Rare within Rare: A Girl with Severe Haemophilia A and Turner Syndrome
Abstract
1. Introduction
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tosetto, A.; Castaman, G.; Rodeghiero, F. Bleeders, bleeding rates, and bleeding score. J. Thromb. Haemost. 2013, 11 (Suppl. S1), 142–150. [Google Scholar] [CrossRef]
- Weyand, A.C.; Sidonio, R.F., Jr.; Sholzberg, M. Health issues in women and girls affected by haemophilia with a focus on nomenclature, heavy menstrual bleeding, and musculoskeletal issues. Haemophilia 2022, 28 (Suppl. S4), 18–25. [Google Scholar] [CrossRef]
- d’Oiron, R.; O’Brien, S.; James, A.H. Women and girls with haemophilia: Lessons learned. Haemophilia 2021, 27 (Suppl. S3), 75–81. [Google Scholar] [CrossRef] [PubMed]
- Khair, K.; Holland, M.; Pollard, D. The experience of girls and young women with inherited bleeding disorders. Haemophilia 2013, 19, e276–e281. [Google Scholar] [CrossRef] [PubMed]
- Sager, R. Women with haemophilia: More than just carriers. J. Haemoph. Pract. 2014, 1, 2–7. [Google Scholar] [CrossRef]
- Presky, K.O.; Kadir, R.A. Women with inherited bleeding disorders—Challenges and strategies for improved care. Thromb. Res. 2020, 196, 569–578. [Google Scholar] [CrossRef] [PubMed]
- Blanchette, V.S.; Srivastava, A. Definitions in hemophilia: Resolved and unresolved issues. Semin. Thromb. Hemost. 2015, 41, 819–825. [Google Scholar] [CrossRef]
- Stonebraker, J.S.; Bolton-Maggs, P.H.; Soucie, J.M.; Walker, I.; Brooker, M. A study of variations in the reported haemophilia A prevalence around the world. Haemophilia 2010, 16, 20–32. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://wfh.org/research-and-data-collection/annual-global-survey/ (accessed on 1 October 2023).
- Iorio, A.; Stonebraker, J.S.; Chambost, H.; Makris, M.; Coffin, D.; Herr, C.; Germini, F.; Data and Demographics Committee of the World Federation of Hemophilia. Establishing the Prevalence and Prevalence at Birth of Hemophilia in Males: A Meta-analytic Approach Using National Registries. Ann. Intern. Med. 2019, 171, 540–546. [Google Scholar] [CrossRef]
- Soucie, J.M.; Miller, C.H.; Dupervil, B.; Le, B.; Buckner, T.W. Occurrence rates of haemophilia among males in the United States based on surveillance conducted in specialized haemophilia treatment centres. Haemophilia 2020, 26, 487–493. [Google Scholar] [CrossRef]
- Inserro, A. Prevalence of Hemophilia Worldwide is Triple That of Previous Estimates. New Study Says, AJMC, 2019, Life Sciencies. Available online: https://www.ajmc.com/view/prevalence-of-hemophilia-worldwide-is-triple-that-of-previous-estimates-new-study-says (accessed on 1 October 2023).
- Soucie, J.M.; Evatt, B.; Jackson, D.; The Hemophilia Surveillance System Project Investigators. Occurrence of hemophilia in the United States. Am. J. Hematol. 1998, 59, 288–294. [Google Scholar] [CrossRef]
- Raso, S.; Lambert, C.; Boban, A.; Napolitano, M.; Siragusa, S.; Hermans, C. Can we compare haemophilia carriers with clotting factor deficiency to male patients with mild haemophilia? Haemophilia 2020, 26, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Blanchette, V.S.; Key, N.S.; Ljung, L.R.; Manco-Johnson, M.J.; van den Berg, H.M.; Srivastava, A. Definitions in hemophilia: Communication from the SSC of the ISTH. J. Thromb. Haemost. 2014, 12, 1935–1939. [Google Scholar] [CrossRef] [PubMed]
- van Galen, K.P.M.; d’Oiron, R.; James, P.; Abdul-Kadir, R.; Kouides, P.A.; Kulkarni, R.; Mahlangu, J.N.; Othman, M.; Peyvandi, F.; Rotellini, D.; et al. A new hemophilia carrier nomenclature to define hemophilia in women and girls: Communication from the SSC of the ISTH. J. Thromb. Haemost. 2021, 19, 1883–1887. [Google Scholar] [CrossRef] [PubMed]
- Annual Report, Women with Haemophilia Also Exist. 2019. Available online: http://www.ukhcdo.org (accessed on 1 October 2023).
- Kasper, C.K.; Lin, J.C. How many carriers are there? Haemophilia 2010, 16, 842. [Google Scholar] [CrossRef] [PubMed]
- Winikoff, R.; Amesse, C.; James, A.; Lee, C.; Pollard, D. The role of haemophilia treatment centres in providing services to women with bleeding disorders. Hemophilia 2004, 10 (Suppl. S4), 196–204. [Google Scholar] [CrossRef] [PubMed]
- Seeler, R.A.; Vnencak-Jones, C.L.; Bassett, L.M.; Gilbert, J.B.; Michaelis, R.C. Severe haemophilia A in a female: A compound heterozygote with nonrandom X-inactivation. Haemophilia 1999, 5, 445–449. [Google Scholar] [CrossRef]
- Miller, C.H.; Bean, C.J. Genetic causes of haemophilia in women and girls. Haemophilia 2021, 27, e164–e179. [Google Scholar] [CrossRef]
- Miller, C.H.; Soucie, J.M.; Byams, V.R.; Payne, A.B.; Sidonio, R.F., Jr.; Buckner, T.W.; Bean, C.J. Women and girls with haemophilia receiving care at specialized haemophilia treatment centres in the United States. Haemophilia 2021, 27, 1037–1044. [Google Scholar] [CrossRef]
- Pavlova, A.; Brondke, H.; Müsebeck, J.; Pollmann, H.; Srivastava, A.; Oldenburg, J. Molecular mechanisms underlying hemophilia A phenotype in seven females. J. Thromb. Haemost. 2009, 7, 976–982. [Google Scholar] [CrossRef]
- Pezeshkpoor, B.; Oldenburg, J.; Pavlova, A. Insights into the Molecular Genetic of Hemophilia A and Hemophilia B: The Relevance of Genetic Testing in Routine Clinical Practice. Hamostaseologie 2022, 42, 390–399. [Google Scholar] [CrossRef]
- Janczar, S.; Babol-Pokora, K.; Jatczak-Pawlik, I.; Taha, J.; Klukowska, A.; Laguna, P.; Windyga, J.; Odnoczko, E.; Zdziarska, J.; Iwaniec, T.; et al. Six molecular patterns leading to hemophilia A phenotype in 18 females from Poland. Thromb. Res. 2020, 193, 9–14. [Google Scholar] [CrossRef]
- Janczar, S.; Kosinska, J.; Ploski, R.; Pastorczak, A.; Wegner, O.; Zalewska-Szewczyk, B.; Paige, A.J.; Borowiec, M.; Mlynarski, W. Haemophilia A and cardiovascular morbidity in a female SHAM syndrome carrier due to skewed X chromosome inactivation. Eur. J. Med. Genet. 2016, 59, 43–47. [Google Scholar] [CrossRef]
- Radic, C.P.; Rossetti, L.C.; Abelleyro, M.M.; Tetzlaff, T.; Candela, M.; Neme, D.; Sciuccati, G.; Bonduel, M.; Medina-Acosta, E.; Larripa, I.B.; et al. Phenotype-genotype correlations in hemophilia A carriers are consistent with the binary role of the phase between F8 and X-chromosome inactivation. J. Thromb. Haemost. 2015, 13, 530–539. [Google Scholar] [CrossRef]
- Coleman, R.; Genet, S.A.; Harper, J.I.; Wilkie, A.O. Interaction of incontinentia pigmenti and factor VIII mutations in a female with biased X inactivation, resulting in haemophilia. J. Med. Genet. 1993, 30, 497–500. [Google Scholar] [CrossRef][Green Version]
- Martín-Salces, M.; Venceslá, A.; Alvárez-Román, M.T.; Rivas, I.; Fernandez, I.; Butta, N.; Baena, M.; Fuentes-Prior, P.; Tizzano, E.F.; Jiménez-Yuste, V. Clinical and genetic findings in five female patients with haemophilia A: Identification of a novel missense mutation, p.Phe2127Ser. Thromb. Haemost. 2010, 104, 718–723. [Google Scholar] [CrossRef]
- Knobe, K.E.; Sjörin, E.; Soller, M.J.; Liljebjörn, H.; Ljung, R.C. Female haemophilia A caused by skewed X inactivation. Haemophilia 2008, 14, 846–848. [Google Scholar] [CrossRef]
- Ljung, R.C.; Sjörin, E. Origin of mutation in sporadic cases of haemophilia A. Br. J. Haematol. 1999, 106, 870–874. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Ma, W.; Xie, B.; Zhu, M.; Zhang, C.; Li, J.; Wang, Y.; Wang, M.; Jin, Y. Severe female hemophilia A patient caused by a nonsense mutation (p. Gln1686X) of F8 gene combined with skewed X-chromosome inactivation. Blood Coagul. Fibrinolysis 2015, 26, 977–978. [Google Scholar] [CrossRef] [PubMed]
- Afrose, S. Haemophilia A in a female patient with Turner syndrome. Haematol. J. Bangladesh 2017, 1, 26–27. [Google Scholar] [CrossRef]
- Chan, J.T.; Cabanas, M.C.C. A Rare Variant of Turner Syndrome with Isodicentric X Chromosome Resulting in Trisomy: A Case Report. J. Endocr. Soc. 2021, 5 (Suppl. S1), A695–A696. [Google Scholar] [CrossRef]
- Cui, X.; Cui, Y.; Shi, L.; Luan, J.; Zhou, X.; Han, J. A basic understanding of Turner syndrome: Incidence, complications, diagnosis, and treatment. Intractable Rare Dis. Res. 2018, 7, 223–228. [Google Scholar] [CrossRef]
- Gursoy, S.; Ercal, D. Turner syndrome and its variants. J. Pediatr. Res. 2017, 4, 171–178. [Google Scholar] [CrossRef]
- Weinspach, S.; Siepermann, M.; Schaper, J.; Sarikaya-Seiwert, S.; Rieder, H.; Gerigk, M.; Höhn, T.; Laws, H.J. Intracranial hemorrhage in a female leading to the diagnosis of severe hemophilia A and Turner syndrome. Klin. Padiatr. 2009, 221, 167–171. [Google Scholar] [CrossRef]
- Berendt, A.; Wójtowicz-Marzec, M.; Wysokińska, B.; Kwaśniewska, A. Severe haemophilia A in a preterm girl with Turner syndrome: Case report—A diagnostic and therapeutic challenge for a paediatrician (Part 2). Ital. J. Pediatr. 2021, 47, 157. [Google Scholar] [CrossRef]
- Panarello, C.; Acquila, M.; Caprino, D.; Gimelli, G.; Pecorara, M.; Mori, P.G. Concomitant Turner syndrome and hemophilia A in a female with an idic(X)(p11) heterozygous at locus DXS52. Cytogenet. Cell Genet. 1992, 59, 241–242. [Google Scholar] [CrossRef]
- Chuansumrit, A.; Sasanakul, W.; Goodeve, A.; Treratvirapong, T.; Parinayok, R.; Pintadit, P.; Hathirat, P. Inversion of intron 22 of the factor VIII gene in a girl with severe hemophilia A and Turner’s syndrome. Thromb. Haemost. 1999, 82, 1379. [Google Scholar]
- Jones, K.L.; McNamara, E.A.; Longoni, M.; Miller, D.E.; Rohanizadegan, M.; Newman, L.A.; Hayes, F.; Levitsky, L.L.; Herrington, B.L.; Lin, A.E. Dual diagnoses in 152 patients with Turner syndrome: Knowledge of the second condition may lead to modification of treatment and/or surveillance. Am. J. Med. Genet. Part A 2018, 176, 2435–2445. [Google Scholar] [CrossRef]
- Byams, V.R.; Kouides, P.A.; Kulkarni, R.; Baker, J.R.; Brown, D.L.; Gill, J.C.; Grant, A.M.; James, A.H.; Konkle, B.A.; Maahs, J.; et al. Surveillance of female patients with inherited bleeding disorders in United States Haemophilia Treatment Centres. Haemophilia 2011, 17 (Suppl. S1), 6–13. [Google Scholar] [CrossRef] [PubMed]
- Chi, C.; Lee, C.A.; Shiltagh, N.; Khan, A.; Pollard, D.; Kadir, R.A. Pregnancy in carriers of haemophilia. Haemophilia 2008, 14, 56–64. [Google Scholar] [CrossRef] [PubMed]
- McLintock, C. Women with bleeding disorders: Clinical and psychological issues. Haemophilia 2018, 24 (Suppl. S6), 22–28. [Google Scholar] [CrossRef] [PubMed]

| Initial Lab Results | Current Investigations | Biomolecular Investigations | Cytogenetics |
|---|---|---|---|
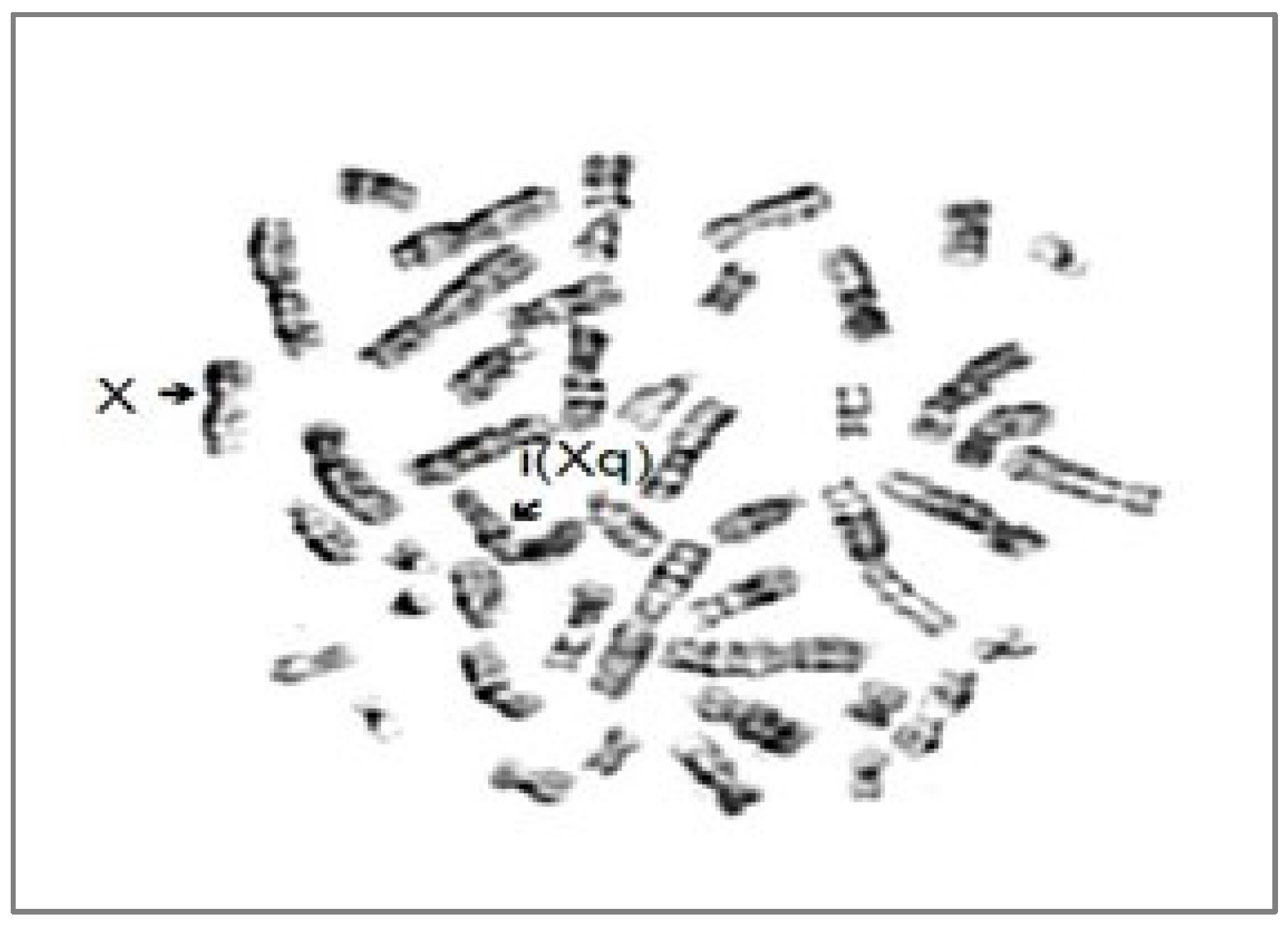
| - aPTT 52 s, prothrombin time-14.4 s, IP 88.2%, INR-1.01; - repeated bruises and hematomas since childhood/prolonged post-dental extraction bleeding | - aPTT-47.5 s, prothrombin time-13.3 s, thrombin time-13.1 s, FVIII <1%, FIX-57.4%, factor von Willebrand activity-48.4%, von Willebrand antigen-46.5%, ristocetin cofactor-75% | missense mutation of F8 within the last base of exon 14, at codon 391—c1172G>A p.R.391H heterozygous | Isochromosome X-46,X,i(Xq) |
| Asymptomatic carrier/symptomatic carrier | Severe haemophilia A | Confirmed severe haemophilia A | Turner syndrome |
| Haemophilia A | Haemophilia B | |
|---|---|---|
| General prevalence/100,000 males | 17.1(14.8–19.3) | 3.8 (3.2–4.4) |
| General prevalence at birth/100,000 males | 23.2(20.1–26.3) | 4.7 (3.4–6.1) |
| Correction for underestimation of diagnosed cases in a 5-year lag time | 24.6 (21.4–27.7) | 5.0 (3.6–6.5) |
| No | Age | Sex | Family History | Patient Diagnosis | Genetic Defect | Publication |
|---|---|---|---|---|---|---|
| 1 | 6 months | female | negative | Mosaic Turner syndrome (45 XO) with ring X (p22, 2q13) with severe haemophilia A and persistent hyperplastic primary vitreous. | 45 XO with ring X chromosome 46X: rX (p22, 2q13); intron-22-inversion (F8, IVS22 INV) hemizygote | Shahriari, 2016 [38] |
| 2 | 2 years | female | negative | Mild haemophilia A and Turner’s syndrome | F8 missense mutation c.5123G>A (p.Arg1708His) in exon 14; hemizygosity for the X-chromosome | Williams, 2012 [38] |
| 3 | 3 months | female | carrier mother | Severe Haemophilia A and Turner Syndrome | intron-22-inversion (F8, IVS22 INV); 45,X0 karyotype | Weinspach, 2009 [37] |
| 4 | 3 months | female | negative | Turner syndrome and moderate haemophilia A | 46,X,idic(X)(p11) karyotype; F8 de novo mutation | Panarello, 1992 [39] |
| 5 | 7 months | female | carrier mother | Severe haemophilia A and Turner’s syndrome | intron-22-inversion (F8, IVS22 INV); 45,X0 karyotype | Sasanakul, 1999 [40] |
| 6 | NA | female | NA | Severe haemophilia A in a phenotypically normal female with 45,X/46,Xr(X) mosaicism | 45,X/46,Xr(X) mosaicism karyotype | Ariyoshi, 1985 [38] |
| 7 | 5 years | female | carrier mother, brother with severe haemophilia A | Severe haemophilia A and Turner’s syndrome | deletion in Xq28 region; 45,X/46,XXr mosaicism karyotype | Gilgenkrantz, 1986 [38] |
| 8 | 6 years | female | carrier mother | Haemophilia A in a phenotypically normal female with XX-XO mosaicism | 45,X/46,XX mosaicism karyotype | Gilchrist, 1965 [38] |
| 9 | preterm, gestational age 28 weeks | female | mother’s brother had severe haemophilia | Severe haemophilia A in a preterm girl with Turner syndrome | mosaic karyotype (46,X + mar,45, X) | Berendt, 2020 [38] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blag, C.; Serban, M.; Ursu, C.E.; Popa, C.; Traila, A.; Jinca, C.; Tomuleasa, C.; Bota, M.; Ionita, I.; Arghirescu, T.S. Rare within Rare: A Girl with Severe Haemophilia A and Turner Syndrome. J. Clin. Med. 2023, 12, 7437. https://doi.org/10.3390/jcm12237437
Blag C, Serban M, Ursu CE, Popa C, Traila A, Jinca C, Tomuleasa C, Bota M, Ionita I, Arghirescu TS. Rare within Rare: A Girl with Severe Haemophilia A and Turner Syndrome. Journal of Clinical Medicine. 2023; 12(23):7437. https://doi.org/10.3390/jcm12237437
Chicago/Turabian StyleBlag, Cristina, Margit Serban, Cristina Emilia Ursu, Cristina Popa, Adina Traila, Cristian Jinca, Ciprian Tomuleasa, Madalina Bota, Ioana Ionita, and Teodora Smaranda Arghirescu. 2023. "Rare within Rare: A Girl with Severe Haemophilia A and Turner Syndrome" Journal of Clinical Medicine 12, no. 23: 7437. https://doi.org/10.3390/jcm12237437
APA StyleBlag, C., Serban, M., Ursu, C. E., Popa, C., Traila, A., Jinca, C., Tomuleasa, C., Bota, M., Ionita, I., & Arghirescu, T. S. (2023). Rare within Rare: A Girl with Severe Haemophilia A and Turner Syndrome. Journal of Clinical Medicine, 12(23), 7437. https://doi.org/10.3390/jcm12237437






