Associations between N-Terminal Pro-B-Type Natriuretic Peptide, Body Fluid Imbalance and Quality of Life in Patients Undergoing Hemodialysis: A Cross-Sectional Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Data Collection
2.3. Assessment of Body Fluid Composition
2.4. QOL Assessment
2.5. Statistical Analyses
3. Results
3.1. Population Characteristics
3.2. Association between Body Fluid Imbalance and NT-proBNP
3.3. Association between NT-proBNP and Echocardiographic Findings
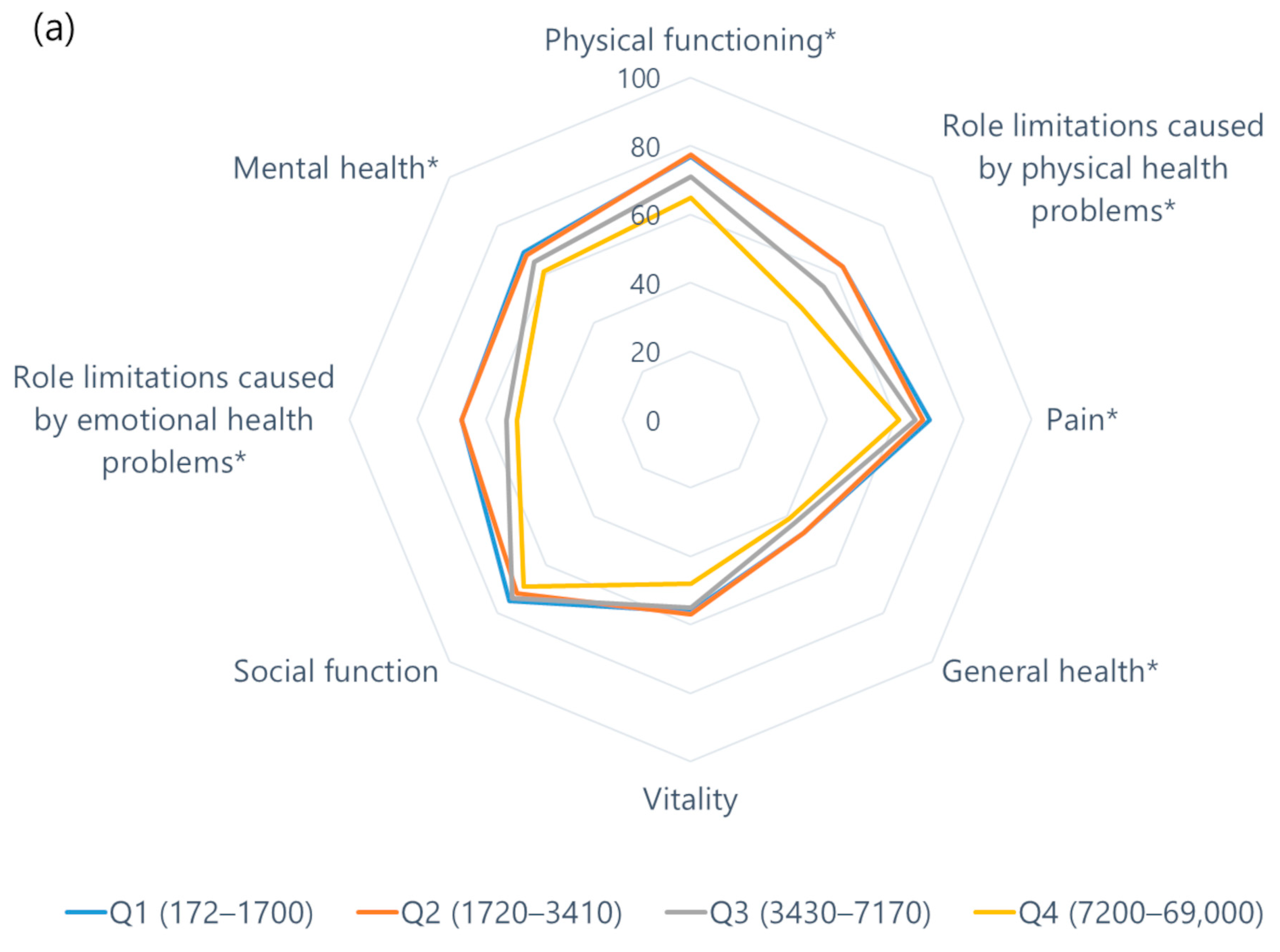
3.4. Association between NT-proBNP and QOL
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cozzolino, M.; Mangano, M.; Stucchi, A.; Ciceri, P.; Conte, F.; Galassi, A. Cardiovascular disease in dialysis patients. Nephrol. Dial. Transplant. 2018, 33, iii28–iii34. [Google Scholar] [CrossRef] [PubMed]
- Madsen, L.H.; Ladefoged, S.; Corell, P.; Schou, M.; Hildebrandt, P.R.; Atar, D. N-terminal pro brain natriuretic peptide predicts mortality in patients with end-stage renal disease in hemodialysis. Kidney Int. 2007, 71, 548–554. [Google Scholar] [CrossRef] [PubMed]
- Paniagua, R.; Ventura, M.D.; Avila-Díaz, M.; Hinojosa-Heredia, H.; Méndez-Durán, A.; Cueto-Manzano, A.; Cisneros, A.; Ramos, A.; Madonia-Juseino, C.; Belio-Caro, F.; et al. NT-proBNP, fluid volume overload and dialysis modality are independent predictors of mortality in ESRD patients. Nephrol. Dial. Transplant. 2010, 25, 551–557. [Google Scholar] [CrossRef] [PubMed]
- Kamano, C.; Osawa, H.; Hashimoto, K.; Nishimura, S.; Saito, S.K.; Kashiwagi, T.; Iino, Y.; Katayama, Y. N-Terminal pro-brain natriuretic peptide as a predictor of heart failure with preserved ejection fraction in hemodialysis patients without fluid overload. Blood Purif. 2012, 33, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, K.; Mizuiri, S.; Nishizawa, Y.; Shigemoto, K.; Doi, S.; Masaki, T. Addition of Novel Biomarkers for Predicting All-Cause and Cardiovascular Mortality in Prevalent Hemodialysis Patients. Ther. Apher. Dial. 2018, 22, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Bárány, P.; Qureshi, A.R.; Snaedal, S.; Heimburger, O.; Stenvinkel, P.; Lindholm, B.; Axelsson, J. N-terminal pro-brain natriuretic peptide independently predicts protein energy wasting and is associated with all-cause mortality in prevalent HD patients. Am. J. Nephrol. 2009, 29, 516–523. [Google Scholar] [CrossRef] [PubMed]
- Wathanavasin, W.; Banjongjit, A.; Avihingsanon, Y.; Praditpornsilpa, K.; Tungsanga, K.; Eiam-Ong, S.; Susantitaphong, P. Prevalence of Sarcopenia and Its Impact on Cardiovascular Events and Mortality among Dialysis Patients: A Systematic Review and Meta-Analysis. Nutrients 2022, 14, 4077. [Google Scholar] [CrossRef]
- Booth, J.; Pinney, J.; Davenport, A. N-terminal proBNP--marker of cardiac dysfunction, fluid overload, or malnutrition in hemodialysis patients? Clin. J. Am. Soc. Nephrol. 2010, 5, 1036–1040. [Google Scholar] [CrossRef]
- Stenberg, J.; Melin, J.; Lindberg, M.; Furuland, H. Brain natriuretic peptide reflects individual variation in hydration status in hemodialysis patients. Hemodial. Int. 2019, 23, 402–413. [Google Scholar] [CrossRef]
- Fang, N.; Che, M.; Shi, L.; Yu, Z.; Ni, Z.; Fang, W.; Pang, H.; Gu, L.; Lin, X. B-type natriuretic peptide levels and volume status in twice-weekly hemodialysis patients. Ren. Fail. 2021, 43, 1259–1265. [Google Scholar] [CrossRef]
- Kawagoe, C.; Sato, Y.; Toida, T.; Nakagawa, H.; Yamashita, Y.; Fukuda, A.; Iwatsubo, S.; Fujimoto, S. N-terminal-pro-B-type-natriuretic peptide associated with 2-year mortality from both cardiovascular and non-cardiovascular origins in prevalent chronic hemodialysis patients. Ren. Fail. 2018, 40, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Cleary, J.; Drennan, J. Quality of life of patients on haemodialysis for end-stage renal disease. J. Adv. Nurs. 2005, 51, 577–586. [Google Scholar] [CrossRef] [PubMed]
- Boateng, E.A.; East, L. The impact of dialysis modality on quality of life: A systematic review. J. Ren. Care 2011, 37, 190–200. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Kim, B.; Park, K.S.; Choi, J.Y.; Seo, J.J.; Park, S.H.; Kim, C.D.; Kim, Y.L. Health-related quality of life with KDQOL-36 and its association with self-efficacy and treatment satisfaction in korean dialysis patients. Qual. Life Res. 2013, 22, 753–758. [Google Scholar] [CrossRef] [PubMed]
- Jhamb, M.; Argyropoulos, C.; Steel, J.L.; Plantinga, L.; Wu, A.W.; Fink, N.E.; Powe, N.R.; Meyer, K.B.; Unruh, M.L.; Choices for Healthy Outcomes in Caring for End-Stage Renal Disease (CHOICE) Study. Correlates and outcomes of fatigue among incident dialysis patients. Clin. J. Am. Soc. Nephrol. 2009, 4, 1779–1786. [Google Scholar] [CrossRef]
- Zyga, S.; Alikari, V.; Sachlas, A.; Fradelos, E.C.; Stathoulis, J.; Panoutsopoulos, G.; Georgopoulou, M.; Theophilou, P.; Lavdaniti, M. Assessment of fatigue in end stage renal disease patients undergoing hemodialysis: Prevalence and associated factors. Med. Arch. 2015, 69, 376–380. [Google Scholar] [CrossRef] [PubMed]
- Inaba, M.; Okuno, S.; Ohno, Y. Importance of considering malnutrition and sarcopenia in order to improve the QOL of elderly hemodialysis patients in japan in the era of 100-year life. Nutrients 2021, 13, 2377. [Google Scholar] [CrossRef]
- Nakayama, Y.; Yamada, Y.; Ishii, S.; Hitaka, M.; Yamazaki, K.; Masai, M.; Joki, N.; Sakai, K.; Ohashi, Y. Association between intra- and extra-cellular water ratio imbalance and natriuretic peptides in patients undergoing hemodialysis. Nutrients 2023, 15, 1274. [Google Scholar] [CrossRef]
- Shinzato, T.; Nakai, S.; Fujita, Y.; Takai, I.; Morita, H.; Nakane, K.; Maeda, K. Determination of Kt/V and protein catabolic rate using pre- and postdialysis blood urea nitrogen concentrations. Nephron 1994, 67, 280–290. [Google Scholar] [CrossRef]
- Bouillanne, O.; Morineau, G.; Dupont, C.; Coulombel, I.; Vincent, J.P.; Nicolis, I.; Benazeth, S.; Cynober, L.; Aussel, C. Geriatric Nutritional Risk Index: A new index for evaluating at-risk elderly medical patients. Am. J. Clin. Nutr. 2005, 82, 777–783. [Google Scholar] [CrossRef]
- Devereux, R.B.; Alonso, D.R.; Lutas, E.M.; Gottlieb, G.J.; Campo, E.; Sachs, I.; Reichek, N. Echocardiographic assessment of left ventricular hypertrophy: Comparison to necropsy findings. Am. J. Cardiol. 1986, 57, 450–458. [Google Scholar] [CrossRef] [PubMed]
- Sorensen, J.; Kondrup, J.; Prokopowicz, J.; Schiesser, M.; Krähenbühl, L.; Meier, R.; Liberda, M.; EuroOOPS Study Group. EuroOOPS: An international, multicentre study to implement nutritional risk screening and evaluate clinical outcome. Clin. Nutr. 2008, 27, 340–349. [Google Scholar] [CrossRef] [PubMed]
- Hays, R.D.; Kallich, J.D.; Mapes, D.L.; Coons, S.J.; Carter, W.B. Development of the kidney disease quality of life (KDQOL) instrument. Qual. Life Res. 1994, 3, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Green, J.; Fukuhara, S.; Shinzato, T.; Miura, Y.; Wada, S.; Hays, R.D.; Tabata, R.; Otsuka, H.; Takai, I.; Maeda, K.; et al. Translation, cultural adaptation, and initial reliability and multitrait testing of the Kidney Disease Quality of Life instrument for use in Japan. Qual. Life Res. 2001, 10, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.C.; Tsai, H.J.; Lee, C.S.; Chiu, Y.W.; Kuo, H.T.; Lee, S.C.; Chen, T.H.; Kuo, M.C. The interaction between N-terminal pro-brain natriuretic peptide and fluid status in adverse clinical outcomes of late stages of chronic kidney disease. PLoS ONE 2018, 13, e0202733. [Google Scholar] [CrossRef] [PubMed]
- Goto, T.; Takase, H.; Toriyama, T.; Sugiura, T.; Kurita, Y.; Tsuru, N.; Masuda, H.; Hayashi, K.; Ueda, R.; Dohi, Y. Increased circulating levels of natriuretic peptides predict future cardiac event in patients with chronic hemodialysis. Nephron 2002, 92, 610–615. [Google Scholar] [CrossRef] [PubMed]
- Naganuma, T.; Sugimura, K.; Wada, S.; Yasumoto, R.; Sugimura, T.; Masuda, C.; Uchida, J.; Nakatani, T. The prognostic role of brain natriuretic peptides in hemodialysis patients. Am. J. Nephrol. 2002, 22, 437–444. [Google Scholar] [CrossRef]
- Ducros, J.; Larifla, L.; Merault, H.; Galantine, V.; Bassien-Capsa, V.; Foucan, L. N-terminal Pro-B-Type Natriuretic Peptide and Malnutrition in Patients on Hemodialysis. Int. J. Nephrol. 2020, 2020, 9528014. [Google Scholar] [CrossRef]
- Tokudome, T.; Otani, K.; Miyazato, M.; Kangawa, K. Ghrelin and the heart. Peptides 2019, 111, 42–46. [Google Scholar] [CrossRef]
- Lexell, J.; Taylor, C.C.; Sjöström, M. What is the cause of the ageing atrophy? Total number, size and proportion of different fiber types studied in whole vastus lateralis muscle from 15- to 83-year-old men. J. Neurol. Sci. 1988, 84, 275–294. [Google Scholar] [CrossRef]
- Gregg, L.P.; Bossola, M.; Ostrosky-Frid, M.; Hedayati, S.S. Fatigue in CKD: Epidemiology, Pathophysiology, and Treatment. Clin. J. Am. Soc. Nephrol. 2021, 16, 1445–1455. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.E.; Curtis, B.M.; Randell, E.W.; Foley, R.N.; Parfrey, P.S. Cardiac biomarkers and health-related quality of life in new hemodialysis patients without symptomatic cardiac disease. Can. J. Kidney Health Dis. 2014, 1, 16. [Google Scholar] [CrossRef] [PubMed]
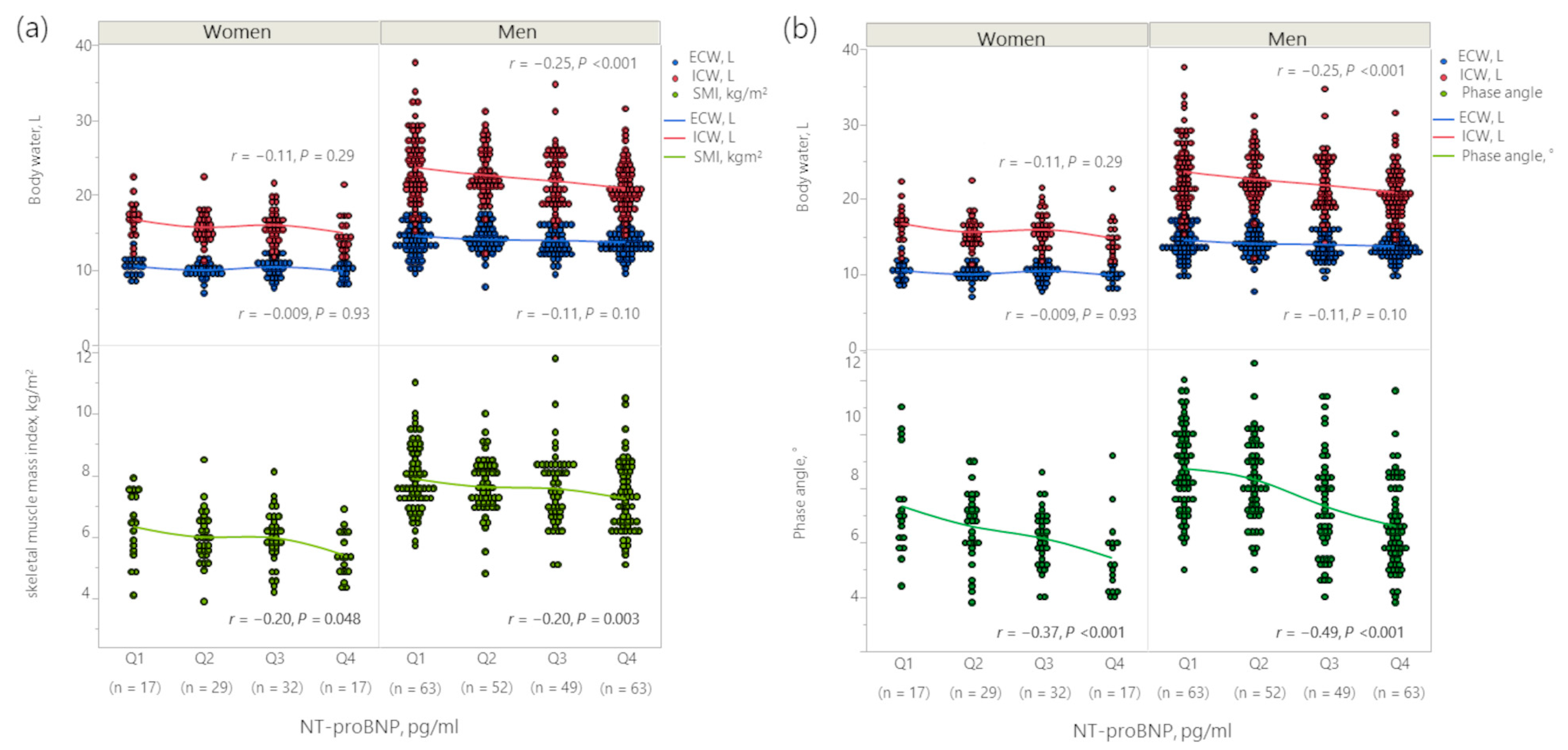
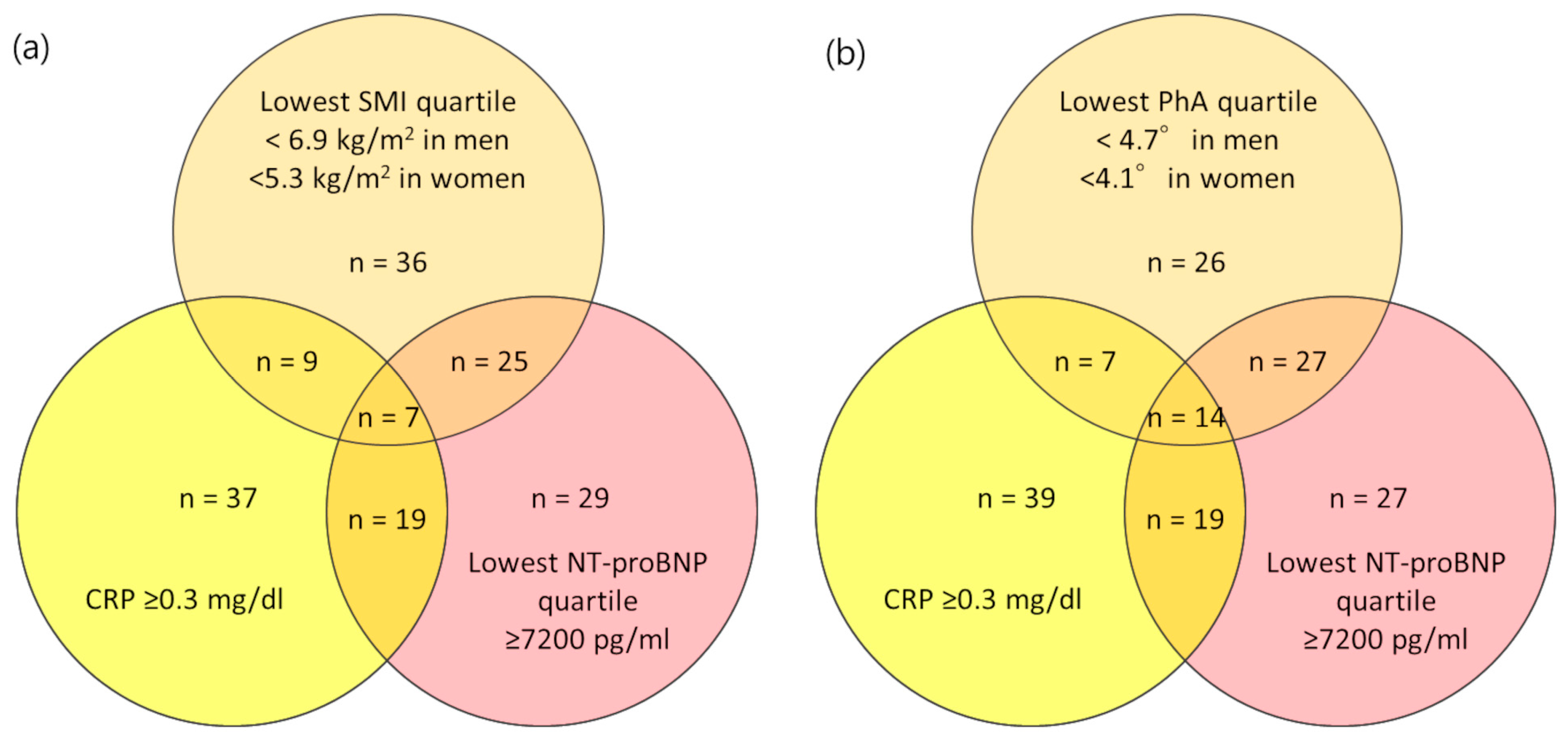

| Patients’ Characteristics | NT-proBNP, pg/mL | p | |||
|---|---|---|---|---|---|
| Quartile 1 172–1700 (n = 80) | Quartile 2 1720–3410 (n = 81) | Quartile 3 3430–7170 (n = 81) | Quartile 4 7200–69,000 (n = 80) | ||
| Age, years | 62 (49–71) | 67 (54–74) | 68 (57–76) | 70 (63–75) | <0.001 |
| Male sex, n (%) | 63 (78.8) | 52 (64.2) | 49 (60.5) | 63 (78.8) | 0.76 |
| Diabetes mellitus, n (%) | 45 (56.3) | 46 (56.8) | 50 (61.7) | 42 (52.5) | 0.81 |
| Dialysis vintage, months | 48 (23–98) | 81 (38–126) | 67 (35–128) | 88 (45–155) | 0.001 |
| Cardiovascular disease, n (%) | 13 (16.3) | 15 (18.5) | 10 (12.4) | 17 (21.3) | 0.48 |
| Smoker, n (%) | 17 (21.3) | 27 (33.3) | 23 (28.4) | 19 (23.8) | 0.76 |
| Body mass index, kg/m2 | 24.3 (20.8–27.3) | 22.9 (20.4–25.1) | 21.1 (19.4–24.3) | 21.0 (19.2–23.9) | <0.001 |
| Pre-dialysis body weight, kg | 69.3 (58.3–82.2) | 62.6 (53.0–72.8) | 60.3 (51.2–67.8) | 60.2 (52.8–68.3) | <0.001 |
| Post-dialysis body weight, kg | 65.9 (56.3–79.0) | 59.9 (50.6–69.2) | 56.5 (48.4–64.1) | 56.7 (49.8–65.1) | <0.001 |
| Ultrafiltration volume, L | 3.2 (2.5–4.3) | 2.9 (2.4–4.0) | 2.9 (2.2–4.0) | 2.9 (2.3–3.7) | 0.06 |
| Ultrafiltration volume, % of body weight | 4.9 (4.0–5.9) | 5.2 (4.4–6.0) | 5.2 (4.1–6.2) | 5.1 (4.1–6.2) | 0.31 |
| Pre-dialysis systolic BP, mmHg | 140 (126–154) | 144 (129–159) | 150 (131–165) | 154 (133–166) | 0.001 |
| Post-dialysis systolic BP, mmHg | 128 (115–148) | 133 (119–150) | 145 (133–165) | 144 (125–164) | <0.001 |
| Pre-dialysis diastolic BP, mmHg | 79 (68–88) | 74 (68–88) | 78 (68–87) | 76 (68–85) | 0.98 |
| Post-dialysis diastolic BP, mmHg | 75 (69–86) | 75 (66–84) | 78 (66–90) | 75 (65–86) | 0.95 |
| Total protein, g/dL | 6.7 (6.4–6.9) | 6.5 (6.2–6.7) | 6.5 (6.2–6.9) | 6.5 (6.2–6.7) | 0.14 |
| Serum albumin, mg/dL | 3.7 (3.5–3.8) | 3.6 (3.5–3.8) | 3.5 (3.4–3.7) | 3.5 (3.3–3.7) | <0.001 |
| Blood urea nitrogen, mg/dL | 59 (50–71) | 61 (53–72) | 58 (49–67) | 53 (45–62) | 0.001 |
| Serum creatinine, mg/dL | 11.36 (9.41–12.87) | 10.67 (9.38–11.89) | 9.93 (8.69–11.60) | 9.56 (8.45–10.79) | <0.001 |
| Serum sodium, mEq/L | 139 (138–143) | 139 (138–141) | 139 (137–141) | 139 (138–140) | 0.037 |
| Serum potassium, mEq/L | 4.7 (4.2–5.0) | 5.0 (4.5–5.6) | 4.8 (4.5–5.3) | 4.8 (4.4–5.2) | 0.24 |
| Serum chloride, mEq/L | 103 (100–105) | 103 (102–105) | 104 (102–106) | 104 (101–106) | 0.60 |
| Serum calcium, mg/dL | 8.7 (8.3–8.9) | 8.7 (8.3–9.0) | 8.5 (8.2–8.9) | 8.7 (8.2–8.9) | 0.78 |
| Serum phosphorus, mg/dL | 5.6 (5.0–6.2) | 5.6 (5.2–6.4) | 5.5 (5.0–6.4) | 5.5 (4.8–6.1) | 0.31 |
| Uric acid, mg/dL | 8.1 (7.3–9.2) | 7.8 (7.1–8.5) | 7.6 (6.9–8.3) | 7.3 (6.5–8.2) | <0.001 |
| Total cholesterol, mg/dL | 169 (151–198) | 169 (142–205) | 159 (140–188) | 153 (133–177) | 0.010 |
| Triglyceride, mg/dL | 138 (81–204) | 104 (67–154) | 91 (65–133) | 95 (62–114) | <0.001 |
| Blood glucose, mg/dL | 117 (98–158) | 109 (89–158) | 111 (94–146) | 112 (96–139) | 0.73 |
| Fe, μg/dL | 62 (50–82) | 71 (56–84) | 65 (54–82) | 65 (51–81) | 0.43 |
| TIBC, μg/dL | 262 (233–314) | 253 (222–277) | 260 (234–286) | 267 (242–302) | 0.31 |
| Ferritin, ng/mL | 49 (29–98) | 66 (40–97) | 54 (33–92) | 59 (33–101) | 0.70 |
| Hemoglobin, g/dL | 11.4 (10.7–12.2) | 11.2 (10.8–11.9) | 11.1 (10.7–11.8) | 11.0 (10.5–11.6) | <0.001 |
| Hematocrit, % | 34.7 (32.6–37.5) | 33.9 (32.4–36.1) | 33.8 (32.8–35.5) | 33.4 (31.4–35.9) | 0.010 |
| C-reactive protein, mg/dL | 0.14 (0.05–0.22) | 0.07 (0.04–0.21) | 0.08 (0.04–0.26) | 0.14 (0.06–0.38) | <0.001 |
| Kt/Vurea | 1.71 (1.54–1.94) | 1.88 (1.67–2.08) | 1.87 (1.68–2.18) | 1.85 (1.71–2.07) | 0.07 |
| Geriatric nutritional risk index | 101 (95–108) | 97 (92–102) | 93 (88–101) | 93 (87–98) | 0.003 |
| CTR in men (n = 227), % | 50.2 (46.4–52.9) | 52.6 (50.8–53.9) | 51.7 (49.4–51.7) | 56.0 (52.6–59.3) | <0.001 |
| CTR in women (n = 95), % | 48.4 (46.0–52.3) | 49.4 (45.1–52.2) | 50.2 (46.7–53.5) | 52.8 (49.4–55.2) | <0.001 |
| Variables | Unadjusted | Model 1 | Model 2 | |||
|---|---|---|---|---|---|---|
| β (95% CI) | p | β (95% CI) | p | β (95% CI) | p | |
| Age | 0.29 (0.007–0.016) | <0.001 | 0.03 (−0.004–0.006) | 0.59 | ||
| Dialysis vintage, months | 0.18 (0.000–0.002) | 0.001 | 0.08 (−0.000–0.001) | 0.08 | 0.05 (−0.000–0.001) | 0.36 |
| Body mass index, kg/m2 | −0.22 (−0.042–−0.014) | <0.001 | −0.01 (−0.013–0.016) | 0.86 | ||
| Post-dialysis systolic BP, mmHg | 0.25 (0.003–0.076) | <0.001 | 0.19 (0.002–0.006) | 0.006 | 0.15 (0.001–0.005) | 0.006 |
| Serum albumin, mg/dL | −0.26 (−0.630–−0.262) | <0.001 | −0.08 (−0.325–0.059) | 0.18 | ||
| Hemoglobin, g/dL | −0.18 (−0.141–−0.037) | <0.001 | −0.09 (−0.097–0.010) | 0.11 | −0.02 (−0.060–0.041) | 0.11 |
| C-reactive protein, mg/dL | 0.22 (0.083–0.243) | <0.001 | 0.16 (0.044–0.201) | 0.002 | 0.13 (0.024–0.178) | 0.002 |
| Geriatric nutritional risk index * | −0.29 (−0.022–−0.010) | <0.001 | −0.20 (−0.016–−0.004) | <0.001 | ||
| Phase angle, ° | −0.45 (−0.253–−0.162) | <0.001 | −0.34 (−0.218–−0.091) | <0.001 | ||
| Patients’ Characteristics | NT-proBNP, pg/mL | p | |||
|---|---|---|---|---|---|
| Quartile 1 172–1700 (n = 80) | Quartile 2 1720–3410 (n = 81) | Quartile 3 3430–7170 (n = 81) | Quartile 4 7200–69,000 (n = 80) | ||
| LAD, mm | 36.0 (32.0–38.6) | 36.2 (33.3–39.2) | 37.0 (33.0–41.5) | 40.2 (36.1–44.4) | <0.001 |
| LVDd, mm | 45.4 (39.9–49.3) | 45.0 (41.5–51.7) | 44.3 (40.4–49.3) | 48.0 (42.0–51.4) | 0.046 |
| LVDs, mm | 29.4 (25.0–32.1) | 29.8 (24.5–34.2) | 28.8 (26.5–33.0) | 31.3 (27.8–35.3) | 0.003 |
| PWT, mm | 10.1 (9.0–11.7) | 10.5 (9.6–12.0) | 10.8 (9.8–12.8) | 11.6 (10.0–12.9) | <0.001 |
| IVST, mm | 10.2 (8.6–12.0) | 11.2 (10.0–12.3) | 11.0 (10.0–12.2) | 11.8 (10.0–13.2) | <0.001 |
| EF | 65.3 (60.4–70.0) | 65.1 (59.6–72.6) | 63.3 (58.9–68.1) | 62.1 (56.1–67.8) | <0.001 |
| LVMI, g/m2 | 87 (72–106) | 107 (93–134) | 107 (89–126) | 124 (104–149) | <0.001 |
| QoL Domains | NT-proBNP, pg/mL | p | |||
|---|---|---|---|---|---|
| Quartile 1 172–1700 (n = 80) | Quartile 2 1720–3410 (n = 81) | Quartile 3 3430–7170 (n = 81) | Quartile 4 7200–69,000 (n = 80) | ||
| Physical health domains | |||||
| Physical functioning | 85 (66–95) | 85 (70–95) | 75 (60–90) | 70 (50–80) | <0.001 |
| Role limitations caused by physical health problems | 75 (25–100) | 75 (13–100) | 75 (0–100) | 38 (0–100) | 0.004 |
| Bodily pain | 78 (55–90) | 70 (45–90) | 68 (45–90) | 59 (45–80) | 0.013 |
| General health | 50 (40–55) | 50 (35–60) | 45 (35–50) | 45 (30–50) | 0.05 |
| Mental health domains | |||||
| Vitality | 58 (40–70) | 60 (45–75) | 55 (40–75) | 50 (31–65) | 0.025 |
| Social functioning | 88 (50–100) | 75 (50–100) | 75 (56–100) | 75 (50–100) | 0.14 |
| Role limitations caused by emotional health problems | 100 (83–100) | 100 (33–100) | 67 (0–100) | 67 (0–100) | 0.008 |
| Emotional well-being | 72 (56–84) | 76 (56–88) | 68 (56–82) | 64 (48–80) | 0.018 |
| Kidney disease-specific domains | |||||
| Symptoms | 83 (73–90) | 88 (78–94) | 83 (74–95) | 79 (71–88) | 0.031 |
| Effects of kidney disease | 78 (69–88) | 81 (69–91) | 78 (63–91) | 77 (63–88) | 0.10 |
| Burden of kidney disease | 38 (25–50) | 44 (25–56) | 38 (25–56) | 31 (25–50) | 0.83 |
| Work status | 50 (0–100) | 50 (0–100) | 50 (0–50) | 50 (0–50) | 0.003 |
| Cognitive function | 93 (87–100) | 93 (87–100) | 93 (80–100) | 93 (80–100) | 0.57 |
| Quality of social interaction | 87 (67–100) | 93 (50–100) | 93 (55–100) | 68 (47–87) | 0.94 |
| Sleep | 55 (43–70) | 68 (50–80) | 63 (53–80) | 59 (48–73) | 0.32 |
| Social support | 67 (67–83) | 67 (67–83) | 67 (67–100) | 67 (67–83) | 0.36 |
| Dialysis staff encouragement | 75 (50–100) | 75 (50–100) | 75 (67–100) | 75 (67–88) | 0.38 |
| Patient satisfaction | 83 (67–100) | 83 (83–100) | 83 (67–100) | 83 (67–83) | 0.60 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamazaki, K.; Ishii, S.; Hitaka, M.; Masai, M.; Ohashi, Y. Associations between N-Terminal Pro-B-Type Natriuretic Peptide, Body Fluid Imbalance and Quality of Life in Patients Undergoing Hemodialysis: A Cross-Sectional Study. J. Clin. Med. 2023, 12, 7356. https://doi.org/10.3390/jcm12237356
Yamazaki K, Ishii S, Hitaka M, Masai M, Ohashi Y. Associations between N-Terminal Pro-B-Type Natriuretic Peptide, Body Fluid Imbalance and Quality of Life in Patients Undergoing Hemodialysis: A Cross-Sectional Study. Journal of Clinical Medicine. 2023; 12(23):7356. https://doi.org/10.3390/jcm12237356
Chicago/Turabian StyleYamazaki, Keisuke, Shingo Ishii, Mai Hitaka, Motoyuki Masai, and Yasushi Ohashi. 2023. "Associations between N-Terminal Pro-B-Type Natriuretic Peptide, Body Fluid Imbalance and Quality of Life in Patients Undergoing Hemodialysis: A Cross-Sectional Study" Journal of Clinical Medicine 12, no. 23: 7356. https://doi.org/10.3390/jcm12237356
APA StyleYamazaki, K., Ishii, S., Hitaka, M., Masai, M., & Ohashi, Y. (2023). Associations between N-Terminal Pro-B-Type Natriuretic Peptide, Body Fluid Imbalance and Quality of Life in Patients Undergoing Hemodialysis: A Cross-Sectional Study. Journal of Clinical Medicine, 12(23), 7356. https://doi.org/10.3390/jcm12237356







