Dual-Layer Spectral CT as Innovative Imaging Guidance in Lung Biopsies: Could Color-Coded Z-Effective Images Allow More Diagnostic Samplings and Biomarkers Information?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients Selection
2.2. Technique of Execution of the Lung Biopsy
2.3. Biopsy Analysis
2.4. Data Analysis and Statistics
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wistuba, I.I.; Gazdar, A.F. Lung Cancer Preneoplasia. Annu. Rev. Pathol. Mech. Dis. 2006, 1, 331–348. [Google Scholar] [CrossRef]
- Montagne, F.; Guisier, F.; Venissac, N.; Baste, J.-M. The Role of Surgery in Lung Cancer Treatment: Present Indications and Future Perspectives—State of the Art. Cancers 2021, 13, 3711. [Google Scholar] [CrossRef]
- Batchelor, T.J.P.; Ljungqvist, O. A surgical perspective of ERAS guidelines in thoracic surgery. Curr. Opin. Anaesthesiol. 2019, 32, 17–22. [Google Scholar] [CrossRef]
- Ma, J.; Li, X.; Zhao, S.; Wang, J.; Zhang, W.; Sun, G. Robot-assisted thoracic surgery versus video-assisted thoracic surgery for lung lobectomy or segmentectomy in patients with non-small cell lung cancer: A meta-analysis. BMC Cancer 2021, 21, 498. [Google Scholar] [CrossRef]
- Vinod, S.K.; Hau, E. Radiotherapy treatment for lung cancer: Current status and future directions. Respirology 2020, 25 (Suppl. S2), 61–71. [Google Scholar] [CrossRef]
- Levy, A.; Mercier, O.; Le Péchoux, C. Indications and Parameters Around Postoperative Radiation Therapy for Lung Cancer. J. Clin. Oncol. 2022, 40, 556–566. [Google Scholar] [CrossRef]
- Bartlett, E.; Rahman, S.; Ridge, C. Percutaneous image-guided thermal ablation of lung cancer: What is the evidence? Lung Cancer 2023, 176, 14–23. [Google Scholar] [CrossRef]
- Sabath, B.F.; Casal, R.F. Bronchoscopic ablation of peripheral lung tumors. J. Thorac. Dis. 2019, 11, 2628–2638. [Google Scholar] [CrossRef]
- Venturini, M.; Cariati, M.; Marra, P.; Masala, S.; Pereira, P.L.; Carrafiello, G. CIRSE Standards of Practice on Thermal Ablation of Primary and Secondary Lung Tumours. Cardiovasc. Interv. Radiol. 2020, 43, 667–683. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, L.; Xiu, Z.; Guo, J.; Wang, L.; Zhou, Y.; Jiao, Y.; Sun, M.; Cai, J. Combination of Immune Checkpoint Inhibitors with Chemotherapy in Lung Cancer. OncoTargets Ther. 2020, 13, 7229–7241. [Google Scholar] [CrossRef]
- El-Hussein, A.; Manoto, S.L.; Ombinda-Lemboumba, S.; Alrowaili, Z.A.; Mthunzi-Kufa, P. A Review of Chemotherapy and Photodynamic Therapy for Lung Cancer Treatment. Anti-Cancer Agents Med. Chem. 2021, 21, 149–161. [Google Scholar] [CrossRef]
- Mamdani, H.; Matosevic, S.; Khalid, A.B.; Durm, G.; Jalal, S.I. Immunotherapy in Lung Cancer: Current Landscape and Future Directions. Front. Immunol. 2022, 13, 823618. [Google Scholar] [CrossRef]
- Ruiz-Cordero, R.; Devine, W.P. Targeted Therapy and Checkpoint Immunotherapy in Lung Cancer. Surg. Pathol. Clin. 2020, 13, 17–33. [Google Scholar] [CrossRef]
- Garelli, E.; Rittmeyer, A.; Putora, P.M.; Glatzer, M.; Dressel, R.; Andreas, S. Abscopal effect in lung cancer: Three case reports and a concise review. Immunotherapy 2019, 11, 1445–1461. [Google Scholar] [CrossRef]
- Yin, L.; Xue, J.; Li, R.; Zhou, L.; Deng, L.; Chen, L.; Zhang, Y.; Li, Y.; Zhang, X.; Xiu, W.; et al. Effect of Low-Dose Radiation Therapy on Abscopal Responses to Hypofractionated Radiation Therapy and Anti-PD1 in Mice and Patients with Non-Small Cell Lung Cancer. Int. J. Radiat. Oncol. 2020, 108, 212–224. [Google Scholar] [CrossRef]
- De Koning, H.J.; Van Der Aalst, C.M.; De Jong, P.A.; Scholten, E.T.; Nackaerts, K.; Heuvelmans, M.A.; Lammers, J.-W.J.; Weenink, C.; Yousaf-Khan, U.; Horeweg, N.; et al. Reduced Lung-Cancer Mortality with Volume CT Screening in a Randomized Trial. N. Engl. J. Med. 2020, 382, 503–513. [Google Scholar] [CrossRef]
- Guerrini, S.; Del Roscio, D.; Zanoni, M.; Cameli, P.; Bargagli, E.; Volterrani, L.; Mazzei, M.A.; Luzzi, L. Lung Cancer Imaging: Screening Result and Nodule Management. Int. J. Environ. Res. Public Health 2022, 19, 2460. [Google Scholar] [CrossRef]
- Griesinger, F.; Eberhardt, W.; Nusch, A.; Reiser, M.; Zahn, M.-O.; Maintz, C.; Bernhardt, C.; Losem, C.; Stenzinger, A.; Heukamp, L.C.; et al. Biomarker testing in non-small cell lung cancer in routine care: Analysis of the first 3717 patients in the German prospective, observational, nation-wide CRISP Registry (AIO-TRK-0315). Lung Cancer 2021, 152, 174–184. [Google Scholar] [CrossRef]
- Stevenson, M.; Christensen, J.; Shoemaker, D.; Foster, T.; Barry, W.T.; Tong, B.C.; Wahidi, M.; Shofer, S.; Datto, M.; Ginsburg, G.; et al. Tumor acquisition for biomarker research in lung cancer. Cancer Investig. 2014, 32, 291–298. [Google Scholar] [CrossRef]
- Mezzapelle, R.; Rrapaj, E.; Gatti, E.; Ceriotti, C.; De Marchis, F.; Preti, A.; Spinelli, A.E.; Perani, L.; Venturini, M.; Valtorta, S.; et al. Human malignant mesothelioma is recapitulated in immunocompetent BALB/c mice injected with murine AB cells. Sci. Rep. 2016, 6, 22850. [Google Scholar] [CrossRef]
- Veltri, A.; Bargellini, I.; Giorgi, L.; Almeida, P.A.M.S.; Akhan, O. CIRSE Guidelines on Percutaneous Needle Biopsy (PNB). Cardiovasc. Interv. Radiol. 2017, 40, 1501–1513. [Google Scholar] [CrossRef] [PubMed]
- Rotolo, N.; Floridi, C.; Imperatori, A.S.; Fontana, F.; Ierardi, A.M.; Mangini, M.; Arlant, V.; De Marchi, G.; Novario, R.; Dominioni, L.; et al. Comparison of cone-beam CT-guided and CT fluoroscopy-guided transthoracic needle biopsy of lung nodules. Eur. Radiol. 2016, 26, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Cerci, J.J.; Bogoni, M.; Cerci, R.J.; Masukawa, M.; Neto, C.C.P.; Krauzer, C.; Fanti, S.; Sakamoto, D.G.; Barreiros, R.B.; Nanni, C.; et al. PET/CT-Guided Biopsy of Suspected Lung Lesions Requires Less Rebiopsy Than CT-Guided Biopsy Due to Inconclusive Results. J. Nucl. Med. 2021, 62, 1057–1061. [Google Scholar] [CrossRef] [PubMed]
- Floridi, C.; Carnevale, A.; Fumarola, E.M.; Schampaert, S.; Fontana, F.; De Palma, D.; Del Sole, A.; Giganti, M.; Carrafiello, G. Percutaneous Lung Tumor Biopsy Under CBCT Guidance with PET-CT Fusion Imaging: Preliminary Experience. Cardiovasc. Interv. Radiol. 2019, 42, 1644–1648. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, S.; Kamei, S.; Tomita, K.; Fujita, C.; Endo, K.; Hiraiwa, S.; Hasebe, T. CT-guided bone biopsy using electron density maps from dual-energy CT. Radiol. Case Rep. 2021, 16, 2343–2346. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Li, S.; Huang, G.; Huang, X.; Zhou, Q.; Wang, W.; Wang, J.; Zhao, F.; Li, Z.; Chen, X.; et al. Role of iodine density value on dual-energy CT for detection of high tumor cell proportion region in lung cancer during CT-guided transthoracic biopsy. Eur. J. Radiol. 2023, 160, 110689. [Google Scholar] [CrossRef]
- Austin, J.H. The Incidental Small Pulmonary Nodule and the Fleischner Criteria 5 Years Later: Have we learned anything more? J. Thorac. Imaging 2011, 26, 88–89. [Google Scholar] [CrossRef]
- Gould, M.K.; Donington, J.; Lynch, W.R.; Mazzone, P.J.; Midthun, D.E.; Naidich, D.P.; Wiener, R.S. Evaluation of individuals with pulmonary nodules: When is it lung cancer?: Diagnosis and management of lung cancer: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013, 143, e93S–e120S. [Google Scholar] [CrossRef]
- Kothary, N.; Lock, L.; Sze, D.Y.; Hofmann, L.V. Computed tomography–guided percutaneous needle biopsy of pulmonary nodules: Impact of nodule size on diagnostic accuracy. Clin. Lung Cancer 2009, 10, 360–363. [Google Scholar] [CrossRef]
- Li, H.; Boiselle, P.M.; O Shepard, J.; Trotman-Dickenson, B.; McLoud, T.C.; Li, P.M.B.H.; Wu, C.C.; Maher, M.M.; Shepard, J.-A.O.; Tsukada, H.; et al. Diagnostic accuracy and safety of CT-guided percutaneous needle aspiration biopsy of the lung: Comparison of small and large pulmonary nodules. Am. J. Roentgenol. 1996, 167, 105–109. [Google Scholar] [CrossRef]
- Fontana, F.; Piacentino, F.; Ierardi, A.M.; Carrafiello, G.; Coppola, A.; Muollo, A.; Beneventi, A.; Floridi, C.; Imperatori, A.S.; Carcano, G.; et al. Comparison Between CBCT and Fusion PET/CT-CBCT Guidance for Lung Biopsies. Cardiovasc. Interv. Radiol. 2021, 44, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Piacentino, F.; Fontana, F.; Zorzetto, G.; Saccomanno, A.; Casagrande, S.; Franzi, F.; Imperatori, A.; Lanza, C.; Carriero, S.; Coppola, A.; et al. Could Maximum SUV be Used as Imaging Guidance in Large Lung Lesions Biopsies? Double Sampling Under PET-CT/XperGuide Fusion Imaging in Inhomogeneous Lung Uptaking Lesions to Show That it can Make a Difference. Technol. Cancer Res. Treat. 2023, 22. [Google Scholar] [CrossRef] [PubMed]
- Rassouli, N.; Etesami, M.; Dhanantwari, A.; Rajiah, P. Detector-based spectral CT with a novel dual-layer technology: Principles and applications. Insights Imaging 2017, 8, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Rajiah, P.; Abbara, S.; Halliburton, S.S. Spectral detector CT for cardiovascular applications. Diagn. Interv. Radiol. 2017, 23, 187–193. [Google Scholar] [CrossRef]
- Kalender, W.A.; Perman, W.H.; Vetter, J.R.; Klotz, E. Evaluation of a prototype dual-energy computed tomographic apparatus. I. Phantom studies. Med. Phys. 1986, 13, 334–339. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, R.E.; Macovski, A. Energy-selective reconstructions in X-ray computerised tomography. Phys. Med. Biol. 1976, 21, 733–744. [Google Scholar] [CrossRef]
- Kaza, R.K.; Ananthakrishnan, L.; Kambadakone, A.; Platt, J.F. Update of Dual-Energy CT Applications in the Genitourinary Tract. Am. J. Roentgenol. 2017, 208, 1185–1192. [Google Scholar] [CrossRef]
- Ho, L.M.; Marin, D.; Neville, A.M.; Barnhart, H.X.; Gupta, R.T.; Paulson, E.K.; Boll, D.T. Characterization of adrenal nodules with dual-energy CT: Can virtual unenhanced attenuation values replace true unenhanced attenuation values? Am. J. Roentgenol. 2012, 198, 840–845. [Google Scholar] [CrossRef]
- Franco, P.N.; Spasiano, C.M.; Maino, C.; De Ponti, E.; Ragusi, M.; Giandola, T.; Terrani, S.; Peroni, M.; Corso, R.; Ippolito, D. Principles and Applications of Dual-Layer Spectral CT in Gastrointestinal Imaging. Diagnostics 2023, 13, 1740. [Google Scholar] [CrossRef]
- Goodsitt, M.M.; Christodoulou, E.G.; Larson, S.C. Accuracies of the synthesized monochromatic CT numbers and effective atomic numbers obtained with a rapid kVp switching dual energy CT scanner. Med. Phys. 2011, 38, 2222–2232. [Google Scholar] [CrossRef]
- Curti, M.; Fontana, F.; Piacentino, F.; Ossola, C.; Coppola, A.; Carcano, G.; Venturini, M. Dual-layer spectral CT fusion imaging for lung biopsies: More accurate targets, diagnostic samplings, and biomarker information? Eur. Radiol. Exp. 2022, 6, 34. [Google Scholar] [CrossRef] [PubMed]
- Gehling, K.; Mokry, T.; Do, T.D.; Giesel, F.L.; Dietrich, S.; Haberkorn, U.; Kauczor, H.-U.; Weber, T.F. Dual-Layer Spectral Detector CT in Comparison with FDG-PET/CT for the Assessment of Lymphoma Activity. ROFO Fortschr. Geb Rontgenstr. Nuklearmed. 2022, 194, 747–754. [Google Scholar] [CrossRef] [PubMed]
- Sedaghat, S.; Langguth, P.; Larsen, N.; Campbell, G.; Both, M.; Jansen, O. Diagnostic Accuracy of Dual-Layer Spectral CT Using Electron Density Images to Detect Post-Traumatic Prevertebral Hematoma of the Cervical Spine. ROFO Fortschr. Geb Rontgenstr. Nuklearmed. 2021, 193, 1445–1450. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Cao, G.; Zhao, L.; Tang, K.; Lin, J.; Miao, S.; Lin, T.; Sun, J.; Zheng, X. Spectral CT Analysis of Solitary Pulmonary Nodules for Differentiating Malignancy from Benignancy: The Value of Iodine Concentration Spatial Distribution Difference. BioMed Res. Int. 2018, 2018, 4830659. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, R.; Gupta, V.; Vyas, S.; Gupta, K.; Singh, V. Correlation of dual energy computed tomography electron density measurements with cerebral glioma grade. Neuroradiol. J. 2022, 35, 352–362. [Google Scholar] [CrossRef]
- Chandarana, H.; Shanbhogue, K. Noninvasive Staging of Liver Fibrosis with Dual-Energy CT: Close but No Cigar. Radiology 2021, 298, 609–610. [Google Scholar] [CrossRef] [PubMed]
- Bicci, E.; Mastrorosato, M.; Danti, G.; Lattavo, L.; Bertelli, E.; Cozzi, D.; Pradella, S.; Agostini, S.; Miele, V. Dual-Energy CT applications in urinary tract cancers: An update. Tumori J. 2023, 109, 148–156. [Google Scholar] [CrossRef]
- Benveniste, A.P.; Faria, S.d.C.; Broering, G.; Ganeshan, D.M.; Tamm, E.P.; Iyer, R.B.; Bhosale, P. Potential Application of Dual-Energy CT in Gynecologic Cancer: Initial Experience. Am. J. Roentgenol. 2017, 208, 695–705. [Google Scholar] [CrossRef]
- Gibney, B.; Murray, N. Dual-Energy CT of Spinal Tophaceous Gout. Radiology 2020, 296, 276. [Google Scholar] [CrossRef]
- Yu, Z.; Mao, T.; Xu, Y.; Li, T.; Wang, Y.; Gao, F.; Sun, W. Diagnostic accuracy of dual-energy CT in gout: A systematic review and meta-analysis. Skelet. Radiol. 2018, 47, 1587–1593. [Google Scholar] [CrossRef]
- Tan, M.T.; Lloyd, T.B. Utility of dual energy computed tomography in the evaluation of infiltrative skeletal lesions and metastasis: A literature review. Skelet. Radiol. 2022, 51, 1731–1741. [Google Scholar] [CrossRef] [PubMed]
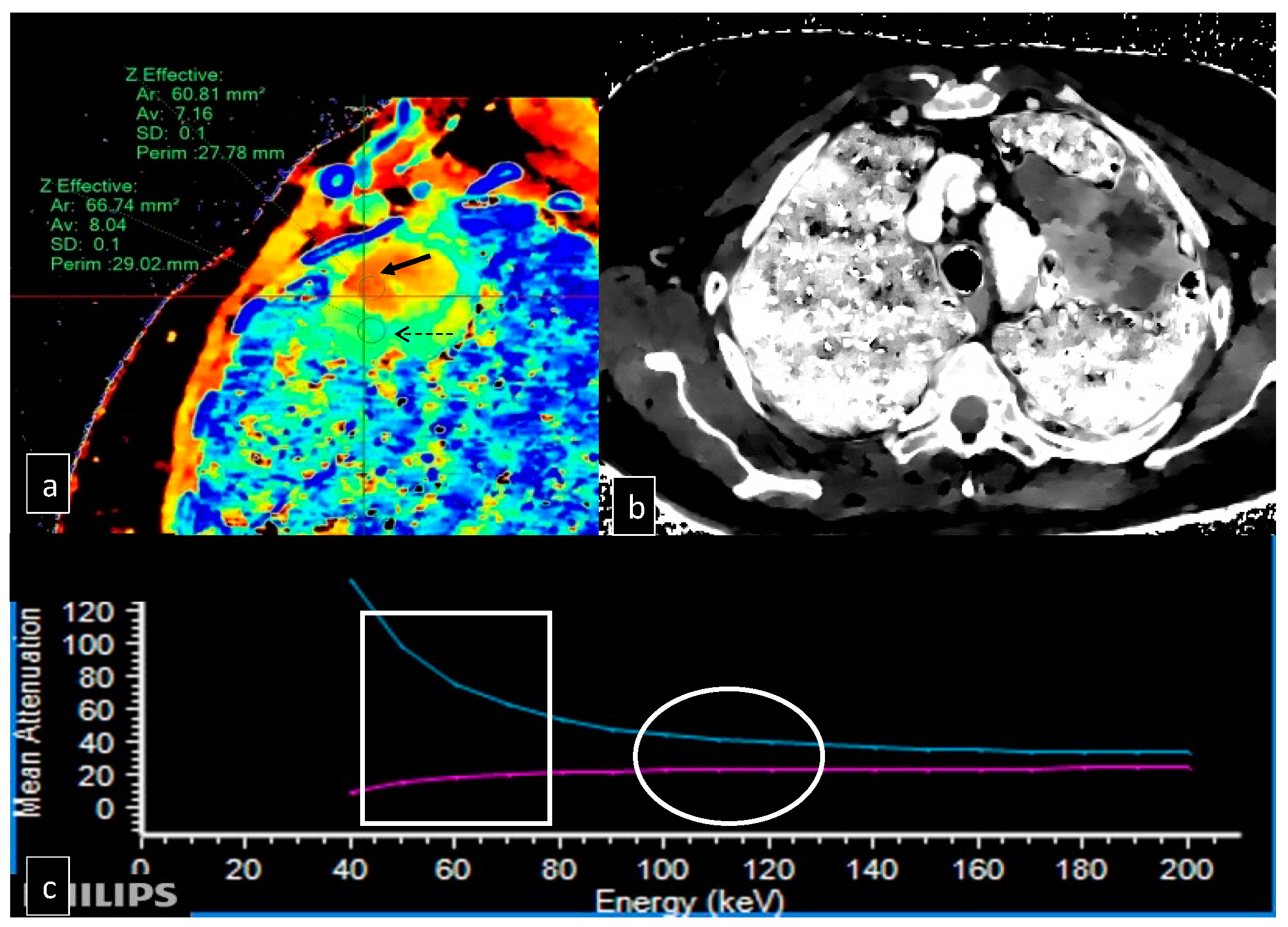
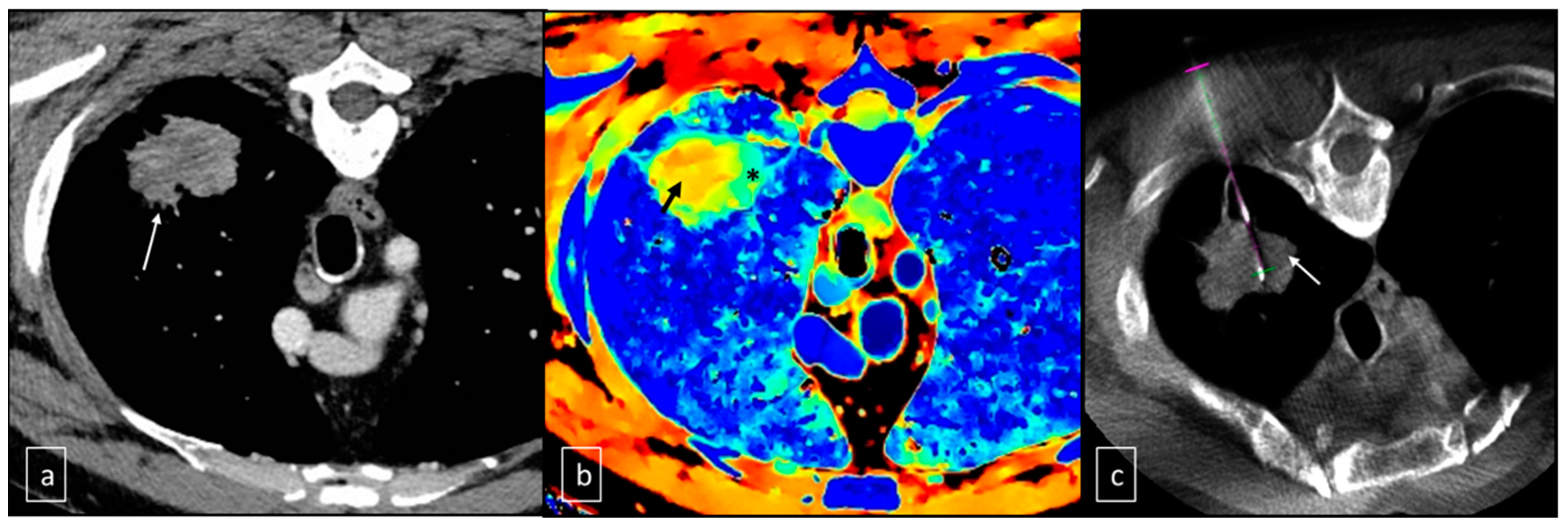

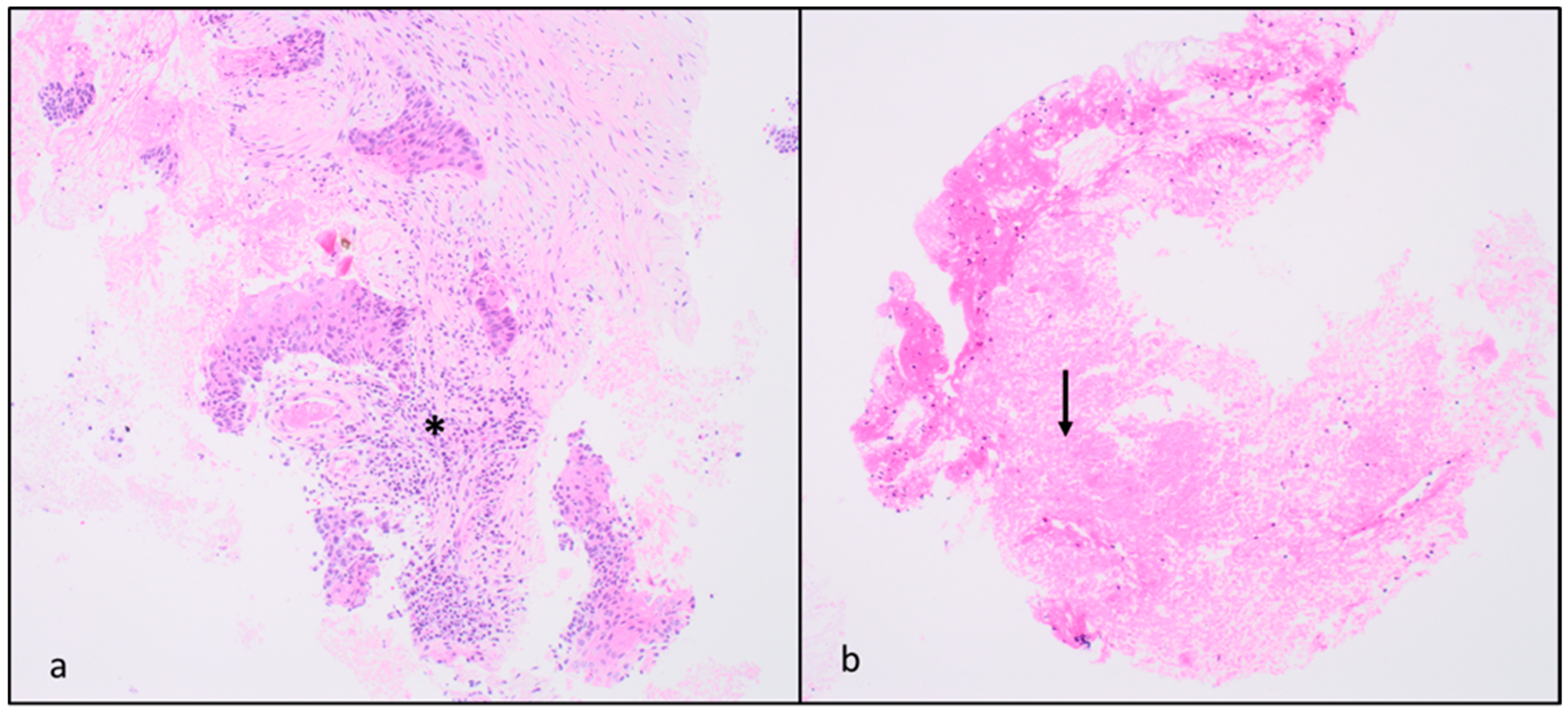
| Patients | Gender | Age | AP Diameter (cm) | CC Diameter (cm) | LL Diameter (cm) | Location | Z Effect max | Z Effect min |
|---|---|---|---|---|---|---|---|---|
| 1 | F | 58 | 4.1 | 8.0 | 3.5 | RLL | 8.21 | 7.59 |
| 2 | M | 76 | 11.5 | 10.0 | 9.1 | RLL | 8.10 | 7.30 |
| 3 | M | 34 | 10.2 | 15.4 | 13.0 | LUL | 7.92 | 7.50 |
| 4 | F | 62 | 8.4 | 9.3 | 9.9 | RML | 8.02 | 7.15 |
| 5 | M | 79 | 8.1 | 9.4 | 7.9 | LUL | 8.23 | 7.40 |
| 6 | F | 71 | 3.1 | 3.4 | 3.3 | LUL | 8.30 | 7.79 |
| 7 | M | 80 | 9.8 | 4.9 | 3.7 | LUL | 8.29 | 7.82 |
| 8 | M | 42 | 10.2 | 16.5 | 11.5 | LUL | 8.29 | 7.15 |
| 9 | F | 64 | 5.1 | 4.9 | 6.2 | LUL | 7.98 | 7.13 |
| 10 | M | 85 | 8.2 | 11.6 | 9.0 | RUP | 8.10 | 7.38 |
| High Z Effective | Low Z Effective | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Patient | Neoplasia | Flogosis | Fibrosis | Necrosis | Lung Parenchyma | Neoplasia | Flogosis | Fibrosis | Necrosis | Lung Parenchyma |
| 1 | 80 | 10 | 0 | 10 | 0 | 40 | 0 | 30 | 30 | 0 |
| 2 | 90 | 5 | 0 | 5 | 0 | non diagnostic | ||||
| 3 | 25 | 60 | 15 | 0 | 0 | 10 | 80 | 10 | 0 | 0 |
| 4 | 40 | 5 | 10 | 45 | 0 | non diagnostic | 80 | 20 | ||
| 5 | 10 | 5 | 70 | 10 | 5 | 20 | 0 | 0 | 80 | 0 |
| 6 | 80 | 5 | 15 | 0 | 0 | 30 | 5 | 65 | 0 | 0 |
| 7 | 80 | 5 | 15 | 0 | 0 | 5 | 15 | 75 | 0 | 5 |
| 8 | 90 | 0 | 5 | 5 | 0 | non diagnostic | ||||
| 9 | 60 | 25 | 0 | 15 | 0 | 60 | 10 | 0 | 30 | 0 |
| 10 | 40 | 5 | 50 | 5 | 0 | 25 | 5 | 70 | 0 | 0 |
| Mean | 59.5 | 12.5 | 18 | 9.5 | 0.5 | 30.83 | 16.43 | 35.71 | 20 | 0.71 |
| SD | 28.91 | 17.99 | 23.48 | 13.43 | 1.58 | 17.44 | 28.54 | 33.72 | 30 | 1.89 |
| Spearman’ Rho | 0.25944 | 0 | 0.10385 | 0.8454 | −0.16667 | |||||
| p-value | 0.57424 | 1 | 0.82465 | 0.01658 | 0.72097 | |||||
| Patients | Diagnosis | High Z Effect | Low Z Effect |
|---|---|---|---|
| Biomolecular Characterization | Biomolecular Characterization | ||
| 1 | Squamous cell carcinoma | yes | yes |
| 2 | Sarcoma (single sampling) | yes | NO |
| 3 | Hodgkin lymphoma | yes | NO |
| 4 | Squamous cell carcinoma | NO | NO |
| 5 | Squamous cell carcinoma | yes | NO |
| 6 | Squamous cell carcinoma | yes | yes |
| 7 | Adenocarcinoma | yes | yes |
| 8 | Diffuse large B-cell lymphoma (single sampling) | yes | NO |
| 9 | Adenocarcinoma | yes | NO |
| 10 | Adenocarcinoma | yes | yes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piacentino, F.; Fontana, F.; Zorzetto, G.; Saccomanno, A.; Gatta, T.; Recaldini, C.; Franzi, F.; Imperatori, A.; Rotolo, N.; Coppola, A.; et al. Dual-Layer Spectral CT as Innovative Imaging Guidance in Lung Biopsies: Could Color-Coded Z-Effective Images Allow More Diagnostic Samplings and Biomarkers Information? J. Clin. Med. 2023, 12, 7426. https://doi.org/10.3390/jcm12237426
Piacentino F, Fontana F, Zorzetto G, Saccomanno A, Gatta T, Recaldini C, Franzi F, Imperatori A, Rotolo N, Coppola A, et al. Dual-Layer Spectral CT as Innovative Imaging Guidance in Lung Biopsies: Could Color-Coded Z-Effective Images Allow More Diagnostic Samplings and Biomarkers Information? Journal of Clinical Medicine. 2023; 12(23):7426. https://doi.org/10.3390/jcm12237426
Chicago/Turabian StylePiacentino, Filippo, Federico Fontana, Giada Zorzetto, Angiola Saccomanno, Tonia Gatta, Chiara Recaldini, Francesca Franzi, Andrea Imperatori, Nicola Rotolo, Andrea Coppola, and et al. 2023. "Dual-Layer Spectral CT as Innovative Imaging Guidance in Lung Biopsies: Could Color-Coded Z-Effective Images Allow More Diagnostic Samplings and Biomarkers Information?" Journal of Clinical Medicine 12, no. 23: 7426. https://doi.org/10.3390/jcm12237426





