Intra-Assessment Resting Metabolic Rate Variability Is Associated with Cardiometabolic Risk Factors in Middle-Aged Adults
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Resting Gas Exchange Measurement
2.3. Maximum-Effort Walking Graded Exercise Test
2.4. Blood Pressure Measurements and Circulating Cardiometabolic Risk Factors
2.5. Resting Heart Rhythm Assessments
2.6. Anthropometry and Body Composition Assessments
2.7. Statistical Analyses
3. Results
4. Discussion
5. Clinical and Practical Implications
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lam, Y.Y.; Ravussin, E. Indirect calorimetry: An indispensable tool to understand and predict obesity. Eur. J. Clin. Nutr. 2017, 71, 318–322. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Delgado, G.; Ravussin, E. Assessment of energy expenditure: Are calories measured differently for different diets? Curr. Opin. Clin. Nutr. Metab. Care 2020, 23, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.Y.; Brychta, R.J.; Linderman, J.D.; Smith, S.; Courville, A.; Dieckmann, W.; Herscovitch, P.; Millo, C.M.; Remaley, A.; Lee, P.; et al. Brown fat activation mediates cold-induced thermogenesis in adult humans in response to a mild decrease in ambient temperature. J. Clin. Endocrinol. Metab. 2013, 98, E1218–E1223. [Google Scholar] [CrossRef] [PubMed]
- Schoffelen, P.F.M.; Plasqui, G. Classical experiments in whole-body metabolism: Open-circuit respirometry—Diluted flow chamber, hood, or facemask systems. Eur. J. Appl. Physiol. 2018, 188, 33–49. [Google Scholar] [CrossRef] [PubMed]
- Burton, T.; Killen, S.S.; Armstrong, J.D.; Metcalfe, N.B. What causes intraspecific variation in resting metabolic rate and what are its ecological consequences? Proc. R. Soc. B Biol. Sci. 2011, 278, 3465–3473. [Google Scholar] [CrossRef] [PubMed]
- White, C.R.; Kearney, M.R. Determinants of inter-specific variation in basal metabolic rate. J. Comp. Physiol. B. 2013, 183, 1–26. [Google Scholar] [CrossRef]
- Konarzewski, M.; Książek, A. Determinants of intra-specific variation in basal metabolic rate. J. Comp. Physiol. B Biochem. Syst. Environ. Physiol. 2013, 183, 27–41. [Google Scholar] [CrossRef]
- Donahoo, W.T.; Levine, J.A.; Melanson, E.L. Variability in energy expenditure and its components. Curr. Opin. Clin. Nutr. Metab. Care 2004, 7, 599–605. [Google Scholar] [CrossRef]
- Alcantara, J.M.A.; Osuna-Prieto, F.J.; Plaza-Florido, A. Associations between Intra-Assessment Resting Metabolic Rate Variability and Health-Related Factors. Metabolites 2022, 12, 1218. [Google Scholar] [CrossRef]
- Nevill, A.; Holder, R.; Markovic, G. Scaling maximum oxygen uptake using lower leg muscle volume provides further insight into the pitfalls of whole body-mass power laws. J. Appl. Physiol. 2006, 101, 1006–1007. [Google Scholar] [CrossRef]
- Tolfrey, K.; Barker, A.; Thom, J.M.; Morse, C.I.; Narici, M.V.; Batterham, A.M. Scaling of maximal oxygen uptake by lower leg muscle volume in boys and men. J. Appl. Physiol. 2006, 100, 1851–1856. [Google Scholar] [CrossRef] [PubMed]
- Halsey, L.G.; Careau, V.; Pontzer, H.; Ainslie, P.N.; Andersen, L.F.; Anderson, L.J.; Arab, L.; Baddou, I.; Bedu-Addo, K.; Blaak, E.E.; et al. Variability in energy expenditure is much greater in males than females. J. Hum. Evol. 2022, 171, 103229. [Google Scholar] [CrossRef] [PubMed]
- Fullmer, S.; Benson-Davies, S.; Earthman, C.P.; Frankenfield, D.C.; Gradwell, E.; Lee, P.S.P.; Piemonte, T.; Trabulsi, J. Evidence analysis library review of best practices for performing indirect calorimetry in healthy and non-critically ill individuals. J. Acad. Nutr. Diet. 2015, 115, 1417–1446.e2. [Google Scholar] [CrossRef] [PubMed]
- Alcantara, J.M.A.; Delgado, G.S.; Gahete, F.J.A.; Galgani, J.E.; Ruiz, J.R. Impact of the Method Used to Select Gas Exchange Data for Estimating the Resting Metabolic Rate, as Supplied by Breath-by-Breath Metabolic Carts. Nutrients 2020, 12, 487. [Google Scholar] [CrossRef] [PubMed]
- Irving, C.J.; Eggett, D.L.; Fullmer, S. Comparing Steady State to Time Interval and Non-Steady State Measurements of Resting Metabolic Rate. Nutr. Clin. Pract. 2017, 32, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Reeves, M.M.; Davies, P.S.W.; Bauer, J.; Battistutta, D. Reducing the time period of steady state does not affect the accuracy of energy expenditure measurements by indirect calorimetry. J. Appl. Physiol. 2004, 97, 130–134. [Google Scholar] [CrossRef] [PubMed]
- Olejnik, L.A.; Peters, E.N.; Parrott, J.S.; Marcus, A.F.; Brody, R.A.; Hand, R.K.; Fiutem, J.J.; Byham-Gray, L.D. Abbreviated Steady State Intervals for Measuring Resting Energy Expenditure in Patients on Maintenance Hemodialysis. JPEN J. Parenter. Enteral Nutr. 2017, 41, 1348–1355. [Google Scholar] [CrossRef]
- McEvoy, C.; Cooke, S.R.; Young, I.S. A reduced abbreviated indirect calorimetry protocol is clinically acceptable for use in spontaneously breathing patients with traumatic brain injury. Nutr. Clin. Pract. 2009, 24, 513–519. [Google Scholar] [CrossRef]
- Mathisen, T.F.; Engen, K.M.; Sundgot-Borgen, J.; Stensrud, T. Evaluation of a short protocol for indirect calorimetry in females with eating disorders and healthy controls. Clin. Nutr. ESPEN 2017, 22, 28–35. [Google Scholar] [CrossRef][Green Version]
- McClave, S.A.; Spain, D.A.; Skolnick, J.L.; Lowen, C.C.; Kleber, M.J.; Wickerham, P.S.; Vogt, J.R.; Looney, S.W. Achievement of steady state optimizes results when performing indirect calorimetry. J. Parenter. Enter. Nutr. 2003, 27, 16–20. [Google Scholar] [CrossRef]
- Popp, C.; Butler, M.; Curran, M.; Illiano, P.; Sevick, M.A.; St-Jules, D. Evaluating steady-state resting energy expenditure using indirect calorimetry in adults with overweight and obesity. Clin. Nutr. 2019, 39, 2220–2226. [Google Scholar] [CrossRef] [PubMed]
- Frankenfield, D.C.; Sarson, G.Y.; Blosser, S.A.; Cooney, R.N.; Smith, J.S. Validation of a 5-minute steady state indirect calorimetry protocol for resting energy expenditure in critically ill patients. J. Am. Coll. Nutr. 1996, 15, 397–402. [Google Scholar] [CrossRef] [PubMed]
- Force, T. Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Eur. Heart J. 1996, 17, 354–381. [Google Scholar]
- Aune, D.; Sen, A.; ó’Hartaigh, B.; Janszky, I.; Romundstad, P.R.; Tonstad, S.; Vatten, L.J. Resting heart rate and the risk of cardiovascular disease, total cancer, and all-cause mortality—A systematic review and dose–response meta-analysis of prospective studies. Nutr. Metab. Cardiovasc. Dis. 2017, 27, 504–517. [Google Scholar] [CrossRef] [PubMed]
- La Rovere, M.T.; Pinna, G.D.; Maestri, R.; Mortara, A.; Capomolla, S.; Febo, O.; Ferrari, R.; Franchini, M.; Gnemmi, M.; Opasich, C.; et al. Short-term heart rate variability strongly predicts sudden cardiac death in chronic heart failure patients. Circulation 2003, 107, 565–570. [Google Scholar] [CrossRef] [PubMed]
- Bigger, J.T.; Fleiss, J.L.; Steinman, R.C.; Rolnitzky, L.M.; Kleiger, R.E.; Rottman, J.N. Frequency domain measures of heart period variability and mortality after myocardial infarction. Circulation 1992, 85, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Manini, T.M. Energy expenditure and aging. Ageing Res. Rev. 2010, 9, 1–11. [Google Scholar] [CrossRef]
- Amaro-Gahete, F.J.; De-la-O, A.; Jurado-Fasoli, L.; Espuch-Oliver, A.; Robles-Gonzalez, L.; Navarro-Lomas, G.; de Haro, T.; Femia, P.; Castillo, M.J.; Gutierrez, A. Exercise training as S-Klotho protein stimulator in sedentary healthy adults: Rationale, design, and methodology. Contemp. Clin. Trials Commun. 2018, 11, 10–19. [Google Scholar] [CrossRef]
- de Weir, J.B.V. New methods for calculating metabolic rate with special reference to protein metabolism. J. Physiol. 1949, 109, 1–9. [Google Scholar] [CrossRef]
- Balke, B.; Ware, R. An experimental study of physical fitness of Air Force personnel. U. S. Armed Forces Med. J. 1959, 10, 675–688. [Google Scholar]
- Alcantara, J.M.A.; Plaza-florido, A.; Amaro-gahete, F.J.; Acosta, F.M.; Migueles, J.H.; Molina-garcia, P.; Sacha, J.; Sanchez-delgado, G.; Martinez-tellez, B. Impact of Using Different Levels of Threshold-Based Artefact Correction on the Quantification of Heart Rate Variability in Three Independent Human Cohorts. J. Clin. Med. 2020, 9, 325. [Google Scholar] [CrossRef] [PubMed]
- Johnstone, A.M.; Murison, S.D.; Duncan, J.S.; Rance, K.A.; Speakman, J.R. Factors influencing variation in basal metabolic rate include fat-free mass, fat mass, age, and circulating thyroxine but not sex, circulating leptin, or triiodothyronine. Am. J. Clin. Nutr. 2005, 82, 941–948. [Google Scholar] [CrossRef]
- Pontzer, H.; Yamada, Y.; Sagayama, H.; Ainslie, P.N.; Andersen, L.F.; Anderson, L.J.; Arab, L.; Baddou, I.; Bedu-Addo, K.; Blaak, E.E.; et al. Daily energy expenditure through the human life course. Science 2021, 373, 808–812. [Google Scholar] [CrossRef] [PubMed]
- Fiuza-Luces, C.; Garatachea, N.; Berger, N.A.; Lucia, A. Exercise is the real polypill. Physiology 2013, 28, 330–358. [Google Scholar] [CrossRef] [PubMed]
- Unger, T.; Borghi, C.; Charchar, F.; Khan, N.A.; Poulter, N.R.; Prabhakaran, D.; Ramirez, A.; Schlaich, M.; Stergiou, G.S.; Tomaszewski, M.; et al. 2020 International Society of Hypertension Global Hypertension Practice Guidelines. Hypertension 2020, 75, 1334–1357. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Tellez, B.; Sanchez-Delgado, G.; Acosta, F.M.; Alcantara, J.M.A.; Amaro-Gahete, F.J.; Martinez-Avila, W.D.; Merchan-Ramirez, E.; Muñoz-Hernandez, V.; Osuna-Prieto, F.J.; Jurado-Fasoli, L.; et al. No evidence of brown adipose tissue activation after 24 weeks of supervised exercise training in young sedentary adults in the ACTIBATE randomized controlled trial. Nat. Commun. 2022, 13, 5259. [Google Scholar] [CrossRef]
- Amaro-Gahete, F.J.; De-La-O, A.; Jurado-Fasoli, L.; Martinez-Tellez, B.; Ruiz, J.R.; Castillo, M.J. Exercise training as a treatment for cardiometabolic risk in sedentary adults: Are physical activity guidelines the best way to improve cardiometabolic health? the fit-ageing randomized controlled trial. J. Clin. Med. 2019, 8, 2097. [Google Scholar] [CrossRef]
- Jandackova, V.K.; Scholes, S.; Britton, A.; Steptoe, A. Are changes in heart rate variability in middle-aged and older people normative or caused by pathological conditions? Findings from a large population-based longitudinal cohort study. J. Am. Heart Assoc. 2016, 5, e002365. [Google Scholar] [CrossRef]
- Chen, X.J.; Barywani, S.B.; Hansson, P.O.; Östgärd Thunström, E.; Rosengren, A.; Ergatoudes, C.; Mandalenakis, Z.; Caidahl, K.; Fu, M.L. Impact of changes in heart rate with age on all-cause death and cardiovascular events in 50-year-old men from the general population. Open Heart 2019, 6, e000856. [Google Scholar] [CrossRef]
- Zhang, J. Effect of Age and Sex on Heart Rate Variability in Healthy Subjects. J. Manip. Physiol. Ther. 2007, 30, 374–379. [Google Scholar] [CrossRef]
- Garavaglia, L.; Gulich, D.; Defeo, M.M.; Mailland, J.T.; Irurzun, I.M. The effect of age on the heart rate variability of healthy subjects. PLoS ONE 2021, 16, e0255894. [Google Scholar] [CrossRef] [PubMed]
- Kostis, J.B.; Moreyra, A.E.; Amendo, M.T.; Di Pietro, J.; Cosgrove, N.; Kuo, P.T. The effect of age on heart rate in subjects free of heart disease. Studies by ambulatory electrocardiography and maximal exercise stress test. Circulation 1982, 65, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Delsoglio, M.; Achamrah, N.; Berger, M.M.; Pichard, C. Indirect Calorimetry in Clinical Practice. J. Clin. Med. 2019, 8, 1387. [Google Scholar] [CrossRef] [PubMed]
- Achamrah, N.; Delsoglio, M.; De Waele, E.; Berger, M.M.; Pichard, C. Indirect calorimetry: The 6 main issues. Clin. Nutr. 2020, 40, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Oshima, T.; Delsoglio, M.; Dupertuis, Y.M.; Singer, P.; De Waele, E.; Veraar, C.; Heidegger, C.P.; Wernermann, J.; Wischmeyer, P.E.; Berger, M.M.; et al. The clinical evaluation of the new indirect calorimeter developed by the ICALIC project. Clin. Nutr. 2020, 39, 3105–3111. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.Y.; Smith, S.; Ravussin, E.; Krakoff, J.; Plasqui, G.; Tanaka, S.; Murgatroyd, P.; Brychta, R.; Bock, C.; Carnero, E.; et al. Room Indirect Calorimetry Operating and Reporting Standards (RICORS 1.0): A Guide to Conducting and Reporting Human Whole-Room Calorimeter Studies. Obesity 2020, 28, 1613–1625. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.W.P.; Jarczok, M.N.; Ellis, R.J.; Hillecke, T.K.; Thayer, J.F.; Koenig, J. Two-week test-retest reliability of the Polar® RS800CXTM to record heart rate variability. Clin. Physiol. Funct. Imaging 2017, 37, 776–781. [Google Scholar] [CrossRef]

| Men (n = 34) | Women (n = 38) | ||||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | p | |
| Anthropometry and body composition | |||||
| Weight (kg) | 87 | 11 | 65 | 9 | <0.001 |
| Height (cm) | 176 | 6 | 161 | 6 | <0.001 |
| BMI (kg/m2) | 28 | 4 | 25 | 3 | <0.001 |
| Fat mass (kg) | 31 | 10 | 29 | 7 | 0.440 |
| Fat mass (%) | 34 | 8 | 45 | 7 | <0.001 |
| Lean mass (kg) | 54 | 6 | 34 | 6 | <0.001 |
| Waist circumference (cm) | 103 | 9 | 88 | 10 | <0.001 |
| Cardiorespiratory fitness | |||||
| CRF (mL/min) | 2920 | 378 | 1790 | 314 | <0.001 |
| CRFBW (mL/[kg/BW]/min) | 33 | 5 | 28 | 5 | <0.001 |
| CRFLM (mL/[kg/lean mass]/min) | 54 | 7 | 53 | 9 | 0.652 |
| Blood pressure and circulating cardiometabolic risk factors | |||||
| Systolic BP (mm Hg) | 134 | 14 | 121 | 15 | 0.001 |
| Diastolic BP (mm Hg) | 85 | 11 | 78 | 12 | 0.009 |
| Glucose (mg/dl) | 95 | 14 | 93 | 9 | 0.450 |
| Insulin (UI/mL) | 9 | 7 | 8 | 5 | 0.466 |
| HOMA index | 2 | 2 | 2 | 1 | 0.357 |
| Total cholesterol (mg/dL) | 202 | 36 | 211 | 30 | 0.231 |
| HDL-C (mg/dL) | 56 | 13 | 62 | 12 | 0.041 |
| LDL-C (mg/dL) | 126 | 31 | 127 | 27 | 0.958 |
| Triglycerides (mg/dL) | 147 | 85 | 126 | 50 | 0.213 |
| Cardiometabolic risk Z-score 1 | 0.4 | 0.9 | −0.3 | 0.5 | <0.001 |
| Cardiometabolic risk Z-score 2 | 0.2 | 0.8 | −0.2 | 0.5 | 0.014 |
| Heart rate and heart rate variability | |||||
| HR (bpm) | 62 | 10 | 64 | 7 | 0.353 |
| RMSSD (ms) | 29 | 17 | 34 | 21 | 0.361 |
| SDNN (ms) | 30 | 14 | 31 | 14 | 0.669 |
| pNN50 (%) | 13 | 16 | 13 | 17 | 0.917 |
| HF (ms2) | 374 | 389 | 548 | 754 | 0.230 |
| CV for VO2 | CV for VCO2 | CV for RER | CV for REE | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Men | Women | Men | Women | Men | Women | Men | Women | |||||||||
| β | p | β | p | β | p | β | p | β | p | β | p | β | p | β | p | |
| Anthropometry and body composition | ||||||||||||||||
| Weight (kg) | 0.14 | 0.41 | −0.04 | 0.84 | 0.18 | 0.30 | −0.08 | 0.65 | 0.28 | 0.10 | −0.13 | 0.45 | 0.14 | 0.39 | −0.06 | 0.76 |
| Height (cm) | 0.10 | 0.57 | 0.22 | 0.21 | 0.03 | 0.87 | 0.21 | 0.21 | −0.16 | 0.35 | 0.08 | 0.63 | 0.06 | 0.71 | 0.21 | 0.22 |
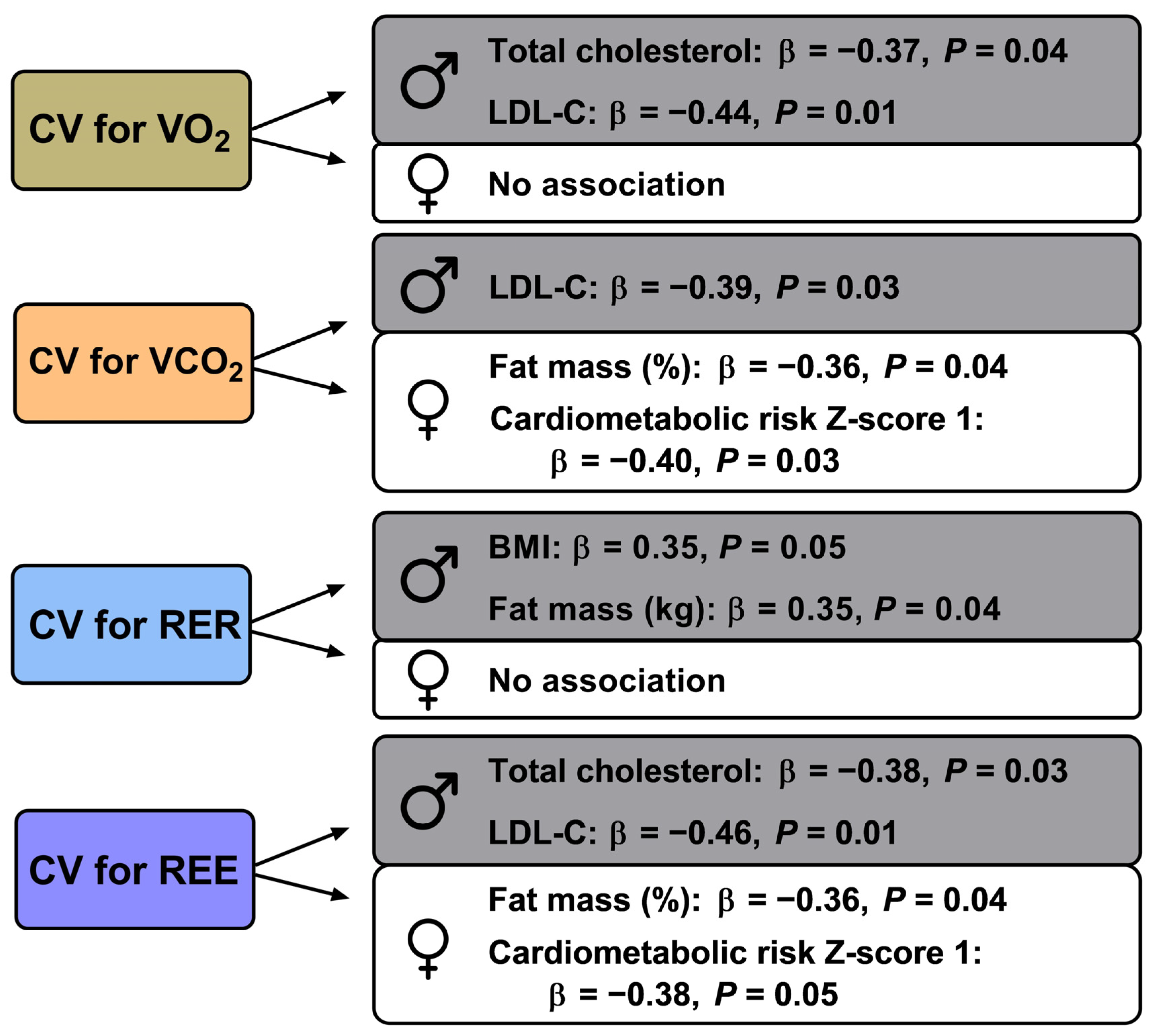
| BMI (kg/m2) | 0.11 | 0.56 | −0.17 | 0.36 | 0.18 | 0.33 | −0.21 | 0.25 | 0.35 | 0.05 | −0.18 | 0.30 | 0.13 | 0.48 | −0.19 | 0.31 |
| Fat mass (kg) | 0.01 | 0.95 | −0.24 | 0.19 | 0.11 | 0.52 | −0.29 | 0.11 | 0.35 | 0.04 | −0.12 | 0.49 | 0.02 | 0.92 | −0.27 | 0.14 |
| Fat mass (%) | −0.07 | 0.70 | −0.33 | 0.06 | 0.04 | 0.82 | −0.36 | 0.04 | 0.34 | 0.06 | −0.09 | 0.62 | −0.06 | 0.73 | −0.36 | 0.04 |
| Lean mass (kg) | 0.23 | 0.19 | 0.23 | 0.20 | 0.14 | 0.44 | 0.22 | 0.21 | −0.06 | 0.75 | −0.06 | 0.73 | 0.23 | 0.19 | 0.23 | 0.18 |
| Waist Circumference (cm) | 0.01 | 0.98 | −0.01 | 0.96 | 0.10 | 0.57 | −0.10 | 0.60 | 0.31 | 0.07 | −0.16 | 0.35 | 0.02 | 0.90 | −0.04 | 0.83 |
| Cardiorespiratory fitness | ||||||||||||||||
| CRF (mL/min) | 0.16 | 0.38 | 0.12 | 0.55 | 0.13 | 0.46 | 0.14 | 0.47 | 0.13 | 0.49 | 0.13 | 0.46 | 0.17 | 0.35 | 0.13 | 0.51 |
| CRFBW (mL/[kg/BW]/min) | 0.15 | 0.41 | 0.22 | 0.27 | 0.09 | 0.62 | 0.25 | 0.19 | −0.05 | 0.77 | 0.26 | 0.14 | 0.16 | 0.40 | 0.23 | 0.23 |
| CRFLM (mL/[kg/lean mass]/min) | 0.01 | 0.99 | −0.02 | 0.94 | 0.05 | 0.79 | −0.01 | 0.98 | 0.22 | 0.24 | 0.24 | 0.19 | 0.01 | 0.98 | −0.02 | 0.92 |
| CV for VO2 | CV for VCO2 | CV for RER | CV for REE | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Men | Women | Men | Women | Men | Women | Men | Women | |||||||||
| β | p | β | p | β | p | β | p | β | p | β | p | β | p | β | p | |
| Blood pressure and circulating cardiometabolic risk factors | ||||||||||||||||
| Systolic BP (mm Hg) | −0.15 | 0.44 | −0.14 | 0.46 | −0.16 | 0.39 | −0.18 | 0.35 | −0.18 | 0.35 | −0.09 | 0.63 | −0.18 | 0.35 | −0.16 | 0.42 |
| Diastolic BP (mm Hg) | −0.21 | 0.28 | −0.22 | 0.26 | −0.14 | 0.48 | −0.23 | 0.22 | −0.05 | 0.80 | −0.06 | 0.72 | −0.23 | 0.24 | −0.23 | 0.25 |
| Glucose (mg/dL) | −0.15 | 0.42 | −0.31 | 0.09 | −0.08 | 0.68 | −0.27 | 0.13 | −0.02 | 0.92 | −0.05 | 0.79 | −0.12 | 0.50 | −0.30 | 0.10 |
| Insulin (UI/mL) | −0.11 | 0.55 | −0.23 | 0.20 | −0.07 | 0.69 | −0.16 | 0.37 | 0.07 | 0.73 | 0.13 | 0.48 | −0.11 | 0.54 | −0.23 | 0.21 |
| HOMA index | −0.13 | 0.49 | −0.28 | 0.13 | −0.08 | 0.66 | −0.2 | 0.27 | 0.06 | 0.77 | 0.11 | 0.52 | −0.13 | 0.5 | −0.27 | 0.14 |
| Total cholesterol (mg/dL) | −0.37 | 0.04 | −0.16 | 0.39 | −0.31 | 0.08 | −0.25 | 0.15 | −0.15 | 0.40 | −0.15 | 0.40 | −0.38 | 0.03 | −0.20 | 0.27 |
| HDL-C (mg/dL) | 0.10 | 0.60 | 0.20 | 0.27 | 0.13 | 0.48 | 0.25 | 0.15 | 0.20 | 0.28 | 0.07 | 0.71 | 0.13 | 0.48 | 0.23 | 0.21 |
| LDL-C (mg/dL) | −0.44 | 0.01 | 0.05 | 0.80 | −0.39 | 0.03 | −0.13 | 0.48 | −0.22 | 0.22 | −0.21 | 0.23 | −0.46 | 0.01 | −0.02 | 0.91 |
| Triglycerides (mg/dL) | −0.03 | 0.87 | −0.26 | 0.16 | −0.11 | 0.57 | −0.29 | 0.10 | −0.21 | 0.26 | −0.19 | 0.28 | −0.07 | 0.71 | −0.29 | 0.11 |
| Cardiometabolic risk Z-score 1 | −0.26 | 0.19 | −0.37 | 0.06 | −0.21 | 0.30 | −0.40 | 0.03 | −0.14 | 0.48 | −0.19 | 0.29 | −0.26 | 0.19 | −0.38 | 0.05 |
| Cardiometabolic risk Z-score 2 | −0.36 | 0.07 | −0.30 | 0.13 | −0.29 | 0.13 | −0.35 | 0.07 | −0.15 | 0.45 | −0.21 | 0.25 | −0.36 | 0.06 | −0.31 | 0.11 |
| HR and HRV parameters | ||||||||||||||||
| HR (bpm) | −0.33 | 0.06 | −0.16 | 0.38 | −0.20 | 0.25 | −0.14 | 0.44 | 0.09 | 0.62 | 0.02 | 0.91 | −0.30 | 0.09 | −0.15 | 0.41 |
| RMSSD (ms) | 0.08 | 0.64 | 0.03 | 0.85 | −0.06 | 0.74 | 0.01 | 0.99 | −0.18 | 0.30 | 0.13 | 0.46 | 0.05 | 0.78 | 0.01 | 0.08 |
| SDNN (ms) | 0.29 | 0.08 | 0.15 | 0.39 | 0.15 | 0.37 | 0.10 | 0.57 | −0.02 | 0.92 | 0.14 | 0.40 | 0.26 | 0.12 | 0.12 | 0.50 |
| pNN50 (%) | 0.16 | 0.35 | 0.04 | 0.8 | 0.02 | 0.92 | 0.03 | 0.87 | −0.16 | 0.37 | 0.13 | 0.41 | 0.13 | 0.47 | 0.02 | 0.91 |
| HF (ms2) | −0.01 | 0.96 | 0.09 | 0.59 | −0.13 | 0.44 | 0.06 | 0.73 | −0.17 | 0.31 | 0.18 | 0.28 | −0.04 | 0.81 | 0.07 | 0.68 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alcantara, J.M.A.; Osuna-Prieto, F.J.; Castillo, M.J.; Plaza-Florido, A.; Amaro-Gahete, F.J. Intra-Assessment Resting Metabolic Rate Variability Is Associated with Cardiometabolic Risk Factors in Middle-Aged Adults. J. Clin. Med. 2023, 12, 7321. https://doi.org/10.3390/jcm12237321
Alcantara JMA, Osuna-Prieto FJ, Castillo MJ, Plaza-Florido A, Amaro-Gahete FJ. Intra-Assessment Resting Metabolic Rate Variability Is Associated with Cardiometabolic Risk Factors in Middle-Aged Adults. Journal of Clinical Medicine. 2023; 12(23):7321. https://doi.org/10.3390/jcm12237321
Chicago/Turabian StyleAlcantara, Juan M. A., Francisco J. Osuna-Prieto, Manuel J. Castillo, Abel Plaza-Florido, and Francisco J. Amaro-Gahete. 2023. "Intra-Assessment Resting Metabolic Rate Variability Is Associated with Cardiometabolic Risk Factors in Middle-Aged Adults" Journal of Clinical Medicine 12, no. 23: 7321. https://doi.org/10.3390/jcm12237321
APA StyleAlcantara, J. M. A., Osuna-Prieto, F. J., Castillo, M. J., Plaza-Florido, A., & Amaro-Gahete, F. J. (2023). Intra-Assessment Resting Metabolic Rate Variability Is Associated with Cardiometabolic Risk Factors in Middle-Aged Adults. Journal of Clinical Medicine, 12(23), 7321. https://doi.org/10.3390/jcm12237321







