A Systematic Review and Bayesian Network Meta-Analysis on the Effect of Different Anticoagulants on the Prophylaxis of Post-Thrombotic Syndrome after Deep Venous Thrombosis
Abstract
:1. Introduction
2. Methods
2.1. Search Strategy
2.2. Selection Criteria
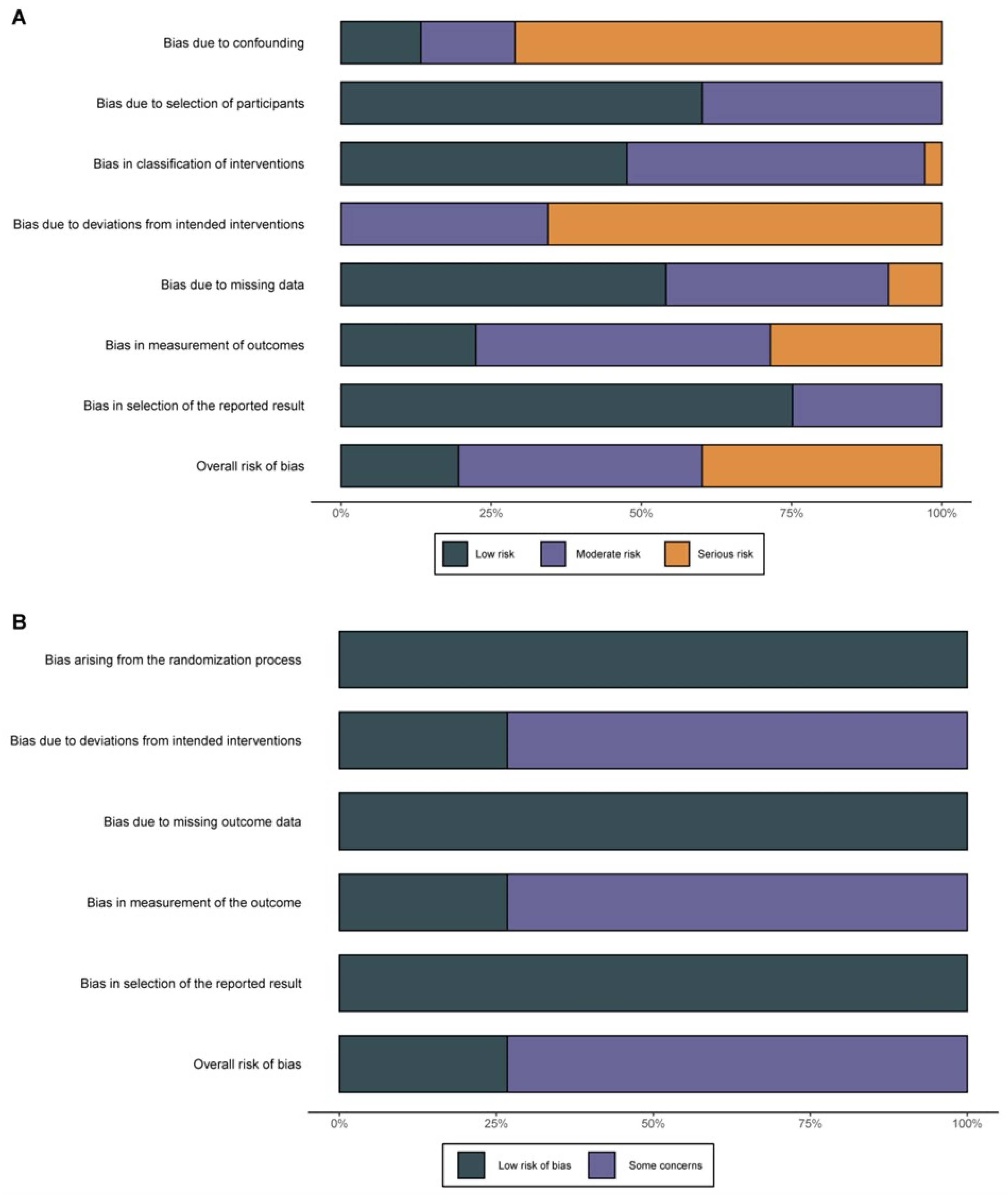
2.3. Data Extraction and Quality Assessment
2.4. Statistical Analysis
2.4.1. Bayesian Network Model
2.4.2. Inconsistency Analysis
2.4.3. Sensitivity Analysis and Meta-Regression
2.4.4. Publication Bias
3. Results
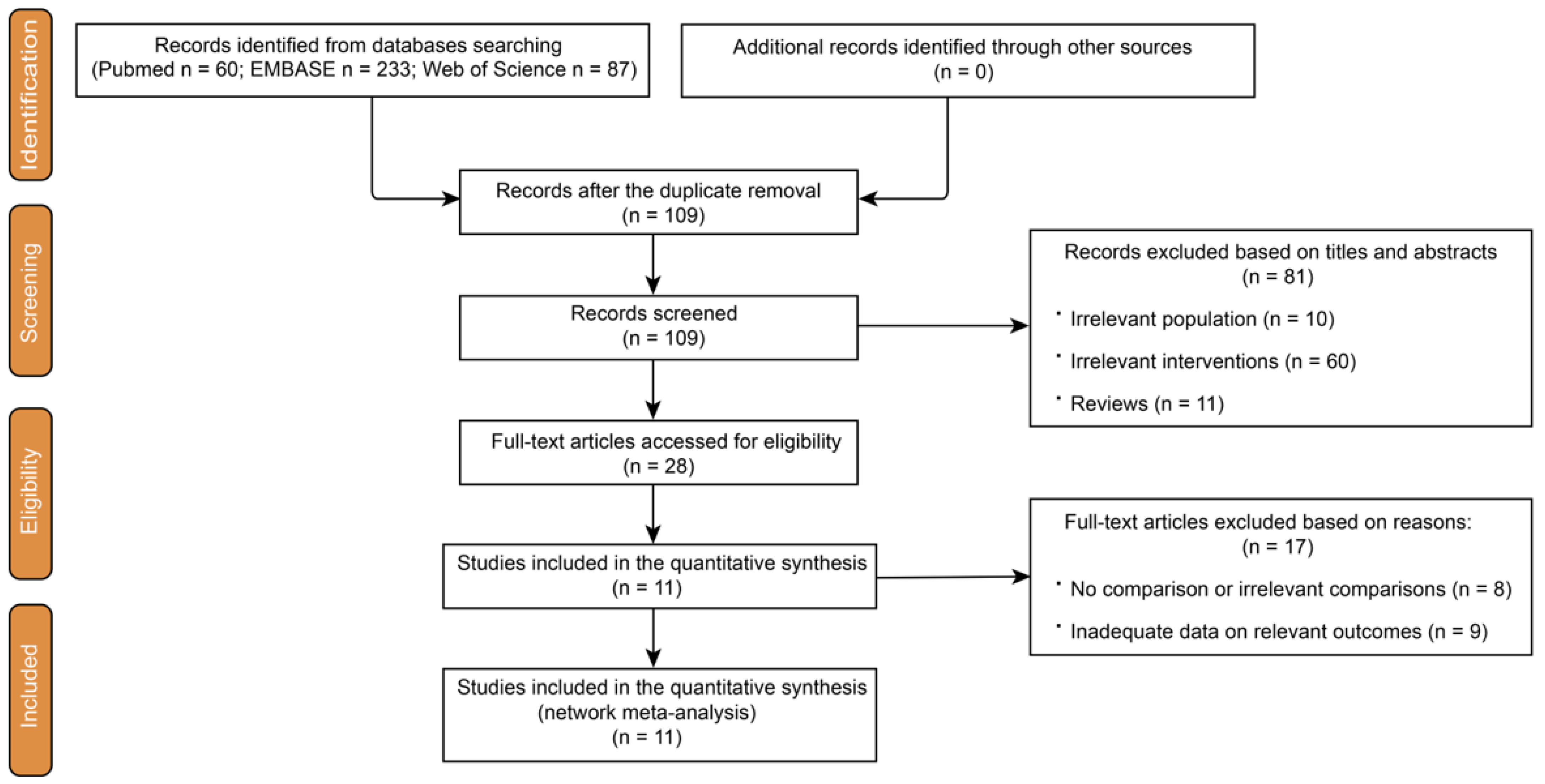
3.1. Study Selection and Quality Assessment
3.2. Characteristics and Baseline Data of the Studies Included in the NMA
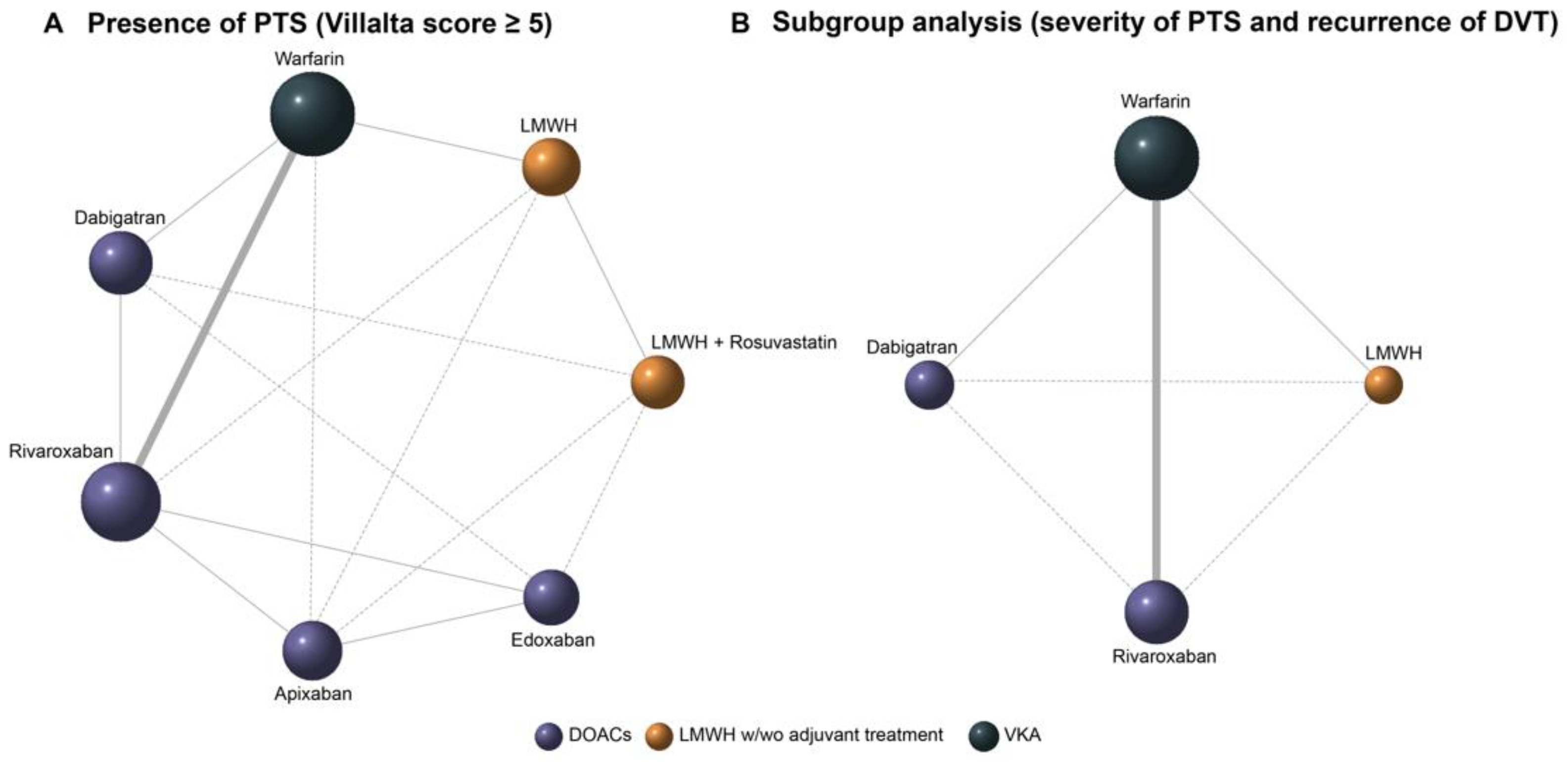
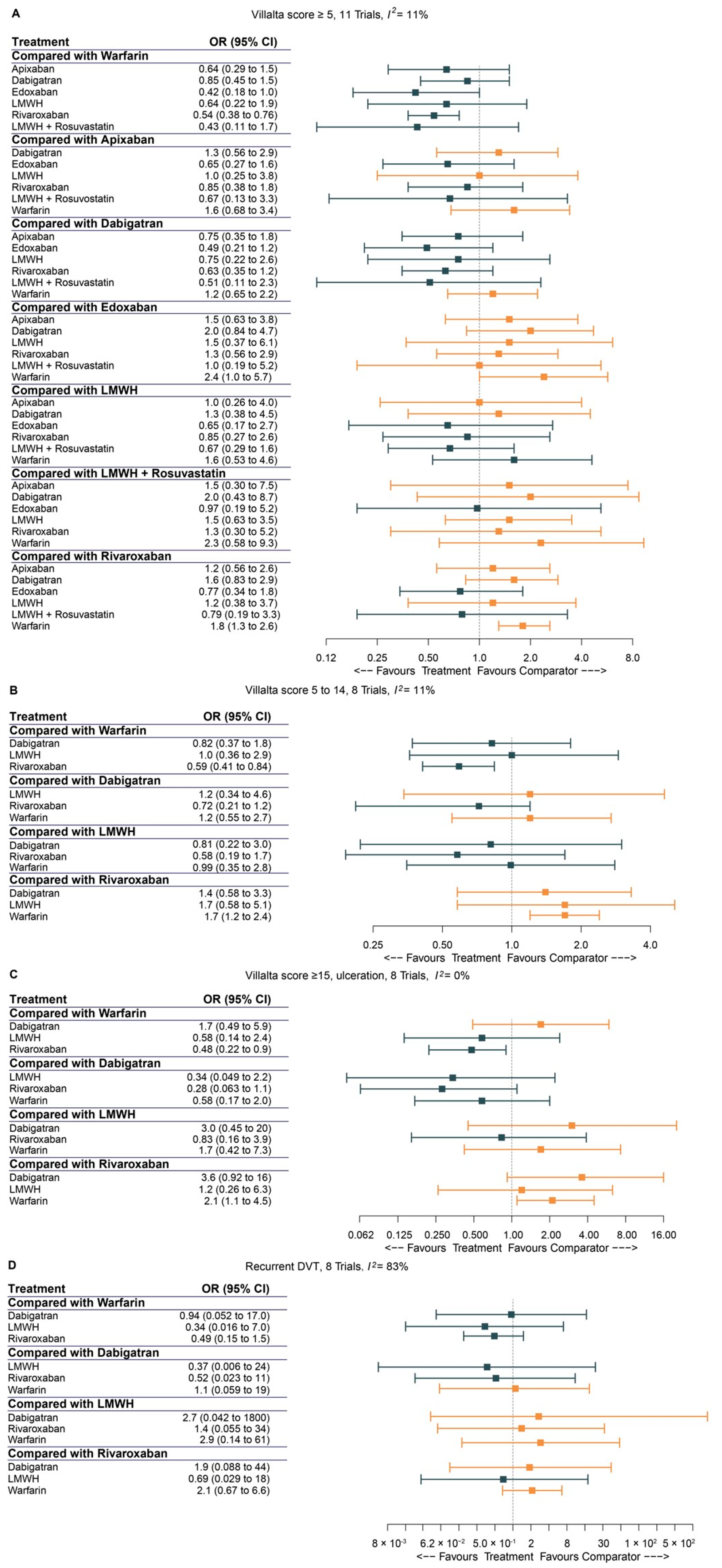
3.3. Bayesian Network Meta-Analysis
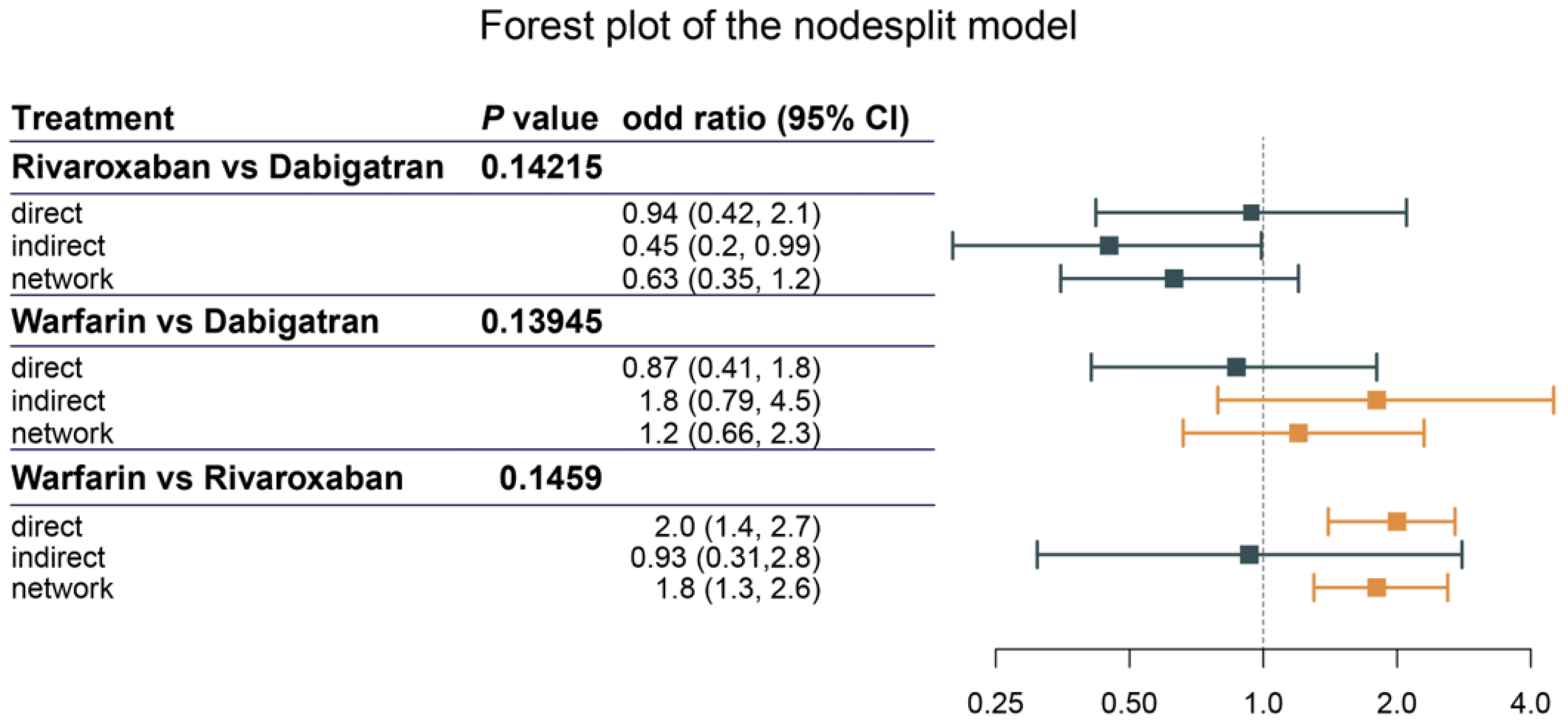
3.4. Assessment of Heterogeneity and Inconsistency
3.5. Pairwise Comparison of Anticoagulants
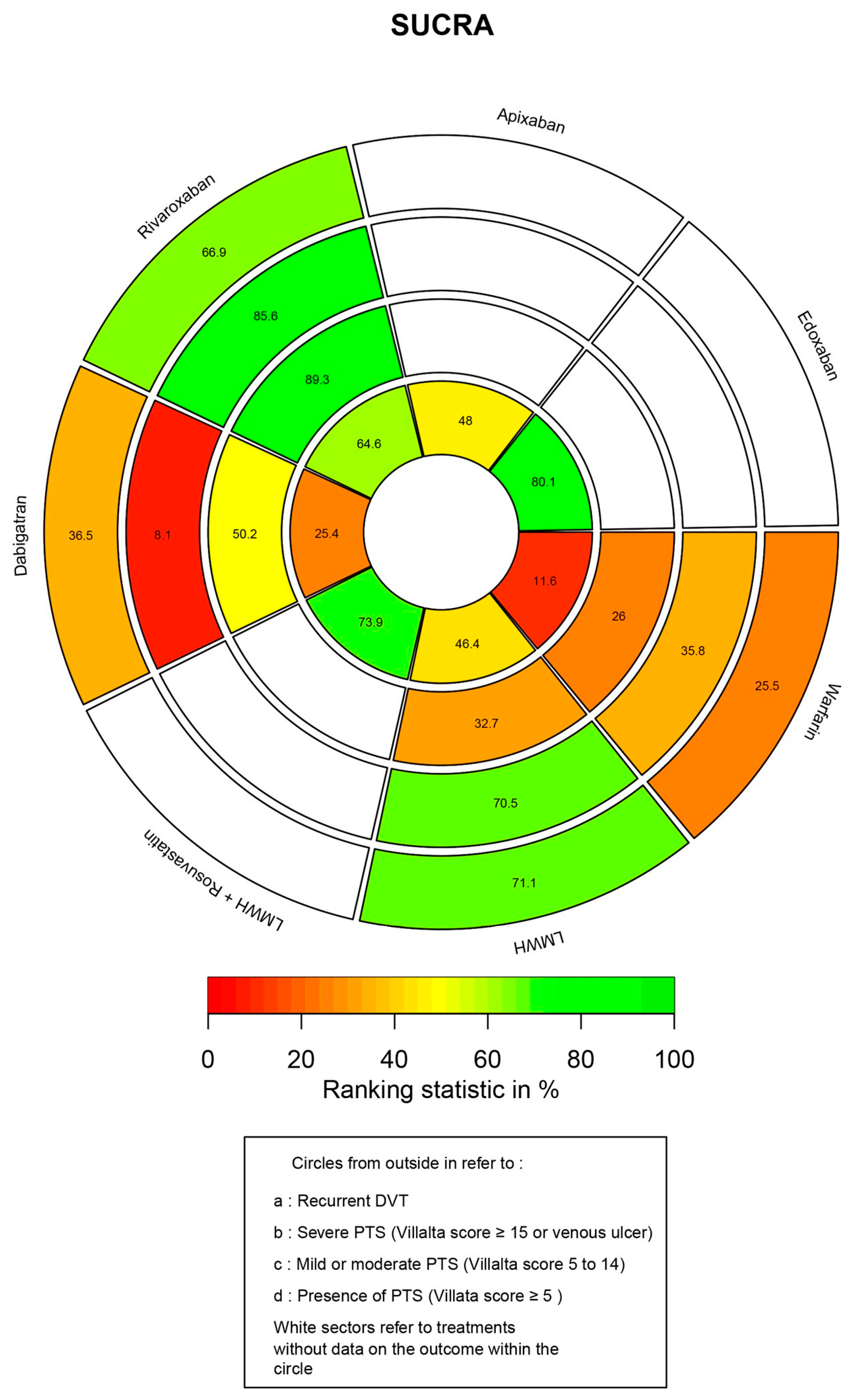
3.6. Ranking Probability and SUCRA
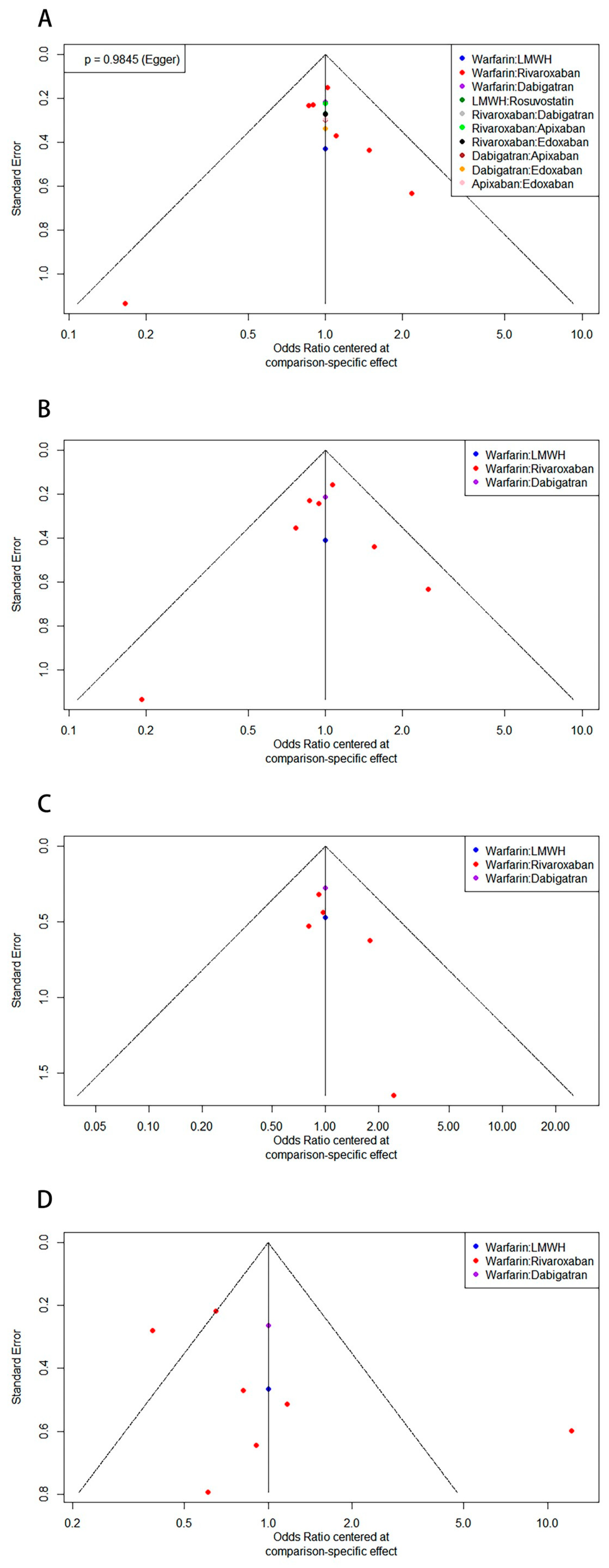
3.7. Publication Bias
3.8. Sensitivity Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Prandoni, P.; Lensing, A.W.; Cogo, A.; Cuppini, S.; Villalta, S.; Carta, M.; Cattelan, A.M.; Polistena, P.; Bernardi, E.; Prins, M.H. The long-term clinical course of acute deep venous thrombosis. Ann. Intern. Med. 1996, 125, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Kahn, S.R. The post-thrombotic syndrome. Hematol. Am. Soc. Hematol. Educ. Program 2016, 2016, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Kahn, S.R. How I treat postthrombotic syndrome. Blood J. Am. Soc. Hematol. 2009, 114, 4624–4631. [Google Scholar] [CrossRef] [PubMed]
- Prandoni, P.; Kahn, S.R. Post-thrombotic syndrome: Prevalence, prognostication and need for progress. Br. J. Haematol. 2009, 145, 286–295. [Google Scholar] [CrossRef] [PubMed]
- Grosse, S.D.; Nelson, R.E.; Nyarko, K.A.; Richardson, L.C.; Raskob, G.E. The economic burden of incident venous thromboembolism in the United States: A review of estimated attributable healthcare costs. Thromb. Res. 2016, 137, 3–10. [Google Scholar] [CrossRef]
- Takase, S.; Pascarella, L.; Lerond, L.; Bergan, J.; Schmid-Schönbein, G. Venous hypertension, inflammation and valve remodeling. Eur. J. Vasc. Endovasc. Surg. 2004, 28, 484–493. [Google Scholar] [CrossRef] [PubMed]
- Kahn, S.R. The post thrombotic syndrome. Thromb. Res. 2011, 127, S89–S92. [Google Scholar] [CrossRef]
- Kahn, S.R.; Ginsberg, J.S. The post-thrombotic syndrome: Current knowledge, controversies, and directions for future research. Blood Rev. 2002, 16, 155–165. [Google Scholar] [CrossRef]
- Schulman, S.; Lindmarker, P.; Holmström, M.; Lärfars, G.; Carlsson, A.; Nicol, P.; Svensson, E.; Ljungberg, B.; Viering, S.; Nordlander, S. Post-thrombotic syndrome, recurrence, and death 10 years after the first episode of venous thromboembolism treated with warfarin for 6 weeks or 6 months. J. Thromb. Haemost. 2006, 4, 734–742. [Google Scholar] [CrossRef]
- Rutherford, R.B.; Padberg, F.T., Jr.; Comerota, A.J.; Kistner, R.L.; Meissner, M.H.; Moneta, G.L. Venous severity scoring: An adjunct to venous outcome assessment. J. Vasc. Surg. 2000, 31, 1307–1312. [Google Scholar] [CrossRef]
- Beebe, H.G.; Bergan, J.J.; Bergqvist, D.; Eklof, B.; Eriksson, I.; Goldman, M.P.; Greenfield, L.J.; Hobson, R.W.; Juhan, C.; Kistner, R.L. Classification and grading of chronic venous disease in the lower limbs. A consensus statement. Eur. J. Vasc. Endovasc. Surg. 1996, 4, 487–492. [Google Scholar] [CrossRef]
- Widmer, L.K. Venen-, Arterien-Krankheiten, Koronare Herzkrankheit bei Berufstatigen: Prospektivepidemiologische Untersuchung. Basler Studie I–III [1959–1978]; Hogrefe AG: Bern, Switzerland, 1981. [Google Scholar]
- Brandjes, D.P.; Büller, H.R.; Heijboer, H.; Huisman, M.V.; de Rijk, M.; Jagt, H.; ten Cate, J.W. Randomised trial of effect of compression stockings in patients with symptomatic proximal-vein thrombosis. Lancet 1997, 349, 759–762. [Google Scholar] [CrossRef]
- Ginsberg, J.S.; Hirsh, J.; Julian, J.; Vander LaandeVries, M.; Magier, D.; MacKinnon, B.; Gent, M. Prevention and treatment of postphlebitic syndrome: Results of a 3-part study. Arch. Intern. Med. 2001, 161, 2105–2109. [Google Scholar] [CrossRef]
- Villalta, S.; Bagatella, P.; Piccioli, A.; Lensing, A.; Prins, M.; Prandoni, P. Assessment of validity and reproducibility of a clinical scale for the post-thrombotic syndrome. Haemostasis 1994, 24, 158a. [Google Scholar]
- Wik, H.S.; Enden, T.R.; Ghanima, W.; Engeseth, M.; Kahn, S.R.; Sandset, P.M. Diagnostic scales for the post-thrombotic syndrome. Thromb. Res. 2018, 164, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Kahn, S.R.; Partsch, H.; Vedantham, S.; Prandoni, P.; Kearon, C. Definition of post-thrombotic syndrome of the leg for use in clinical investigations: A recommendation for standardization. J. Thromb. Haemost. 2009, 7, 879–883. [Google Scholar] [CrossRef] [PubMed]
- Strijkers, R.; Wittens, C.; Kahn, S. Viilaita scale: Goals and limitations. Phlebology 2012, 27, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Notten, P.; de Smet, A.A.; Tick, L.W.; van de Poel, M.H.; Wikkeling, O.R.; Vleming, L.J.; Koster, A.; Jie, K.S.G.; Jacobs, E.M.; Ebben, H.P. CAVA (ultrasound-accelerated catheter-directed thrombolysis on preventing post-thrombotic syndrome) trial: Long-term follow-up results. J. Am. Heart Assoc. 2021, 10, e018973. [Google Scholar] [CrossRef] [PubMed]
- Haig, Y.; Enden, T.; Grøtta, O.; Kløw, N.-E.; Slagsvold, C.-E.; Ghanima, W.; Sandvik, L.; Hafsahl, G.; Holme, P.A.; Holmen, L.O. Post-thrombotic syndrome after catheter-directed thrombolysis for deep vein thrombosis (CaVenT): 5-year follow-up results of an open-label, randomised controlled trial. Lancet Haematol. 2016, 3, e64–e71. [Google Scholar] [CrossRef]
- Vedantham, S.; Goldhaber, S.Z.; Julian, J.A.; Kahn, S.R.; Jaff, M.R.; Cohen, D.J.; Magnuson, E.; Razavi, M.K.; Comerota, A.J.; Gornik, H.L. Pharmacomechanical catheter-directed thrombolysis for deep-vein thrombosis. N. Engl. J. Med. 2017, 377, 2240–2252. [Google Scholar] [CrossRef]
- Kakkos, S.K.; Gohel, M.; Baekgaard, N.; Bauersachs, R.; Bellmunt-Montoya, S.; Black, S.A.; ten Cate-Hoek, A.J.; Elalamy, I.; Enzmann, F.K.; Geroulakos, G. Editor’s Choice—European Society for Vascular Surgery (ESVS) 2021 clinical practice guidelines on the management of venous thrombosis. Eur. J. Vasc. Endovasc. Surg. 2021, 61, 9–82. [Google Scholar] [CrossRef] [PubMed]
- Kahn, S.R.; Comerota, A.J.; Cushman, M.; Evans, N.S.; Ginsberg, J.S.; Goldenberg, N.A.; Gupta, D.K.; Prandoni, P.; Vedantham, S.; Walsh, M.E. The postthrombotic syndrome: Evidence-based prevention, diagnosis, and treatment strategies: A scientific statement from the American Heart Association. Circulation 2014, 130, 1636–1661. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; Moher, D. Evaluations of the uptake and impact of the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) Statement and extensions: A scoping review. Syst. Rev. 2017, 6, 263. [Google Scholar] [CrossRef]
- Harrer, M.; Cuijpers, P.; Furukawa, T.A.; Ebert, D.D. Doing Meta-Analysis with R: A Hands-On Guide; CRC Press: Boca Raton, FL, USA, 2021. [Google Scholar]
- Veroniki, A.A.; Straus, S.E.; Fyraridis, A.; Tricco, A.C. The rank-heat plot is a novel way to present the results from a network meta-analysis including multiple outcomes. J. Clin. Epidemiol. 2016, 76, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Dias, S.; Welton, N.J.; Caldwell, D.M.; Ades, A.E. Checking consistency in mixed treatment comparison meta-analysis. Stat. Med. 2010, 29, 932–944. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, T.; Huber, S.C.; de Moraes Martinelli, B.; Junior, A.L.; Menezes, F.H.; Orsi, F.A.; Bittar, L.F.; de Oliveira, L.F.G.; Sodre, L.R.; Mello, T.T. Low prevalence of Post-thrombotic syndrome in patients treated with rivaroxaban. Vasc. Pharmacol. 2020, 124, 106608. [Google Scholar] [CrossRef]
- González-Fajardo, J.A.; Martin-Pedrosa, M.; Castrodeza, J.; Tamames, S.; Vaquero-Puerta, C. Effect of the anticoagulant therapy in the incidence of post-thrombotic syndrome and recurrent thromboembolism: Comparative study of enoxaparin versus coumarin. J. Vasc. Surg. 2008, 48, 953–959.e952. [Google Scholar] [CrossRef]
- Utne, K.; Dahm, A.; Wik, H.; Jelsness-Jørgensen, L.; Sandset, P.; Ghanima, W. Rivaroxaban versus warfarin for the prevention of post-thrombotic syndrome. Thromb. Res. 2018, 163, 6–11. [Google Scholar] [CrossRef]
- Jeraj, L.; Jezovnik, M.; Poredos, P. Rivaroxaban versus warfarin in the prevention of post-thrombotic syndrome. Thromb. Res. 2017, 157, 46–48. [Google Scholar] [CrossRef]
- Cheung, Y.W.; Middeldorp, S.; Prins, M.H.; Pap, A.F.; Lensing, A.W.; ten Cate-Hoek, A.J.; Villalta, S.; Milan, M.; Beyer-Westendorf, J.; Verhamme, P. Post-thrombotic syndrome in patients treated with rivaroxaban or enoxaparin/vitamin K antagonists for acute deep-vein thrombosis. Thromb. Haemost. 2016, 116, 733–738. [Google Scholar]
- Prandoni, P.; Ageno, W.; Ciammaichella, M.; Mumoli, N.; Zanatta, N.; Imberti, D.; Visonà, A.; Bucherini, E.; Di Nisio, M.; Noventa, F. The risk of post-thrombotic syndrome in patients with proximal deep vein thrombosis treated with the direct oral anticoagulants. Intern. Emerg. Med. 2020, 15, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Sebastian, T.; Hakki, L.O.; Spirk, D.; Baumann, F.A.; Périard, D.; Banyai, M.; Spescha, R.S.; Kucher, N.; Engelberger, R.P. Rivaroxaban or vitamin-K antagonists following early endovascular thrombus removal and stent placement for acute iliofemoral deep vein thrombosis. Thromb. Res. 2018, 172, 86–93. [Google Scholar] [CrossRef]
- San Norberto, E.M.; Gastambide, M.V.; Taylor, J.H.; García-Saiz, I.; Vaquero, C. Effects of rosuvastatin as an adjuvant treatment for deep vein thrombosis. Vasa 2016, 45, 133–140. [Google Scholar] [CrossRef]
- De Athayde Soares, R.; Matielo, M.F.; Neto, F.C.B.; Nogueira, M.P.; Almeida, R.D.; Sacilotto, R. Comparison of the recanalization rate and postthrombotic syndrome in patients with deep venous thrombosis treated with rivaroxaban or warfarin. Surgery 2019, 166, 1076–1083. [Google Scholar] [CrossRef] [PubMed]
- Spiezia, L.; Campello, E.; Simion, C.; Poretto, A.; Dalla Valle, F.; Simioni, P. Risk factors for post-thrombotic syndrome in patients with a first proximal deep venous thrombosis treated with direct oral anticoagulants. Angiology 2022, 73, 649–654. [Google Scholar] [CrossRef]
- Wik, H.S.; Kahn, S.R.; Eriksson, H.; Morrison, D.; Ghanima, W.; Schulman, S.; Sandset, P.M. Post-thrombotic syndrome in patients with venous thromboembolism treated with dabigatran or warfarin: A long-term cross-sectional follow-up of RE-COVER study patients. J. Thromb. Haemost. 2021, 19, 2495–2503. [Google Scholar] [CrossRef] [PubMed]
- Kahn, S.R.; Ginsberg, J.S. Relationship between deep venous thrombosis and the postthrombotic syndrome. Arch. Intern. Med. 2004, 164, 17–26. [Google Scholar] [CrossRef]
- Henke, P.K.; Comerota, A.J. An update on etiology, prevention, and therapy of postthrombotic syndrome. J. Vasc. Surg. 2011, 53, 500–509. [Google Scholar] [CrossRef]
- Haig, Y.; Enden, T.; Slagsvold, C.-E.; Sandvik, L.; Sandset, P.M.; Kløw, N.E. Residual rates of reflux and obstruction and their correlation to post-thrombotic syndrome in a randomized study on catheter-directed thrombolysis for deep vein thrombosis. J. Vasc. Surg. Venous Lymphat. Disord. 2014, 2, 123–130. [Google Scholar] [CrossRef]
- Rabinovich, A.; Kahn, S. The postthrombotic syndrome: Current evidence and future challenges. J. Thromb. Haemost. 2017, 15, 230–241. [Google Scholar] [CrossRef]
- Enden, T.; Haig, Y.; Kløw, N.-E.; Slagsvold, C.-E.; Sandvik, L.; Ghanima, W.; Hafsahl, G.; Holme, P.A.; Holmen, L.O.; Njaastad, A.M. Long-term outcome after additional catheter-directed thrombolysis versus standard treatment for acute iliofemoral deep vein thrombosis (the CaVenT study): A randomised controlled trial. Lancet 2012, 379, 31–38. [Google Scholar] [CrossRef]
- Notten, P.; ten Cate-Hoek, A.J.; Arnoldussen, C.W.; Strijkers, R.H.; de Smet, A.A.; Tick, L.W.; van de Poel, M.H.; Wikkeling, O.R.; Vleming, L.-J.; Koster, A. Ultrasound-accelerated catheter-directed thrombolysis versus anticoagulation for the prevention of post-thrombotic syndrome (CAVA): A single-blind, multicentre, randomised trial. Lancet Haematol. 2020, 7, e40–e49. [Google Scholar] [CrossRef]
- Galanaud, J.-P.; Monreal, M.; Kahn, S.R. Epidemiology of the post-thrombotic syndrome. Thromb. Res. 2018, 164, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Rabinovich, A.; Cohen, J.; Cushman, M.; Wells, P.; Rodger, M.; Kovacs, M.; Anderson, D.; Tagalakis, V.; Lazo-Langner, A.; Solymoss, S. Inflammation markers and their trajectories after deep vein thrombosis in relation to risk of post-thrombotic syndrome. J. Thromb. Haemost. 2015, 13, 398–408. [Google Scholar] [CrossRef] [PubMed]
- Bittar, L.F.; de Moraes Mazetto, B.; Orsi, F.L.A.; Collela, M.P.; De Paula, E.V.; Annichino-Bizzacchi, J.M. Long-term increased factor VIII levels are associated to interleukin-6 levels but not to post-thrombotic syndrome in patients with deep venous thrombosis. Thromb. Res. 2015, 135, 497–501. [Google Scholar] [CrossRef] [PubMed]
- Roumen-Klappe, E.; Janssen, M.; van Rossum, J.; Holewijn, S.; Van Bokhoven, M.; Kaasjager, K.; Wollersheim, H.; den Heijer, M. Inflammation in deep vein thrombosis and the development of post-thrombotic syndrome: A prospective study. J. Thromb. Haemost. 2009, 7, 582–587. [Google Scholar] [CrossRef]
- Jezovnik, M.; Poredos, P. Factors influencing the recanalisation rate of deep venous thrombosis. Int. Angiol. J. Int. Union Angiol. 2012, 31, 169–175. [Google Scholar]
- Purdy, M.; Obi, A.; Myers, D.; Wakefield, T. P-and E-selectin in venous thrombosis and non-venous pathologies. J. Thromb. Haemost. 2022, 20, 1056–1066. [Google Scholar] [CrossRef] [PubMed]
- Aleksandrov, A.P.; Mirkov, I.; Zolotarevski, L.; Ninkov, M.; Mileusnic, D.; Kataranovski, D.; Kataranovski, M. Oral warfarin intake affects skin inflammatory cytokine responses in rats. Environ. Toxicol. Pharmacol. 2017, 54, 93–98. [Google Scholar] [CrossRef]
- Makedonov, I.; Kahn, S.R.; Abdulrehman, J.; Schulman, S.; Delluc, A.; Gross, P.; Galanaud, J.-P. Prevention of the postthrombotic syndrome with anticoagulation: A narrative review. Thromb. Haemost. 2022, 122, 1255–1264. [Google Scholar] [CrossRef]
- Hull, R.D.; Pineo, G.F.; Brant, R.; Liang, J.; Cook, R.; Solymoss, S.; Poon, M.-C.; Raskob, G.; Investigators, L.T. Home therapy of venous thrombosis with long-term LMWH versus usual care: Patient satisfaction and post-thrombotic syndrome. Am. J. Med. 2009, 122, 762–769.e763. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Cohen, A.; Edmondson, R.; Melissari, E.; Kakkar, V. Low-molecular-weight heparin versus warfarin for prevention of recurrent venous thromboembolism: A randomized trial. World J. Surg. 1996, 20, 521–527. [Google Scholar] [CrossRef]
- Gonzalez-Fajardo, J.A.; Arreba, E.; Castrodeza, J.; Perez, J.L.; Fernandez, L.; Agundez, I.; Mateo, A.M.; Carrera, S.; Gutiérrez, V.; Vaquero, C. Venographic comparison of subcutaneous low–molecular weight heparin with oral anticoagulant therapy in the long-term treatment of deep venous thrombosis. J. Vasc. Surg. 1999, 30, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Hull, R.D.; Liang, J.; Townshend, G. Long-term low-molecular-weight heparin and the post-thrombotic syndrome: A systematic review. Am. J. Med. 2011, 124, 756–765. [Google Scholar] [CrossRef]
- Kessinger, C.W.; Qi, G.; Hassan, M.Z.; Henke, P.K.; Tawakol, A.; Jaffer, F.A. FDG-PET/CT imaging predicts vein wall scarring and statin benefit in murine venous thrombosis. Circulation Cardiovasc. Imaging 2021, 14, e011898. [Google Scholar] [CrossRef] [PubMed]
- Glynn, R.J.; Danielson, E.; Fonseca, F.A.; Genest, J.; Gotto Jr, A.M.; Kastelein, J.J.; Koenig, W.; Libby, P.; Lorenzatti, A.J.; MacFadyen, J.G. A randomized trial of rosuvastatin in the prevention of venous thromboembolism. N. Engl. J. Med. 2009, 360, 1851–1861. [Google Scholar] [CrossRef] [PubMed]
- Khemasuwan, D.; DiVietro, M.L.; Tangdhanakanond, K.; Pomerantz, S.C.; Eiger, G. Statins decrease the occurrence of venous thromboembolism in patients with cancer. Am. J. Med. 2010, 123, 60–65. [Google Scholar] [CrossRef]
- Ridker, P.M.; Danielson, E.; Fonseca, F.A.; Genest, J.; Gotto Jr, A.M.; Kastelein, J.J.; Koenig, W.; Libby, P.; Lorenzatti, A.J.; MacFadyen, J.G. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N. Engl. J. Med. 2008, 359, 2195–2207. [Google Scholar] [CrossRef]
- Delluc, A.; Ghanima, W.; Kovacs, M.J.; Shivakumar, S.; Kahn, S.R.; Sandset, P.M.; Kearon, C.; Mallick, R.; Rodger, M.A. Prevention of post-thrombotic syndrome with rosuvastatin: A multicenter randomized controlled pilot trial (SAVER). Thromb. Res. 2022, 213, 119–124. [Google Scholar] [CrossRef]
- Van Es, N.; Coppens, M.; Schulman, S.; Middeldorp, S.; Büller, H.R. Direct oral anticoagulants compared with vitamin K antagonists for acute venous thromboembolism: Evidence from phase 3 trials. Blood J. Am. Soc. Hematol. 2014, 124, 1968–1975. [Google Scholar] [CrossRef]
- Schulman, S.; Kakkar, A.K.; Goldhaber, S.Z.; Schellong, S.; Eriksson, H.; Mismetti, P.; Christiansen, A.V.; Friedman, J.; Le Maulf, F.; Peter, N. Treatment of acute venous thromboembolism with dabigatran or warfarin and pooled analysis. Circulation 2014, 129, 764–772. [Google Scholar] [CrossRef] [PubMed]
- Schulman, S.; Kearon, C.; Kakkar, A.K.; Mismetti, P.; Schellong, S.; Eriksson, H.; Baanstra, D.; Schnee, J.; Goldhaber, S.Z. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N. Engl. J. Med. 2009, 361, 2342–2352. [Google Scholar] [CrossRef] [PubMed]
- Raskob, G.E.; van Es, N.; Verhamme, P.; Carrier, M.; Di Nisio, M.; Garcia, D.; Grosso, M.A.; Kakkar, A.K.; Kovacs, M.J.; Mercuri, M.F. Edoxaban for the treatment of cancer-associated venous thromboembolism. N. Engl. J. Med. 2018, 378, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Marston, X.; Wang, R.; Yeh, Y.; Zimmermann, L.; Ye, X.; Gao, X.; Unverdorben, M. Comparison of clinical outcomes with edoxaban versus apixaban, dabigatran, rivaroxaban, and vitamin K antagonist in patients with atrial fibrillation in Germany: A real-world cohort study. Eur. Heart J. 2020, 41, ehaa946.0401. [Google Scholar] [CrossRef]
- Lee, S.-R.; Choi, E.-K.; Han, K.-D.; Jung, J.-H.; Oh, S.; Lip, G.Y. Comparison of once-daily administration of edoxaban and rivaroxaban in Asian patients with atrial fibrillation. Sci. Rep. 2019, 9, 6690. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shao, J.; Zhou, Q.; Jin, F.; Reissfelder, C.; Sigl, M.; Yagublu, V.; Keese, M. A Systematic Review and Bayesian Network Meta-Analysis on the Effect of Different Anticoagulants on the Prophylaxis of Post-Thrombotic Syndrome after Deep Venous Thrombosis. J. Clin. Med. 2023, 12, 7450. https://doi.org/10.3390/jcm12237450
Shao J, Zhou Q, Jin F, Reissfelder C, Sigl M, Yagublu V, Keese M. A Systematic Review and Bayesian Network Meta-Analysis on the Effect of Different Anticoagulants on the Prophylaxis of Post-Thrombotic Syndrome after Deep Venous Thrombosis. Journal of Clinical Medicine. 2023; 12(23):7450. https://doi.org/10.3390/jcm12237450
Chicago/Turabian StyleShao, Jingbo, Qianwen Zhou, Fukang Jin, Christoph Reissfelder, Martin Sigl, Vugar Yagublu, and Michael Keese. 2023. "A Systematic Review and Bayesian Network Meta-Analysis on the Effect of Different Anticoagulants on the Prophylaxis of Post-Thrombotic Syndrome after Deep Venous Thrombosis" Journal of Clinical Medicine 12, no. 23: 7450. https://doi.org/10.3390/jcm12237450
APA StyleShao, J., Zhou, Q., Jin, F., Reissfelder, C., Sigl, M., Yagublu, V., & Keese, M. (2023). A Systematic Review and Bayesian Network Meta-Analysis on the Effect of Different Anticoagulants on the Prophylaxis of Post-Thrombotic Syndrome after Deep Venous Thrombosis. Journal of Clinical Medicine, 12(23), 7450. https://doi.org/10.3390/jcm12237450




