russell, S.D. Lack of Relationship Between Serum Cardiac Troponin I Level and Giant Cell Myocarditis Diagnosis and Outcomes. J. Card. Fail. 2016, 22, 583–585. [Google Scholar] [CrossRef]
- Okura, Y.; Dec, G.W.; Hare, J.M.; Kodama, M.; Berry, G.J.; Tazelaar, H.D.; Bailey, K.R.; Cooper, L.T. A clinical and histopathologic comparison of cardiac sarcoidosis and idiopathic giant cell myocarditis. J. Am. Coll. Cardiol. 2003, 41, 322–329. [Google Scholar] [CrossRef]
- Paitazoglou, C.; Bergmann, M.W.; Tiemann, K.; Wiese, A.; Schäfer, U.; Schwarz, A.; Eitel, I.; Montenbruck, M. Atrial Giant Cell Myocarditis as a Cause of Heart Failure: Diagnostic Pathway and Successful Treatment. JACC Case Rep. 2022, 4, 66–71. [Google Scholar] [CrossRef]
- Ekström, K.; Lehtonen, J.; Kandolin, R.; Räisänen-Sokolowski, A.; Salmenkivi, K.; Kupari, M. Incidence, Risk Factors, and Outcome of Life-Threatening Ventricular Arrhythmias in Giant Cell Myocarditis. Circ. Arrhythm. Electrophysiol. 2016, 9, e004559. [Google Scholar] [CrossRef]
- Bobbio, E.; Amundsen, J.; Oldfors, A.; Bollano, E.; Bergh, N.; Björkenstam, M.; Astengo, M.; Karason, K.; Gao, S.A.; Polte, C.L. Echocardiography in inflammatory heart disease: A comparison of giant cell myocarditis, cardiac sarcoidosis, and acute non-fulminant myocarditis. IJC Heart Vasc. 2023, 46, 101202. [Google Scholar] [CrossRef]
- Poyhonen, P.; Nordenswan, H.K.; Lehtonen, J.; Syvaranta, S.; Shenoy, C.; Kupari, M. Cardiac magnetic resonance in giant cell myocarditis: A matched comparison with cardiac sarcoidosis. Eur. Heart J. Cardiovasc. Imaging 2023, 24, 404–412. [Google Scholar] [CrossRef]
- Bobbio, E.; Bollano, E.; Oldfors, A.; Hedner, H.; Björkenstam, M.; Svedlund, S.; Karason, K.; Bergh, N.; Polte, C.L. Phenotyping of giant cell myocarditis versus cardiac sarcoidosis using cardiovascular magnetic resonance. Int. J. Cardiol. 2023, 387, 131143. [Google Scholar] [CrossRef]
- Polte, C.L.; Bollano, E.; Oldfors, A.; Dudás, A.; Lagerstrand, K.M.; Himmelman, J.; Bobbio, E.; Karason, K.; van Essen, M.; Bergh, N. Somatostatin Receptor Positron Emission Tomography/Computed Tomography in Giant Cell Myocarditis: A Promising Approach to Molecular Myocardial Inflammation Imaging. Circ. Cardiovasc. Imaging 2022, 15, e013551. [Google Scholar] [CrossRef]
- Al Ali, A.M.; Straatman, L.P.; Allard, M.F.; Ignaszewski, A.P. Eosinophilic myocarditis: Case series and review of literature. Can. J. Cardiol. 2006, 22, 1233–1237. [Google Scholar] [CrossRef]
- Pieroni, M.; Cavallaro, R.; Chimenti, C.; Smeraldi, E.; Frustaci, A. Clozapine-induced hypersensitivity myocarditis. Chest 2004, 126, 1703–1705. [Google Scholar] [CrossRef]
- Ammirati, E.; Cipriani, M.; Musca, F.; Bonacina, E.; Pedrotti, P.; Roghi, A.; Astaneh, A.; Schroeder, J.W.; Nonini, S.; Russo, C.F.; et al. A life-threatening presentation of eosinophilic granulomatosis with polyangiitis. J. Cardiovasc. Med. 2016, 17 (Suppl. 2), e109–e111. [Google Scholar] [CrossRef]
- Roehrl, M.H.; Alexander, M.P.; Hammond, S.B.; Ruzinova, M.; Wang, J.Y.; O’Hara, C.J. Eosinophilic myocarditis in hypereosinophilic syndrome. Am. J. Hematol. 2011, 86, 607–608. [Google Scholar] [CrossRef]
- Brambatti, M.; Matassini, M.V.; Adler, E.D.; Klingel, K.; Camici, P.G.; Ammirati, E. Eosinophilic Myocarditis: Characteristics, Treatment, and Outcomes. J. Am. Coll. Cardiol. 2017, 70, 2363–2375. [Google Scholar] [CrossRef]
- Intarasupht, J.; Kanchanomai, A.; Leelasattakul, W.; Chantrarat, T.; Nakakes, A.; Tiyanon, W. Prevalence, risk factors, and mortality outcome in the drug reaction with eosinophilia and systemic symptoms patients with cardiac involvement. Int. J. Dermatol. 2018, 57, 1187–1191. [Google Scholar] [CrossRef]
- Radovanovic, M.; Jevtic, D.; Calvin, A.D.; Petrovic, M.; Paulson, M.; Rueda Prada, L.; Sprecher, L.; Savic, I.; Dumic, I. “Heart in DRESS”: Cardiac Manifestations, Treatment and Outcome of Patients with Drug Reaction with Eosinophilia and Systemic Symptoms Syndrome: A Systematic Review. J. Clin. Med. 2022, 11, 704. [Google Scholar] [CrossRef]
- Morikawa, D.; Hiraoka, E.; Obunai, K.; Norisue, Y. Myocarditis Associated with Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS) Syndrome: A Case Report and Review of the Literature. Am. J. Case Rep. 2018, 19, 978–984. [Google Scholar] [CrossRef]
- Bourgeois, G.P.; Cafardi, J.A.; Groysman, V.; Hughey, L.C. A review of DRESS-associated myocarditis. J. Am. Acad. Dermatol. 2012, 66, e229–e236. [Google Scholar] [CrossRef]
- Zhong, Z.; Yang, Z.; Peng, Y.; Wang, L.; Yuan, X. Diagnosis and treatment of eosinophilic myocarditis. J. Transl. Autoimmun. 2021, 4, 100118. [Google Scholar] [CrossRef]
- Séguéla, P.E.; Iriart, X.; Acar, P.; Montaudon, M.; Roudaut, R.; Thambo, J.B. Eosinophilic cardiac disease: Molecular, clinical and imaging aspects. Arch. Cardiovasc. Dis. 2015, 108, 258–268. [Google Scholar] [CrossRef]
- Morimoto, S.; Kubo, N.; Hiramitsu, S.; Uemura, A.; Ohtsuki, M.; Kato, S.; Kato, Y.; Sugiura, A.; Miyagishima, K.; Mori, N.; et al. Changes in the peripheral eosinophil count in patients with acute eosinophilic myocarditis. Heart Vessel. 2003, 18, 193–196. [Google Scholar] [CrossRef]
- Arima, M.; Kanoh, T. Eosinophilic myocarditis associated with dense deposits of eosinophil cationic protein (ECP) in endomyocardium with high serum ECP. Heart 1999, 81, 669–671. [Google Scholar] [CrossRef]
- Arima, M.; Kanoh, T.; Kawano, Y.; Oigawa, T.; Yamagami, S.; Matsuda, S. Serum levels of eosinophil cationic protein in patients with eosinophilic myocarditis. Int. J. Cardiol. 2002, 84, 97–99. [Google Scholar] [CrossRef]
- Ommen, S.R.; Seward, J.B.; Tajik, A.J. Clinical and echocardiographic features of hypereosinophilic syndromes. Am. J. Cardiol. 2000, 86, 110–113. [Google Scholar] [CrossRef]
- Simonnet, B.; Jacquier, A.; Salaun, E.; Hubert, S.; Habib, G. Cardiac involvement in hypereosinophilic syndrome: Role of multimodality imaging. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 228. [Google Scholar] [CrossRef][Green Version]
- Gottdiener, J.S.; Maron, B.J.; Schooley, R.T.; Harley, J.B.; Roberts, W.C.; Fauci, A.S. Two-dimensional echocardiographic assessment of the idiopathic hypereosinophilic syndrome. Anatomic basis of mitral regurgitation and peripheral embolization. Circulation 1983, 67, 572–578. [Google Scholar] [CrossRef]
- Dedieu, N.; Giardini, A.; Khambadkone, S.; Marek, J. Eosinophilic heart disease in a paediatric patient. Eur. J. Echocardiogr. 2011, 12, E3. [Google Scholar] [CrossRef]
- Antonopoulos, A.S.; Azzu, A.; Androulakis, E.; Tanking, C.; Papagkikas, P.; Mohiaddin, R.H. Eosinophilic heart disease: Diagnostic and prognostic assessment by cardiac magnetic resonance. Eur. Heart J. Cardiovasc. Imaging 2021, 22, 1273–1284. [Google Scholar] [CrossRef]
- Rotoli, B.; Catalano, L.; Galderisi, M.; Luciano, L.; Pollio, G.; Guerriero, A.; D’Errico, A.; Mecucci, C.; La Starza, R.; Frigeri, F.; et al. Rapid reversion of Loeffler’s endocarditis by imatinib in early stage clonal hypereosinophilic syndrome. Leuk. Lymphoma 2004, 45, 2503–2507. [Google Scholar] [CrossRef]
- Moslehi, J.; Salem, J.E. Immune Checkpoint Inhibitor Myocarditis Treatment Strategies and Future Directions. JACC CardioOncol 2022, 4, 704–707. [Google Scholar] [CrossRef]
- Mahmood, S.S.; Fradley, M.G.; Cohen, J.V.; Nohria, A.; Reynolds, K.L.; Heinzerling, L.M.; Sullivan, R.J.; Damrongwatanasuk, R.; Chen, C.L.; Gupta, D.; et al. Myocarditis in Patients Treated with Immune Checkpoint Inhibitors. J. Am. Coll. Cardiol. 2018, 71, 1755–1764. [Google Scholar] [CrossRef]
- Schneider, B.J.; Naidoo, J.; Santomasso, B.D.; Lacchetti, C.; Adkins, S.; Anadkat, M.; Atkins, M.B.; Brassil, K.J.; Caterino, J.M.; Chau, I.; et al. Management of Immune-Related Adverse Events in Patients Treated with Immune Checkpoint Inhibitor Therapy: ASCO Guideline Update. J. Clin. Oncol. 2021, 39, 4073–4126. [Google Scholar] [CrossRef]
- Jain, V.; Bahia, J.; Mohebtash, M.; Barac, A. Cardiovascular Complications Associated with Novel Cancer Immunotherapies. Curr. Treat. Options Cardiovasc. Med. 2017, 19, 36. [Google Scholar] [CrossRef]
- Johnson, D.B.; Balko, J.M.; Compton, M.L.; Chalkias, S.; Gorham, J.; Xu, Y.; Hicks, M.; Puzanov, I.; Alexander, M.R.; Bloomer, T.L.; et al. Fulminant Myocarditis with Combination Immune Checkpoint Blockade. N. Engl. J. Med. 2016, 375, 1749–1755. [Google Scholar] [CrossRef]
- Zhang, L.; Zlotoff, D.A.; Awadalla, M.; Mahmood, S.S.; Nohria, A.; Hassan, M.Z.O.; Thuny, F.; Zubiri, L.; Chen, C.L.; Sullivan, R.J.; et al. Major Adverse Cardiovascular Events and the Timing and Dose of Corticosteroids in Immune Checkpoint Inhibitor-Associated Myocarditis. Circulation 2020, 141, 2031–2034. [Google Scholar] [CrossRef]
- Arangalage, D.; Delyon, J.; Lermuzeaux, M.; Ekpe, K.; Ederhy, S.; Pages, C.; Lebbe, C. Survival After Fulminant Myocarditis Induced by Immune-Checkpoint Inhibitors. Ann. Intern. Med. 2017, 167, 683–684. [Google Scholar] [CrossRef]
- Lehmann, L.H.; Cautela, J.; Palaskas, N.; Baik, A.H.; Meijers, W.C.; Allenbach, Y.; Alexandre, J.; Rassaf, T.; Müller, O.J.; Aras, M.; et al. Clinical Strategy for the Diagnosis and Treatment of Immune Checkpoint Inhibitor-Associated Myocarditis: A Narrative Review. JAMA Cardiol. 2021, 6, 1329–1337. [Google Scholar] [CrossRef]
- Salem, J.E.; Allenbach, Y.; Vozy, A.; Brechot, N.; Johnson, D.B.; Moslehi, J.J.; Kerneis, M. Abatacept for Severe Immune Checkpoint Inhibitor-Associated Myocarditis. N. Engl. J. Med. 2019, 380, 2377–2379. [Google Scholar] [CrossRef]
- Wang, D.Y.; Okoye, G.D.; Neilan, T.G.; Johnson, D.B.; Moslehi, J.J. Cardiovascular Toxicities Associated with Cancer Immunotherapies. Curr. Cardiol. Rep. 2017, 19, 21. [Google Scholar] [CrossRef]
- Kondapalli, L.; Medina, T.; Groves, D.W. Practical cardiovascular imaging approach to diagnose immune checkpoint inhibitor myocarditis. Eur. Heart J. Cardiovasc. Imaging 2021, 22, 372–374. [Google Scholar] [CrossRef]
- Peters, L.J.F.; Biessen, E.A.L.; Hohl, M.; Weber, C.; van der Vorst, E.P.C.; Santovito, D. Small Things Matter: Relevance of MicroRNAs in Cardiovascular Disease. Front. Physiol. 2020, 11, 793. [Google Scholar] [CrossRef]
- Neuži, P.; Giselbrecht, S.; Länge, K.; Huang, T.J.; Manz, A. Revisiting lab-on-a-chip technology for drug discovery. Nat. Rev. Drug Discov. 2012, 11, 620–632. [Google Scholar] [CrossRef]
- Lewandowski, P.; Goławski, M.; Baron, M.; Reichman-Warmusz, E.; Wojnicz, R. A Systematic Review of miRNA and cfDNA as Potential Biomarkers for Liquid Biopsy in Myocarditis and Inflammatory Dilated Cardiomyopathy. Biomolecules 2022, 12, 1476. [Google Scholar] [CrossRef]
- Calderon-Dominguez, M.; Belmonte, T.; Quezada-Feijoo, M.; Ramos, M.; Calderon-Dominguez, J.; Campuzano, O.; Mangas, A.; Toro, R. Plasma microrna expression profile for reduced ejection fraction in dilated cardiomyopathy. Sci. Rep. 2021, 11, 7517. [Google Scholar] [CrossRef]
- Husain, H.; Velculescu, V.E. Cancer DNA in the Circulation: The Liquid Biopsy. JAMA 2017, 318, 1272–1274. [Google Scholar] [CrossRef]
- Page, K.; Shaw, J.A.; Guttery, D.S. The liquid biopsy: Towards standardisation in preparation for prime time. Lancet Oncol. 2019, 20, 758–760. [Google Scholar] [CrossRef]
- Shaw, J.A.; Brown, J.; Coombes, R.C.; Jacob, J.; Payne, R.; Lee, B.; Page, K.; Hava, N.; Stebbing, J. Circulating tumor cells and plasma DNA analysis in patients with indeterminate early or metastatic breast cancer. Biomark. Med. 2011, 5, 87–91. [Google Scholar] [CrossRef]
- Jylhava, J.; Lehtimaki, T.; Jula, A.; Moilanen, L.; Kesaniemi, Y.A.; Nieminen, M.S.; Kahonen, M.; Hurme, M. Circulating cell-free DNA is associated with cardiometabolic risk factors: The Health 2000 Survey. Atherosclerosis 2014, 233, 268–271. [Google Scholar] [CrossRef]
- Xie, J.; Yang, J.; Hu, P. Correlations of Circulating Cell-Free DNA With Clinical Manifestations in Acute Myocardial Infarction. Am. J. Med. Sci. 2018, 356, 121–129. [Google Scholar] [CrossRef]
- Salzano, A.; Israr, M.Z.; Garcia, D.F.; Middleton, L.; D’Assante, R.; Marra, A.M.; Arcopinto, M.; Yazaki, Y.; Bernieh, D.; Cassambai, S.; et al. Circulating cell-free DNA levels are associated with adverse outcomes in heart failure: Testing liquid biopsy in heart failure. Eur. J. Prev. Cardiol. 2021, 28, e28–e31. [Google Scholar] [CrossRef]
- De Vlaminck, I.; Valantine, H.A.; Snyder, T.M.; Strehl, C.; Cohen, G.; Luikart, H.; Neff, N.F.; Okamoto, J.; Bernstein, D.; Weisshaar, D.; et al. Circulating cell-free DNA enables noninvasive diagnosis of heart transplant rejection. Sci. Transl. Med. 2014, 6, 241ra277. [Google Scholar] [CrossRef]
- Macher, H.C.; Garcia-Fernandez, N.; Adsuar-Gomez, A.; Porras-Lopez, M.; Gonzalez-Calle, A.; Noval-Padillo, J.; Guerrero, J.M.; Molinero, P.; Borrego-Dominguez, J.M.; Herruzo-Aviles, A.; et al. Donor-specific circulating cell free DNA as a noninvasive biomarker of graft injury in heart transplantation. Clin. Chim. Acta 2019, 495, 590–597. [Google Scholar] [CrossRef]
- Khush, K.K.; Patel, J.; Pinney, S.; Kao, A.; Alharethi, R.; DePasquale, E.; Ewald, G.; Berman, P.; Kanwar, M.; Hiller, D.; et al. Noninvasive detection of graft injury after heart transplant using donor-derived cell-free DNA: A prospective multicenter study. Am. J. Transplant. 2019, 19, 2889. [Google Scholar] [CrossRef]
- Coronado, M.J.; Bruno, K.A.; Blauwet, L.A.; Tschöpe, C.; Cunningham, M.W.; Pankuweit, S.; van Linthout, S.; Jeon, E.S.; McNamara, D.M.; Krejčí, J.; et al. Elevated Sera sST2 Is Associated with Heart Failure in Men ≤50 Years Old with Myocarditis. J. Am. Heart Assoc. 2019, 8, e008968. [Google Scholar] [CrossRef]
- Obradovic, D.M.; Büttner, P.; Rommel, K.P.; Blazek, S.; Loncar, G.; von Haehling, S.; von Roeder, M.; Lücke, C.; Gutberlet, M.; Thiele, H.; et al. Soluble ST2 Receptor: Biomarker of Left Ventricular Impairment and Functional Status in Patients with Inflammatory Cardiomyopathy. Cells 2022, 11, 414. [Google Scholar] [CrossRef]
- Besler, C.; Lang, D.; Urban, D.; Rommel, K.P.; von Roeder, M.; Fengler, K.; Blazek, S.; Kandolf, R.; Klingel, K.; Thiele, H.; et al. Plasma and Cardiac Galectin-3 in Patients with Heart Failure Reflects Both Inflammation and Fibrosis: Implications for Its Use as a Biomarker. Circ. Heart Fail. 2017, 10, e003804. [Google Scholar] [CrossRef]
- Saric, P.; Young, K.A.; Rodriguez-Porcel, M.; Chareonthaitawee, P. PET Imaging in Cardiac Sarcoidosis: A Narrative Review with Focus on Novel PET Tracers. Pharmaceuticals 2021, 14, 1286. [Google Scholar] [CrossRef]
- Jahandideh, A.; Uotila, S.; Stahle, M.; Virta, J.; Li, X.G.; Kyto, V.; Marjamaki, P.; Liljenback, H.; Taimen, P.; Oikonen, V.; et al. Folate Receptor beta-Targeted PET Imaging of Macrophages in Autoimmune Myocarditis. J. Nucl. Med. 2020, 61, 1643–1649. [Google Scholar] [CrossRef]
- Lapa, C.; Reiter, T.; Li, X.; Werner, R.A.; Samnick, S.; Jahns, R.; Buck, A.K.; Ertl, G.; Bauer, W.R. Imaging of myocardial inflammation with somatostatin receptor based PET/CT—A comparison to cardiac MRI. Int. J. Cardiol. 2015, 194, 44–49. [Google Scholar] [CrossRef]
- Kindermann, I.; Kindermann, M.; Kandolf, R.; Klingel, K.; Bültmann, B.; Müller, T.; Lindinger, A.; Böhm, M. Predictors of outcome in patients with suspected myocarditis. Circulation 2008, 118, 639–648. [Google Scholar] [CrossRef]
- Caforio, A.L.; Calabrese, F.; Angelini, A.; Tona, F.; Vinci, A.; Bottaro, S.; Ramondo, A.; Carturan, E.; Iliceto, S.; Thiene, G.; et al. A prospective study of biopsy-proven myocarditis: Prognostic relevance of clinical and aetiopathogenetic features at diagnosis. Eur. Heart J. 2007, 28, 1326–1333. [Google Scholar] [CrossRef]
- Caforio, A.L.; Mahon, N.G.; Baig, M.K.; Tona, F.; Murphy, R.T.; Elliott, P.M.; McKenna, W.J. Prospective familial assessment in dilated cardiomyopathy: Cardiac autoantibodies predict disease development in asymptomatic relatives. Circulation 2007, 115, 76–83. [Google Scholar] [CrossRef]
- Wolff, P.G.; Kühl, U.; Schultheiss, H.P. Laminin distribution and autoantibodies to laminin in dilated cardiomyopathy and myocarditis. Am. Heart J. 1989, 117, 1303–1309. [Google Scholar] [CrossRef]
- Staudt, Y.; Mobini, R.; Fu, M.; Felix, S.B.; Kühn, J.P.; Staudt, A. Beta1-adrenoceptor antibodies induce apoptosis in adult isolated cardiomyocytes. Eur. J. Pharmacol. 2003, 466, 1–6. [Google Scholar] [CrossRef]
- Klein, R.; Maisch, B.; Kochsiek, K.; Berg, P.A. Demonstration of organ specific antibodies against heart mitochondria (anti-M7) in sera from patients with some forms of heart diseases. Clin. Exp. Immunol. 1984, 58, 283–292. [Google Scholar]
- Ansari, A.A.; Neckelmann, N.; Villinger, F.; Leung, P.; Danner, D.J.; Brar, S.S.; Zhao, S.; Gravanis, M.B.; Mayne, A.; Gershwin, M.E. Epitope mapping of the branched chain alpha-ketoacid dehydrogenase dihydrolipoyl transacylase (BCKD-E2) protein that reacts with sera from patients with idiopathic dilated cardiomyopathy. J. Immunol. 1994, 153, 4754–4765. [Google Scholar] [CrossRef]


| Community Screening | Diagnosis | Phenotyping | Risk Stratification | Management | Treatment | |
|---|---|---|---|---|---|---|
| Infectious Myocarditis [2,3,4,5,6,8,13,14,17,18,22,23,24,25,37,38,39,40,41,42,43,44,45,46,47] | CK CK-MB Troponins | Blood cell count CRP Erythrocyte sedimentation rate Troponins Virus serology | IgM IgG | Troponins NP CA-125 * Uric acid * CRP * IL-8 * IL-1b * IL-12 * Mir 223-5p * | Troponins NP | CRP |
| TTE CMRI | TTE | |||||
| COVID-19 and Post-vaccination Associated Myocarditis [26,48,49,50,51,52,53,54,55] | BNP CRP Troponins | |||||
| ECG CMRI | ||||||
| Sarcoidotic Myocarditis [3,10,23,31,51,53,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76] | ACE | ACE Lysozyme NT-pro-BNP Troponins | Lysozyme sIL-2R | sIL-2R Troponins | Troponins | |
| ECG TTE CMRI 67Ga-citrate scintigraphy 18F-FDG PET | CMRI 18F-FDG PET | 67Ga-citrate scintigraphy 18F-FDG PET | 18F-FDG PET | |||
| Giant Cell Myocarditis [58,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90] | Troponins | hs-cTnT NT-pro-BNP | hs-cTnT NT-pro-BNP | hs-cTnT | ||
| TTE CMRI 18F-FDG PET ¶ | ||||||
| Eosinophilic Myocarditis [91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106] | Peripheral eosinophilia | CK-MB ECP Peripheral eosinophilia Troponins | CMRI | ECP | ECP Peripheral eosinophilia | |
| CMRI | ECG TTE CMRI | TTE CMRI | TTE CMRI | |||
| Check Point Inhibitors [107,108,109,110,111,112,113,114,115,116,117] | CK Troponins NP | CMRI | ||||
| ECG CMRI TTE | ||||||
| Future Perspectives and Research [118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142,143] | miR-Chr8:96 miR-155 miR-206 CMRI Novel PET tracers for inflammation imaging, e.g., SSTR PET/CT | cfDNA s-ST2 CMRI Novel PET tracers for inflammation imaging, e.g., SSTR PET/CT | cfDNA Galectin-3 CMRI Novel PET tracers for inflammation imaging, e.g., SSTR PET/CT | cfDNA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Crisci, G.; Bobbio, E.; Gentile, P.; Bromage, D.I.; Bollano, E.; Ferone, E.; Israr, M.Z.; Heaney, L.M.; Polte, C.L.; Cannatà, A.; et al. Biomarkers in Acute Myocarditis and Chronic Inflammatory Cardiomyopathy: An Updated Review of the Literature. J. Clin. Med. 2023, 12, 7214. https://doi.org/10.3390/jcm12237214
Crisci G, Bobbio E, Gentile P, Bromage DI, Bollano E, Ferone E, Israr MZ, Heaney LM, Polte CL, Cannatà A, et al. Biomarkers in Acute Myocarditis and Chronic Inflammatory Cardiomyopathy: An Updated Review of the Literature. Journal of Clinical Medicine. 2023; 12(23):7214. https://doi.org/10.3390/jcm12237214
Chicago/Turabian StyleCrisci, Giulia, Emanuele Bobbio, Piero Gentile, Daniel I. Bromage, Entela Bollano, Emma Ferone, Muhammad Zubair Israr, Liam M. Heaney, Christian L. Polte, Antonio Cannatà, and et al. 2023. "Biomarkers in Acute Myocarditis and Chronic Inflammatory Cardiomyopathy: An Updated Review of the Literature" Journal of Clinical Medicine 12, no. 23: 7214. https://doi.org/10.3390/jcm12237214
APA StyleCrisci, G., Bobbio, E., Gentile, P., Bromage, D. I., Bollano, E., Ferone, E., Israr, M. Z., Heaney, L. M., Polte, C. L., Cannatà, A., & Salzano, A. (2023). Biomarkers in Acute Myocarditis and Chronic Inflammatory Cardiomyopathy: An Updated Review of the Literature. Journal of Clinical Medicine, 12(23), 7214. https://doi.org/10.3390/jcm12237214






