A Comprehensive Review of the Diagnosis and Management of Acute Liver Failure
Abstract
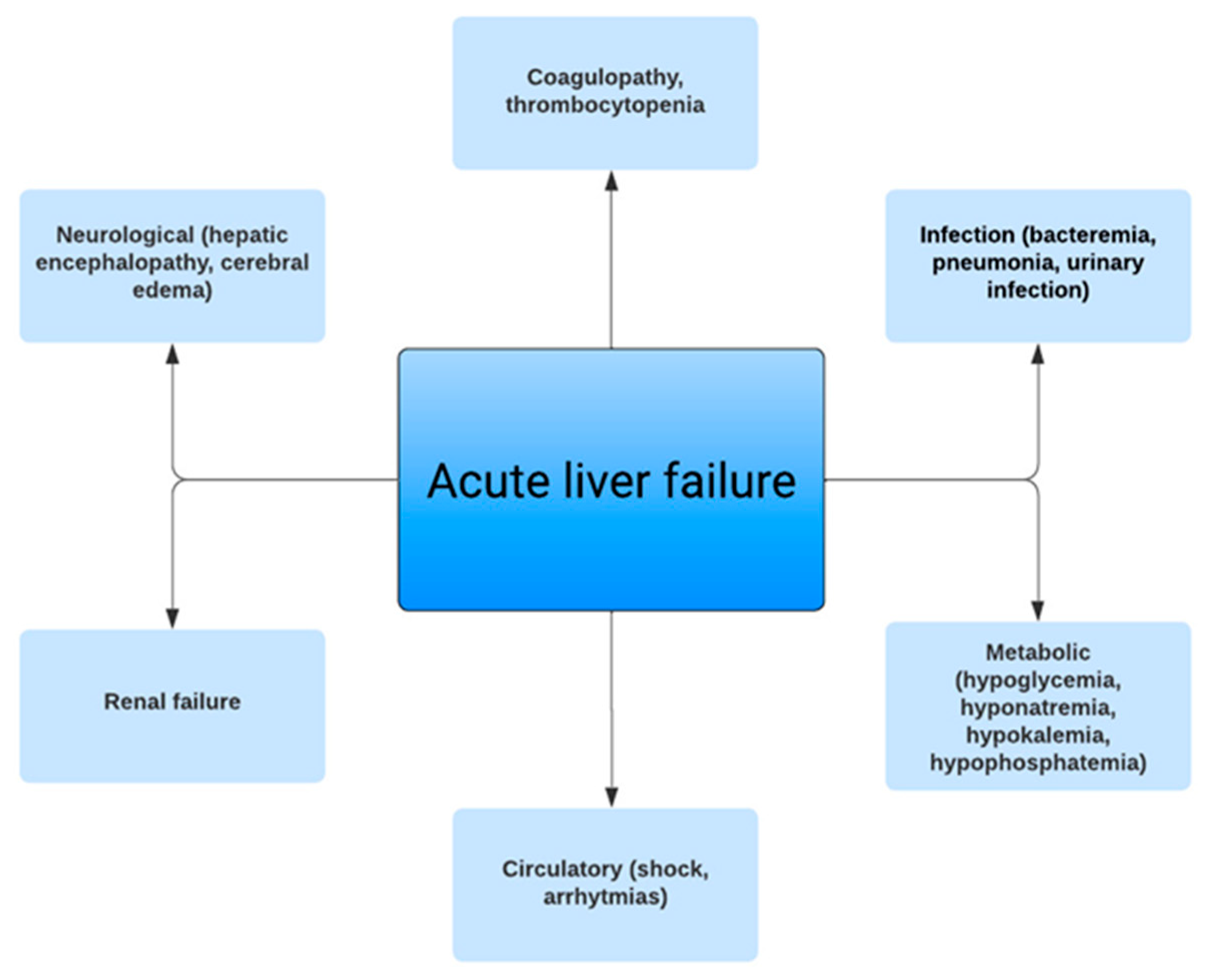
:1. Introduction
2. Causes of Acute Liver Failure
3. Clinical Manifestations
4. Diagnosis
5. Imaging Findings
6. Management of Acute Liver Failure and the Role of Prognostic Scores
7. Role of Liver Transplantation
8. Outcomes
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, W.M.; Stravitz, T.R.; Larson, A.M. Introduction to the revised American Association for the Study of Liver Diseases position paper on acute liver failure 2011. Hepatology 2012, 55, 965–967. [Google Scholar] [CrossRef] [PubMed]
- Shingina, A.; Mukhtar, N.; Wakim-Fleming, J.; Alqahtani, S.; Wong, R.J.; Limketkai, B.N.; Larson, A.M.; Grant, L. Acute Liver Failure Guidelines. Am. J. Gastroenterol. 2023, 118, 1128–1153. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver; Clinical Practice Guidelines Panel; Wendon, J.; Cordoba, J.; Dhawan, A.; Larsen, F.S.; Manns, M.; Nevens, F.; Samuel, D.; Simpson, K.J.; et al. EASL Clinical Practical Guidelines on the management of acute (fulminant) liver failure. J. Hepatol. 2017, 66, 1047–1081. [Google Scholar] [PubMed]
- Stravitz, R.T.; Lee, W.M. Acute liver failure. Lancet 2019, 394, 869–881. [Google Scholar]
- Bernal, W.; Wendon, J. Acute Liver Failure. N. Engl. J. Med. 2013, 369, 2525–2534. [Google Scholar] [CrossRef]
- Bernal, W.; Hyyrylainen, A.; Gera, A.; Audimoolam, V.K.; McPhail, M.J.; Auzinger, G.; Rela, M.; Heaton, N.; O’Grady, J.G.; Wendon, J.; et al. Lessons from look-back in acute liver failure? A single centre experience of 3300 patients. J. Hepatol. 2013, 59, 74–80. [Google Scholar] [CrossRef]
- Germani, G.; Theocharidou, E.; Adam, R.; Karam, V.; Wendon, J.; O’Grady, J.; Burra, P.; Senzolo, M.; Mirza, D.; Castaing, D.; et al. Liver transplantation for acute liver failure in Europe: Outcomes over 20 years from the ELTR database. J. Hepatol. 2012, 57, 288–296. [Google Scholar] [CrossRef]
- Anand, A.C.; Nandi, B.; Acharya, S.K.; Arora, A.; Babu, S.; Batra, Y.; Chawla, Y.K.; Chowdhury, A.; Chaoudhuri, A.; Eapen, E.C.; et al. Indian National Association for the Study of the Liver Consensus Statement on Acute Liver Failure (Part 1): Epidemiology, Pathogenesis, Presentation and Prognosis. J. Clin. Exp. Hepatol. 2020, 10, 339–376. [Google Scholar]
- Acharya, S.K. Acute Liver Failure: Indian Perspective. Clin. Liver Dis. 2021, 18, 143–149. [Google Scholar]
- Shen, T.; Liu, Y.; Shang, J.; Xie, Q.; Li, J.; Yan, M.; Xu, J.; Niu, J.; Liu, J.; Watkins, P.B.; et al. Incidence and Etiology of Drug-Induced Liver Injury in Mainland China. Gastroenterology 2019, 156, 2230–2241.e11. [Google Scholar] [CrossRef]
- Dong, V.; Nanchal, R.; Karvellas, C.J. Pathophysiology of Acute Liver Failure. Nutr. Clin. Pr. 2020, 35, 24–29. [Google Scholar] [CrossRef]
- Andrade, R.J.; Lucena, M.I.; Fernández, M.C.; Pelaez, G.; Pachkoria, K.; García-Ruiz, E.; García-Muñoz, B.; González-Grande, R.; Pizarro, A.; Durán, J.A.; et al. Drug-Induced Liver Injury: An Analysis of 461 Incidences Submitted to the Spanish Registry Over a 10-Year Period. Gastroenterology 2005, 129, 512–521. [Google Scholar] [CrossRef] [PubMed]
- Fontana, R.J.; Bjornsson, E.S.; Reddy, R.; Andrade, R.J. The Evolving Profile of Idiosyncratic Drug-Induced Liver Injury. Clin. Gastroenterol. Hepatol. 2023, 21, 2088–2099. [Google Scholar] [CrossRef] [PubMed]
- Santi, L.; Maggioli, C.; Mastroroberto, M.; Tufoni, M.; Napoli, L.; Caraceni, P. Acute Liver Failure Caused byAmanita phalloidesPoisoning. Int. J. Hepatol. 2012, 2012, e487480. [Google Scholar] [CrossRef] [PubMed]
- Enke, T.; Livingston, S.; Rule, J.; Stravitz, T.; Rakela, J.; Bass, N.; Reuben, A.; Tujios, S.; Larson, A.; Sussman, N.; et al. Autoimmune hepatitis presenting as acute liver failure: A 20-year retrospective review of North America. Liver Transplant. 2023, 29, 570–580. [Google Scholar] [CrossRef] [PubMed]
- Casey, L.C.; Fontana, R.J.; Aday, A.; Nelson, D.B.; Rule, J.A.; Gottfried, M.; Tran, M.; Lee, W.M.; Acute Liver Failure Study Group. Acute Liver Failure (ALF) in Pregnancy: How Much Is Pregnancy Related? Hepatology 2020, 72, 1366–1377. [Google Scholar] [CrossRef] [PubMed]
- Parekh, J.; Matei, V.M.; Canas-Coto, A.; Friedman, D.; Lee, W.M. The Acute Liver Failure Study Group Budd-chiari syndrome causing acute liver failure: A multicenter case series. Liver Transplant. 2017, 23, 135–142. [Google Scholar] [CrossRef]
- Lim, K.B.L.; Schiano, T.D. Still Disease and the Liver—An Underappreciated Association. Gastroenterol Hepatol. 2011, 7, 844–846. [Google Scholar]
- Nanchal, R.; Subramanian, R.; Karvellas, C.J.; Hollenberg, S.M.; Peppard, W.J.; Singbartl, K.; Truwit, J.; Al-Khafaji, A.H.; Killian, A.J.; Alquraini, M.; et al. Guidelines for the Management of Adult Acute and Acute-on-Chronic Liver Failure in the ICU: Cardiovascular, Endocrine, Hematologic, Pulmonary and Renal Considerations: Executive Summary. Crit. Care Med. 2020, 48, 415. [Google Scholar] [CrossRef]
- Fontana, R.J. Acute Liver Failure Including Acetaminophen Overdose. Med. Clin. North Am. 2008, 92, 761–794. [Google Scholar] [CrossRef]
- Flamm, S.L.; Wong, F.; Ahn, J.; Kamath, P.S. AGA Clinical Practice Update on the Evaluation and Management of Acute Kidney Injury in Patients With Cirrhosis: Expert Review. Clin. Gastroenterol. Hepatol. 2022, 20, 2707–2716. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.M.; Hynan, L.S.; Rossaro, L.; Fontana, R.J.; Stravitz, R.T.; Larson, A.M.; Davern, T.; Schilsky, M.; McCashland, T.; Hay, J.E.; et al. Intravenous N-Acetylcysteine Improves Transplant-Free Survival in Early Stage Non-Acetaminophen Acute Liver Failure. Gastroenterology 2009, 137, 856–864.e1. [Google Scholar] [CrossRef] [PubMed]
- Walayat, S.; Shoaib, H.; Asghar, M.N.; Kim, M.; Dhillon, S. Role of N-acetylcysteine in non-acetaminophen-related acute liver failure: An updated meta-analysis and systematic review. Ann. Gastroenterol. 2021, 34, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Sanabria-Cabrera, J.; Tabbai, S.; Niu, H.; Alvarez-Alvarez, I.; Licata, A.; Björnsson, E.; Andrade, R.J.; Lucena, M.I. N-Acetylcysteine for the Management of Non-Acetaminophen Drug-Induced Liver Injury in Adults: A Systematic Review. Front. Pharmacol. 2022, 13, 876868. [Google Scholar] [CrossRef] [PubMed]
- Chayanupatkul, M.; Schiano, T.D. Acute Liver Failure Secondary to Drug-Induced Liver Injury. Clin. Liver Dis. 2020, 24, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Niu, H.; Sanabria-Cabrera, J.; Alvarez-Alvarez, I.; Robles-Diaz, M.; Stankevičiūtė, S.; Aithal, G.P.; Björnsson, E.S.; Andrade, R.J.; Lucena, M.I. Prevention and management of idiosyncratic drug-induced liver injury: Systematic review and meta-analysis of randomised clinical trials. Pharmacol. Res. 2021, 164, 105404. [Google Scholar] [CrossRef] [PubMed]
- Lisman, T.; Bakhtiari, K.; Adelmeijer, J.; Meijers, J.C.M.; Porte, R.J.; Stravitz, R.T. Intact thrombin generation and decreased fibrinolytic capacity in patients with acute liver injury or acute liver failure. J. Thromb. Haemost. 2012, 10, 1312–1319. [Google Scholar] [CrossRef]
- Stravitz, R.T.; Lisman, T. Rebalanced Hemostasis in Patients with Acute Liver Failure. Semin. Thromb. Hemost. 2015, 41, 468–473. [Google Scholar] [CrossRef]
- Stravitz, R.T.; Ellerbe, C.; Durkalski, V.; Schilsky, M.; Fontana, R.J.; Peterseim, C.; Lee, W.M.; the Acute Liver Failure Study Group. Bleeding complications in acute liver failure. Hepatology 2018, 67, 1931–1942. [Google Scholar] [CrossRef]
- Pan, J.J.; Fontana, R.J. CAQ Corner: Acute liver failure management and liver transplantation. Liver Transplant. 2022, 28, 1664–1673. [Google Scholar] [CrossRef]
- Torres, D.M.; Stevens, R.D.; Gurakar, A. Acute liver failure: A management challenge for the practicing gastroenterologist. Gastroenterol. Hepatol. 2010, 6, 444–450. [Google Scholar]
- Schaden, E.; Saner, F.H.; Goerlinger, K. Coagulation pattern in critical liver dysfunction. Curr. Opin. Crit. Care 2013, 19, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Saner, F.H.; Hoyer, D.P.; Hartmann, M.; Nowak, K.M.; Bezinover, D. The Edge of Unknown: Postoperative Critical Care in Liver Transplantation. J. Clin. Med. 2022, 11, 4036. [Google Scholar] [CrossRef] [PubMed]
- Saner, F.H.; Stueben, B.-O.; Hoyer, D.P.; Broering, D.C.; Bezinover, D. Use or Misuse of Albumin in Critical Ill Patients. Diseases 2023, 11, 68. [Google Scholar] [CrossRef] [PubMed]
- Abuelkasem, E.; Hasan, S.; Mazzeffi, M.A.; Planinsic, R.M.; Sakai, T.; Tanaka, K.A. Reduced Requirement for Prothrombin Complex Concentrate for the Restoration of Thrombin Generation in Plasma From Liver Transplant Recipients. Obstet. Anesthesia Dig. 2017, 125, 609–615. [Google Scholar] [CrossRef] [PubMed]
- Görlinger, K.; Shore-Lesserson, L.; Dirkmann, D.; Hanke, A.A.; Rahe-Meyer, N.; Tanaka, K.A. Management of hemorrhage in cardiothoracic surgery. J. Cardiothorac. Vasc. Anesth. 2013, 27 (Suppl. S4), S20–S34. [Google Scholar] [CrossRef] [PubMed]
- Karvellas, C.J.; Fix, O.K.; Battenhouse, H.; Durkalski, V.; Sanders, C.; Lee, W.M. Outcomes and complications of intracranial pressure monitoring in acute liver failure: A retrospective cohort study. Crit. Care Med. 2014, 42, 1157–1167. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.M.; Larson, A.M.; Stravitz, R.T. AASLD Position Paper: The Management of Acute Liver Failure: Update 2011. Hepatology 2011, 55, 965–967. [Google Scholar] [CrossRef]
- Kok, B.; Karvellas, C.J. Management of Cerebral Edema in Acute Liver Failure. Semin. Respir. Crit. Care Med. 2017, 38, 821–829. [Google Scholar] [CrossRef]
- Tujios, S.R.; Hynan, L.S.; Vazquez, M.A.; Larson, A.M.; Seremba, E.; Sanders, C.M.; Lee, W.M.; Acute Liver Failure Study Group. Risk factors and outcomes of acute kidney injury in patients with acute liver failure. Clin. Gastroenterol. Hepatol. 2015, 13, 352–359. [Google Scholar] [CrossRef]
- Stravitz, R.T.; Fontana, R.J.; Karvellas, C.; Durkalski, V.; McGuire, B.; Rule, J.A.; Tujios, S.; Lee, W.M.; for the Acute Liver Failure Study Group. Future directions in acute liver failure. Hepatology 2023, 78, 1266–1289. [Google Scholar] [CrossRef] [PubMed]
- Niranjan-Azadi, A.M.; Araz, F.; Patel, Y.A.; Alachkar, N.; Alqahtani, S.; Cameron, A.M.; Stevens, R.D.; Gurakar, A. Ammonia Level and Mortality in Acute Liver Failure: A Single-Center Experience. Ann. Transplant. 2016, 21, 479–483. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, F.S.; Gottfried, M.; Tujios, S.; Olson, J.C.; Karvellas, C.J. For the US Acute Liver Failure Study Group Continuous renal replacement therapy is associated with reduced serum ammonia levels and mortality in acute liver failure. Hepatology 2018, 67, 711–720. [Google Scholar] [CrossRef] [PubMed]
- Karvellas, C.J.; Cavazos, J.; Battenhouse, H.; Durkalski, V.; Balko, J.; Sanders, C.; Lee, W.M. Effects of Antimicrobial Prophylaxis and Blood Stream Infections in Patients With Acute Liver Failure: A Retrospective Cohort Study. Clin. Gastroenterol. Hepatol. 2014, 12, 1942–1949.e1. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Anand, U.; Priyadarshi, R.N. Liver transplantation in acute liver failure: Dilemmas and challenges. World J. Transplant. 2021, 11, 187–202. [Google Scholar] [CrossRef]
- O’Grady, J.G.; Alexander, G.J.; Hayllar, K.M.; Williams, R. Early indicators of prognosis in fulminant hepatic failure. Gastroenterology 1989, 97, 439–445. [Google Scholar] [CrossRef]
- Bernal, W.; Donaldson, N.; Wyncoll, D.; Wendon, J. Blood lactate as an early predictor of outcome in paracetamol-induced acute liver failure: A cohort study. Lancet 2002, 359, 558–563. [Google Scholar] [CrossRef]
- McPhail, M.J.W.; Farne, H.; Senvar, N.; Wendon, J.A.; Bernal, W. Ability of King’s College Criteria and Model for End-Stage Liver Disease Scores to Predict Mortality of Patients With Acute Liver Failure: A Meta-analysis. Clin. Gastroenterol. Hepatol. 2016, 14, 516–525.e5. [Google Scholar] [CrossRef]
- Bernuau, J.; Goudeau, A.; Poynard, T.; Dubois, F.; Lesage, G.; Yvonnet, B.; Degott, C.; Bezeaud, A.; Rueff, B.; Benhamou, J.-P. Multivariate analysis of prognostic factors in fulminant hepatitis B. Hepatology 1986, 6, 648–651. [Google Scholar] [CrossRef]
- Reddy, K.R.; Ellerbe, C.; Schilsky, M.; Stravitz, R.T.; Fontana, R.J.; Durkalski, V.; Lee, W.M.; The Acute Liver Failure Study Group. Determinants of outcome among patients with acute liver failure listed for liver transplantation in the United States. Liver Transplant. 2016, 22, 505–515. [Google Scholar] [CrossRef]
- Rifaie, N.; Saner, F.H. Critical care management in patients with acute liver failure. Best Pr. Res. Clin. Anaesthesiol. 2020, 34, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Wong, N.Z.; Schaubel, D.E.; Reddy, K.R.; Bittermann, T. Transplant center experience influences spontaneous survival and waitlist mortality in acute liver failure: An analysis of the UNOS database. Am. J. Transplant. 2021, 21, 1092–1099. [Google Scholar] [CrossRef] [PubMed]
- Akamatsu, N.; Sugawara, Y.; Kokudo, N. Acute liver failure and liver transplantation. Intractable Rare Dis. Res. 2013, 2, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Gurakar, A.; Ozturk, N.B.; Muhammad, H.; Gurakar, M.; Aslan, A.; Dao, D. Liver Transplantation in Developing Countries. Hepatol. Forum 2022, 3, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Mendizabal, M.; Silva, M.O. Liver transplantation in acute liver failure: A challenging scenario. World J. Gastroenterol. 2016, 22, 1523–1531. [Google Scholar] [CrossRef] [PubMed]
- Karvellas, C.J.; Leventhal, T.M.; Rakela, J.L.; Zhang, J.; Durkalski, V.; Reddy, K.R.; Fontana, R.J.; Stravitz, R.T.; Lake, J.R.; Lee, W.M.; et al. Outcomes of patients with acute liver failure listed for liver transplantation: A multicenter prospective cohort analysis. Liver Transplant. 2023, 29, 318–330. [Google Scholar]
- O’Grady, J. Liver transplantation for acute liver failure. Best Pract. Res. Clin. Gastroenterol. 2012, 26, 27–33. [Google Scholar] [CrossRef]
- Shingina, A.; A Ziogas, I.; Vutien, P.; Uleryk, E.; Shah, P.S.; Renner, E.; Bhat, M.; Tinmouth, J.; Kim, J. Adult-to-adult living donor liver transplantation in acute liver failure. Transplant. Rev. 2022, 36, 100691. [Google Scholar] [CrossRef]
- Dawwas, M.F.; Gimson, A.E.; Lewsey, J.D.; Copley, L.P.; Van der Meulen, J.H.P.; on behalf of the UK and Ireland Liver Transplant Audit. Survival after liver transplantation in the United Kingdom and Ireland compared with the United States. Gut 2007, 56, 1606–1613. [Google Scholar] [CrossRef]
| Viruses | Hepatitis A, B, C, D, E |
| Cytomegalovirus | |
| Epstein–Barr virus | |
| Herpes simplex virus 1 and 2 | |
| Varicella-zoster virus | |
| Adenovirus | |
| Dengue virus | |
| Medications | Acetaminophen |
| Anti-tuberculosis (Isoniazid, Rifampin, Pyrazinamide) | |
| Statins | |
| Non-steroidal anti-inflammatory drugs | |
| Sulfa drugs | |
| Phenytoin | |
| Carbamazepine | |
| MDMA (ecstasy) | |
| Flucloxacillin | |
| Ketoconazole | |
| Nitrofurantoin | |
| Immune checkpoint inhibitors | |
| Idiosyncratic drug reactions | |
| Genetic/Autoimmune | Wilson disease |
| Autoimmune hepatitis | |
| Vascular | Budd–Chiari syndrome |
| Veno-occlusive disease | |
| Ischemic hepatitis | |
| Herbals/Nutritional Supplements | Multiple agents (Kava Kava, Hydroxycut, Ma Huang, etc.) |
| Dietary and weight loss supplements | |
| Multivitamins | |
| Toxins | Mushroom (most commonly Amanita phalloides) |
| Carbon tetrachloride | |
| Yellow phosphorus | |
| Infiltration | Breast cancer |
| Small-cell lung cancer | |
| Lymphoma | |
| Colon cancer | |
| Melanoma | |
| Multiple myeloma | |
| Pregnancy-related | Acute fatty liver of pregnancy |
| HELLP syndrome | |
| Pre-eclamptic liver rupture | |
| Other | Sepsis |
| Partial hepatectomy | |
| Heat stroke | |
| Hemophagocytic lymphohistiocytosis |
| Acetaminophen toxicity | Very high AST and ALT (often >3500 IU/L) |
| High INR | |
| Low bilirubin | |
| Ischemic hepatic injury | Very high AST and ALT (25–250 times of upper limit of normal) |
| Elevated serum LDH | |
| Hepatitis B virus | Aminotransferase levels: 1000–2000 IU/L ALT usually > AST |
| Wilson disease | Coombs-negative hemolytic anemia |
| Aminotransferase levels <2000 IU/L | |
| AST/ALT ratio >2 | |
| Markedly low ALP (<40 IU/L) | |
| ALP/total bilirubin ratio <4 | |
| Rapidly progressive renal failure | |
| Low uric acid levels | |
| Acute fatty liver of pregnancy/HELLP syndrome | Aminotransferase levels <1000 IU/L |
| Elevated bilirubin | |
| Low platelet count | |
| Herpes simplex virus | Markedly elevated aminotransferases |
| Leukopenia | |
| Low bilirubin | |
| Reye syndrome/Valproate or doxycycline toxicity | Minor to moderate elevations in aminotransferase and bilirubin levels |
| Etiology | Management |
|---|---|
| Acetaminophen | IV N-acetylcysteine |
| Activated charcoal if ingestion is within <4 h | |
| Drug-induced liver injury | Discontinue offending medication |
| Consider N-acetylcysteine if hepatic encephalopathy | |
| Corticosteroids if hypersensitivity or autoimmune features (typically with minocycline or nitrofurantoin) | |
| Supportive care | |
| Hepatitis B virus | Tenofovir/entecavir in reactivation of hepatitis B virus |
| Herpes simplex virus/varicella-zoster virus | IV Acyclovir |
| Cytomegalovirus | IV Ganciclovir |
| Mushroom poisoning | IV silybin |
| IV penicillin if IV silybin is not available | |
| Wilson disease | Continuous hemofiltration |
| Plasma exchange | |
| Autoimmune hepatitis | IV corticosteroids |
| AFLP/HELLP syndrome | Prompt delivery of fetus |
| Budd–Chiari syndrome | Anticoagulation |
| Transjugular intrahepatic portosystemic shunt | |
| Angioplasty, stenting, or shunt creation |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ozturk, N.B.; Herdan, E.; Saner, F.H.; Gurakar, A. A Comprehensive Review of the Diagnosis and Management of Acute Liver Failure. J. Clin. Med. 2023, 12, 7451. https://doi.org/10.3390/jcm12237451
Ozturk NB, Herdan E, Saner FH, Gurakar A. A Comprehensive Review of the Diagnosis and Management of Acute Liver Failure. Journal of Clinical Medicine. 2023; 12(23):7451. https://doi.org/10.3390/jcm12237451
Chicago/Turabian StyleOzturk, Nazli Begum, Emre Herdan, Fuat H. Saner, and Ahmet Gurakar. 2023. "A Comprehensive Review of the Diagnosis and Management of Acute Liver Failure" Journal of Clinical Medicine 12, no. 23: 7451. https://doi.org/10.3390/jcm12237451
APA StyleOzturk, N. B., Herdan, E., Saner, F. H., & Gurakar, A. (2023). A Comprehensive Review of the Diagnosis and Management of Acute Liver Failure. Journal of Clinical Medicine, 12(23), 7451. https://doi.org/10.3390/jcm12237451






