The Psychological, Social and Behavioral Impact of Intravitreal Anti-VEGF Therapy: An Analysis from the ALBATROS Data
Abstract
:1. Introduction
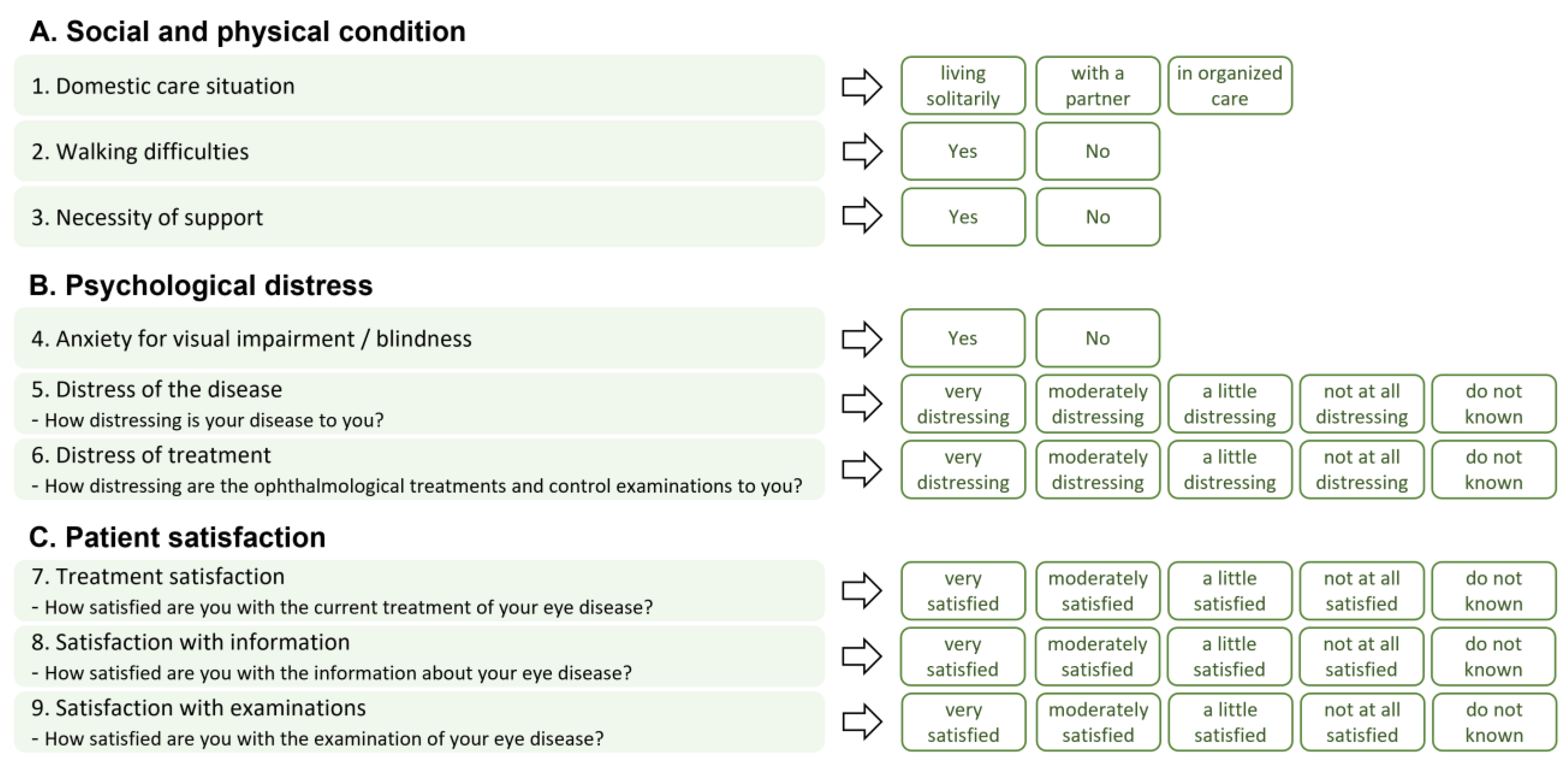
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brown, D.M.; Campochiaro, P.A.; Singh, R.P.; Li, Z.; Gray, S.; Saroj, N.; Rundle, A.C.; Rubio, R.G.; Murahashi, W.Y.; CRUISE Investigators. Ranibizumab for Macular Edema Following Central Retinal Vein Occlusion: Six-Month Primary End Point Results of a Phase III Study. Ophthalmology 2010, 117, 1124–1133.e1. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, P.; Bandello, F.; Schmidt-Erfurth, U.; Lang, G.E.; Massin, P.; Schlingemann, R.O.; Sutter, F.; Simader, C.; Burian, G.; Gerstner, O.; et al. The RESTORE Study: Ranibizumab Monotherapy or Combined with Laser versus Laser Monotherapy for Diabetic Macular Edema. Ophthalmology 2011, 118, 615–625. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, Q.D.; Brown, D.M.; Marcus, D.M.; Boyer, D.S.; Patel, S.; Feiner, L.; Gibson, A.; Sy, J.; Rundle, A.C.; Hopkins, J.J.; et al. Ranibizumab for Diabetic Macular Edema: Results from 2 Phase III Randomized Trials: RISE and RIDE. Ophthalmology 2012, 119, 789–801. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeld, P.J.; Brown, D.M.; Heier, J.S.; Boyer, D.S.; Kaiser, P.K.; Chung, C.Y.; Kim, R.Y.; MARINA Study Group. Ranibizumab for Neovascular Age-Related Macular Degeneration. N. Engl. J. Med. 2006, 355, 1419–1431. [Google Scholar] [CrossRef]
- Delcourt, C.; Le Goff, M.; Von Hanno, T.; Mirshahi, A.; Khawaja, A.P.; Verhoeven, V.J.M.; Hogg, R.E.; Anastosopoulos, E.; Cachulo, M.L.; Höhn, R.; et al. The Decreasing Prevalence of Nonrefractive Visual Impairment in Older Europeans. Ophthalmology 2018, 125, 1149–1159. [Google Scholar] [CrossRef]
- Finger, R.P.; Bertram, B.; Wolfram, C.; Holz, F.G. Blindness and Visual Impairment in Germany. Dtsch. Ärzteblatt Int. 2012, 109, 484. [Google Scholar] [CrossRef]
- Hashimoto, Y.; Okada, A.; Matsui, H.; Yasunaga, H.; Aihara, M.; Obata, R. Recent Trends in Anti-Vascular Endothelial Growth Factor Intravitreal Injections: A Large Claims Database Study in Japan. JPN J. Ophthalmol. 2023, 67, 109–118. [Google Scholar] [CrossRef]
- Reitan, G.; Kjellevold Haugen, I.B.; Andersen, K.; Bragadottir, R.; Bindesbøll, C. Through the Eyes of Patients: Understanding Treatment Burden of Intravitreal Anti-VEGF Injections for nAMD Patients in Norway. Clin. Ophthalmol. 2023, 17, 1465–1474. [Google Scholar] [CrossRef]
- Ehlken, C.; Ziemssen, F.; Eter, N.; Lanzl, I.; Kaymak, H.; Lommatzsch, A.; Schuster, A.K. Systematic Review: Non-Adherence and Non-Persistence in Intravitreal Treatment. Graefes Arch. Clin. Exp. Ophthalmol. 2020, 258, 2077–2090. [Google Scholar] [CrossRef]
- Müller, S.; Junker, S.; Wilke, T.; Lommatzsch, A.; Schuster, A.K.; Kaymak, H.; Ehlken, C.; Ziemssen, F. Questionnaire for the Assessment of Adherence Barriers of Intravitreal Therapy: The ABQ-IVT. Int. J. Retin. Vitr. 2021, 7, 43. [Google Scholar] [CrossRef]
- Ziemssen, F.; Wachtlin, J.; Kuehlewein, L.; Gamulescu, M.-A.; Bertelmann, T.; Feucht, N.; Voegeler, J.; Koch, M.; Liakopoulos, S.; Schmitz-Valckenberg, S.; et al. Intravitreal Ranibizumab Therapy for Diabetic Macular Edema in Routine Practice: Two-Year Real-Life Data from a Non-Interventional, Multicenter Study in Germany. Diabetes Ther. 2018, 9, 2271–2289. [Google Scholar] [CrossRef] [PubMed]
- Wecker, T.; Ehlken, C.; Bühler, A.; Lange, C.; Agostini, H.; Böhringer, D.; Stahl, A. Five-Year Visual Acuity Outcomes and Injection Patterns in Patients with pro-Re-Nata Treatments for AMD, DME, RVO and Myopic CNV. Br. J. Ophthalmol. 2017, 101, 353–359. [Google Scholar] [CrossRef]
- Pesudovs, K.; Gothwal, V.K.; Wright, T.; Lamoureux, E.L. Remediating Serious Flaws in the National Eye Institute Visual Function Questionnaire. J. Cataract. Refract. Surg. 2010, 36, 718–732. [Google Scholar] [CrossRef] [PubMed]
- Schmid, A.; Bucher, F.; Liczenczias, E.; Maslanka Figueroa, S.; Müller, B.; Agostini, H. nAMD: Optimization of Patient Care and Patient-Oriented Information with the Help of an Internet-Based Survey. Graefes Arch. Clin. Exp. Ophthalmol. 2022, 260, 3241–3253. [Google Scholar] [CrossRef]
- Schuster, A.K.; Wolfram, C.; Hudde, T.; Klatt, A.; Schnegelsberg, B.; Midani-Oezkan, H.; Ross, M.; Ziemssen, F.; Pfeiffer, N. Impact of Routinely Performed Optical Coherence Tomography Examinations on Quality of Life in Patients with Retinal Diseases-Results from the ALBATROS Data Collection. J. Clin. Med. 2023, 12, 3881. [Google Scholar] [CrossRef]
- Ziemssen, F.; Feltgen, N.; Holz, F.G.; Guthoff, R.; Ringwald, A.; Bertelmann, T.; Wiedon, A.; Korb, C. Demographics of Patients Receiving Intravitreal Anti-VEGF Treatment in Real-World Practice: Healthcare Research Data versus Randomized Controlled Trials. BMC Ophthalmol. 2017, 17, 7. [Google Scholar] [CrossRef]
- Varano, M.; Eter, N.; Winyard, S.; Wittrup-Jensen, K.U.; Navarro, R.; Heraghty, J. The Emotional and Physical Impact of Wet Age-Related Macular Degeneration: Findings from the wAMD Patient and Caregiver Survey. Clin. Ophthalmol. 2016, 10, 257–267. [Google Scholar] [CrossRef]
- Müller, S.; Ehlken, C.; Bauer-Steinhusen, U.; Lechtenfeld, W.; Hasanbasic, Z.; Agostini, H.; Wilke, T. Treatment of Age-Related Neovascular Macular Degeneration: The Patient’s Perspective. Graefes Arch. Clin. Exp. Ophthalmol. 2017, 255, 2237–2246. [Google Scholar] [CrossRef]
- Giocanti-Aurégan, A.; García-Layana, A.; Peto, T.; Gentile, B.; Chi, G.C.; Mirt, M.; Kosmas, C.E.; Lambert, J.; Lanar, S.; Lewis, H.B.; et al. Drivers of and Barriers to Adherence to Neovascular Age-Related Macular Degeneration and Diabetic Macular Edema Treatment Management Plans: A Multi-National Qualitative Study. Patient Prefer. Adherence 2022, 16, 587–604. [Google Scholar] [CrossRef]
- Chang, A.; Stokes, J.; Priestman, L.; Holmes, C.; Said, P. Impact of a Patient Support Program on Patient Beliefs About Neovascular Age-Related Macular Degeneration and Persistence to Anti-Vascular Endothelial Growth Factor Therapy. Patient Prefer. Adherence 2021, 15, 511–521. [Google Scholar] [CrossRef]
- Gale, R.P.; Finger, R.P.; Eldem, B.; Aslam, T.; Barratt, J.; Daien, V.; Kodjikian, L.; Loewenstein, A.; Okada, M.; Wong, T.Y.; et al. The Management of Neovascular Age-Related Macular Degeneration: A Systematic Literature Review of Patient-Reported Outcomes, Patient Mental Health and Caregiver Burden. Acta Ophthalmol. 2022, 101, e26–e42. [Google Scholar] [CrossRef] [PubMed]
- Senra, H.; Ali, Z.; Balaskas, K.; Aslam, T. Psychological Impact of Anti-VEGF Treatments for Wet Macular Degeneration-a Review. Graefes Arch. Clin. Exp. Ophthalmol. 2016, 254, 1873–1880. [Google Scholar] [CrossRef] [PubMed]
- Ozdemir, S.; Finkelstein, E.; Lee, J.J.; Too, I.H.K.; Teo, K.Y.C.; Tan, A.C.S.; Wong, T.Y.; Cheung, G.C.M. Understanding Patient Preferences in Anti-VEGF Treatment Options for Age-Related Macular Degeneration. PLoS ONE 2022, 17, e0272301. [Google Scholar] [CrossRef] [PubMed]
- McClard, C.K.; Wang, R.; Windham, V.; Munoz, J.; Gomez, S.; Fried, S.; Saroj, N.; Regillo, C.; Wykoff, C.C.; Strutt, A.M. Questionnaire to Assess Life Impact of Treatment by Intravitreal Injections (QUALITII): Development of a Patient-Reported Measure to Assess Treatment Burden of Repeat Intravitreal Injections. BMJ Open Ophth. 2021, 6, e000669. [Google Scholar] [CrossRef] [PubMed]
- Skelly, A.; Taylor, N.; Fasser, C.; Malkowski, J.-P.; Goswami, P.; Downey, L. Patient Preferences in the Management of Wet Age-Related Macular Degeneration: A Conjoint Analysis. Adv. Ther. 2023, 39, 4808–4820. [Google Scholar] [CrossRef]
- Midena, E.; Varano, M.; Pilotto, E.; Staurenghi, G.; Camparini, M.; Pece, A.; Battaglia Parodi, M.; Vadalà, M.; Donati, S.; Frizziero, L.; et al. Real-Life Patient Journey in Neovascular Age-Related Macular Degeneration: A Narrative Medicine Analysis in the Italian Setting. Eye 2022, 36, 182–192. [Google Scholar] [CrossRef]
- Angermann, R.; Rauchegger, T.; Nowosielski, Y.; Casazza, M.; Bilgeri, A.; Ulmer, H.; Zehetner, C. Treatment Compliance and Adherence among Patients with Diabetic Retinopathy and Age-Related Macular Degeneration Treated by Anti-Vascular Endothelial Growth Factor under Universal Health Coverage. Graefes Arch. Clin. Exp. Ophthalmol. 2019, 257, 2119–2125. [Google Scholar] [CrossRef]
- Sii, S.; Aspinall, P.; Borooah, S.; Dhillon, B. Exploring Factors Predicting Changes in Patients’ Expectations and Psychosocial Issues during the Course of Treatment with Intravitreal Injections for Wet Age-Related Macular Degeneration. Eye 2018, 32, 673–678. [Google Scholar] [CrossRef]
- Okada, M.; Mitchell, P.; Finger, R.P.; Eldem, B.; Talks, S.J.; Hirst, C.; Paladini, L.; Barratt, J.; Wong, T.Y.; Loewenstein, A. Nonadherence or Nonpersistence to Intravitreal Injection Therapy for Neovascular Age-Related Macular Degeneration: A Mixed-Methods Systematic Review. Ophthalmology 2021, 128, 234–247. [Google Scholar] [CrossRef]
- Polat, O.; İnan, S.; Özcan, S.; Doğan, M.; Küsbeci, T.; Yavaş, G.F.; İnan, Ü.Ü. Factors Affecting Compliance to Intravitreal Anti-Vascular Endothelial Growth Factor Therapy in Patients with Age-Related Macular Degeneration. Turk. J. Ophthalmol. 2017, 47, 205–210. [Google Scholar] [CrossRef]
- Sobolewska, B.; Sabsabi, M.; Ziemssen, F. Importance of Treatment Duration: Unmasking Barriers and Discovering the Reasons for Undertreatment of Anti-VEGF Agents in Neovascular Age-Related Macular Degeneration. Clin. Ophthalmol. 2021, 15, 4317–4326. [Google Scholar] [CrossRef] [PubMed]
- Angermann, R.; Franchi, A.; Frede, K.; Stöckl, V.; Palme, C.; Kralinger, M.; Zehetner, C. Long-Term Persistence with Aflibercept Therapy among Treatment-Naïve Patients with Exudative Age-Related Macular Degeneration in a Universal Health Care System: A Retrospective Study. BMC Ophthalmol. 2022, 22, 372. [Google Scholar] [CrossRef] [PubMed]





| Total | nAMD | DME | BRVO | CRVO | |
|---|---|---|---|---|---|
| Demographics and Baseline Characteristics | |||||
| Patients, n | 1.478 | 964 | 272 | 140 | 102 |
| Age, years | 74.5 ± 10.9 | 78.3 ± 8.0 | 65.9 ± 12.3 | 68.7 ± 11.4 | 70.4 ± 11.8 |
| Sex, female (%) | 54.9 | 61.1 | 38.6 | 52.1 | 43.1 |
| BCVA study [fellow] eye, letters | 56.1 ± 19.6 [65.8 ± 25.0] | 54.4 ± 19.6 [62.2 ± 27.2] | 65 ± 14.2 [69.2 ± 20.4] | 59.1 ± 19.1 [77.2 ± 13.9] | 44.9 ± 23.1 [75.3 ± 16.4] |
| Better eye at baseline is fellow eye, n (%) | 1028 (69.6) | 643 (66.7) | 172 (63.2) | 123 (87.9) | 90 (88.2) |
| Neovascular/exudative disease of the partner eye (%) | 23.9 | 32.8 | 9.9 | 4.3 | 3.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wolfram, C.; Pfeiffer, N.; Hudde, T.; Klatt, A.; Schnegelsberg, B.; Ross, M.; Ziemssen, F.; Schuster, A.K. The Psychological, Social and Behavioral Impact of Intravitreal Anti-VEGF Therapy: An Analysis from the ALBATROS Data. J. Clin. Med. 2023, 12, 7435. https://doi.org/10.3390/jcm12237435
Wolfram C, Pfeiffer N, Hudde T, Klatt A, Schnegelsberg B, Ross M, Ziemssen F, Schuster AK. The Psychological, Social and Behavioral Impact of Intravitreal Anti-VEGF Therapy: An Analysis from the ALBATROS Data. Journal of Clinical Medicine. 2023; 12(23):7435. https://doi.org/10.3390/jcm12237435
Chicago/Turabian StyleWolfram, Christian, Norbert Pfeiffer, Tobias Hudde, Alexander Klatt, Birthe Schnegelsberg, Mike Ross, Focke Ziemssen, and Alexander K. Schuster. 2023. "The Psychological, Social and Behavioral Impact of Intravitreal Anti-VEGF Therapy: An Analysis from the ALBATROS Data" Journal of Clinical Medicine 12, no. 23: 7435. https://doi.org/10.3390/jcm12237435





