The Influence of Interval Training Combined with Occlusion and Cooling on Selected Indicators of Blood, Muscle Metabolism and Oxidative Stress
Abstract
1. Introduction
2. Materials and Methods
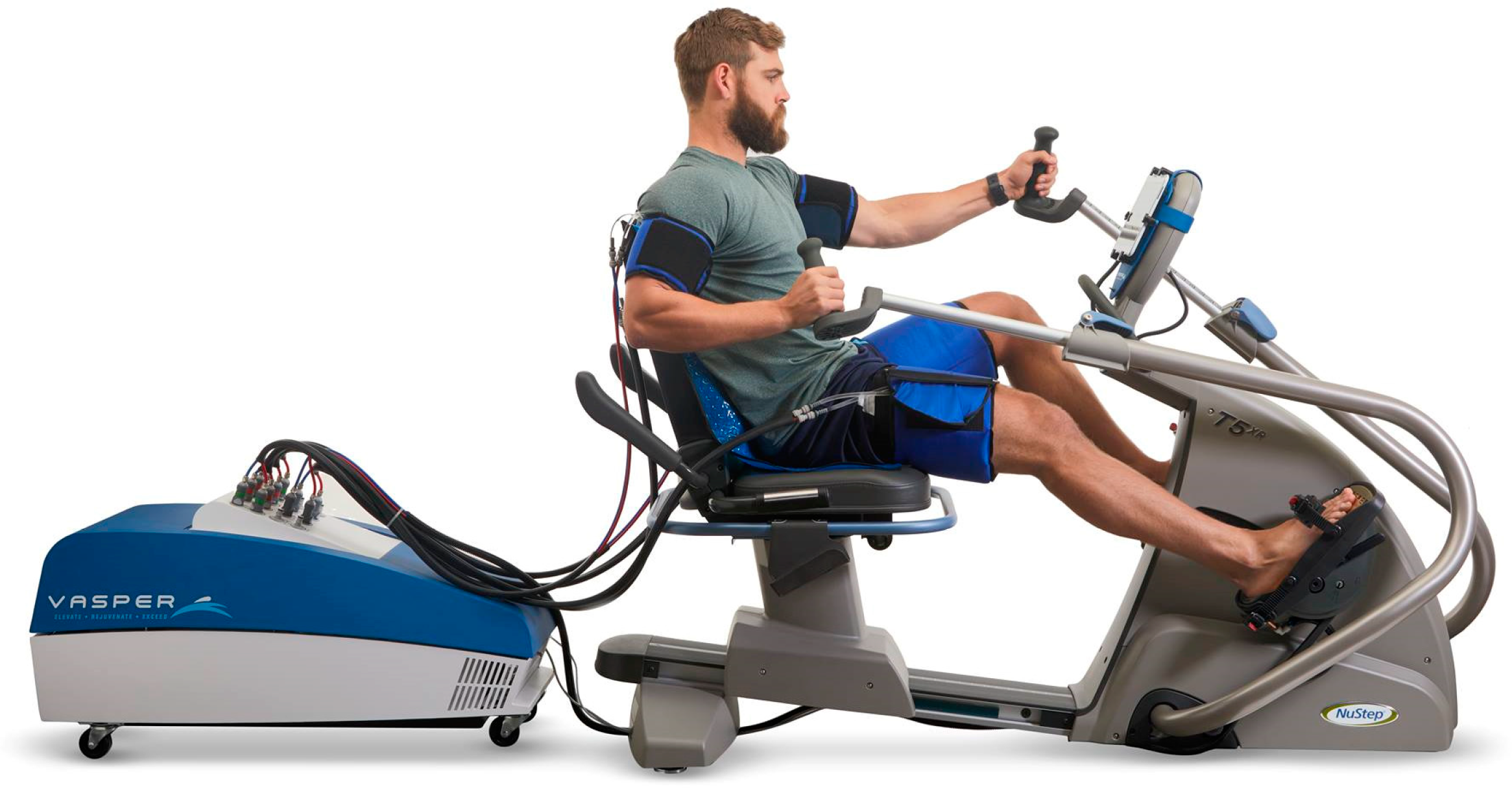
- A total of 10 people performed VASPER Systems LLC (Limited Liability Company) training (Figure 2) without occlusion and cooling (Group CONT)—age [years]: 23.00 ± 0.00; body height [cm]: 164.50 ± 6.44; body mass [kg]: 58.96 ± 10.34.
- A total of 10 people performed VASPER Systems LLC training using occlusion without cooling—the pressure in the occlusion cuffs was 10 mmHg lower than the systolic pressure (Group OCC)—age [years]: 23.22 ± 0.44; body height [cm]: 169.83 ± 9.81; body mass [kg]: 66.31 ± 11.77.
- A total of 10 people performed VASPER Systems LLC training using occlusion and cooling—occlusion as in point 2, additionally combined with activation of the cooling system provided by cuffs and cooling mats located under the feet and in the seat of the subject (Group OCC-COO)—age [years]: 23.25 ± 0.46; body height [cm]: 172.13 ± 9.07; body mass [kg]: 69.45 ± 13.99.
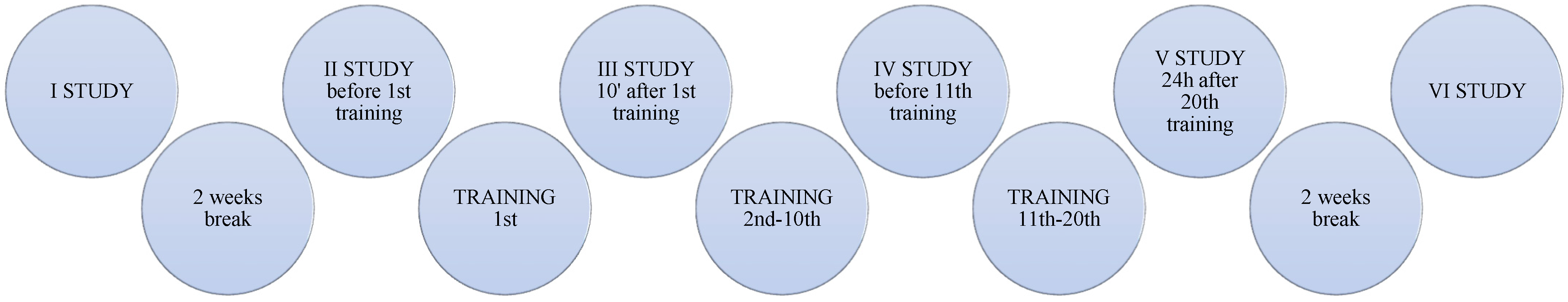
2.1. Analysis of Blood Parameters
2.2. Description of the Intervention
- OCC-COO group—HIIT training combined with occlusion (on the arms and thighs) and local cooling (built-in cryotherapy system under the feet and under the seat). The pressure in the occlusive cuffs (constant) was 10 mmHg lower than the systolic pressure. The cooling and occlusion system was activated for the entire duration of each training unit. Each session consisted of
- ∘
- Introductory part (approx. 2 min)—warm-up, preparation for exercise;
- ∘
- Main part (approx. 20 min)—training consisting of 3 × 6 min and 1 min of rest between intervals;
- ∘
- Final part (approx. 2 min)—calming down, conscious muscle relaxation;
- Group OCC—like OCC-COO training, with an occlusion variant, but without cooling;
- CONT group—training as in the previous groups, but without the occlusion and cooling variant.
2.3. Statistical Analysis
3. Results
- Increase in IGF-1 in the OCC-COO group (F = 2.85, p = 0.03), XOD in the OCC-COO group (F = 10.69, p = 0.00), OCC group (F = 4.42, p = 0.00) and in the CONT group (F = 6.48, p = 0.00), fibrinogen in the OCC-COO group (F = 2.81, p = 0.03), and D-Dimer in the OCC-COO group (F = 2.66, p = 0.04);
- Decrease in MDA in the OCC-COO group (F = 3.74, p = 0.01) and CONT group (F = 3.12, p = 0.03); T-AOC in the OCC-COO group (F = 3.96, p = 0.01), OCC group (F = 7.24, p = 0.00) and the CONT group (F = 2.76, p = 0.04); PT Quick in the OCC-COO group (F = 17.45, p = 0.00), the OCC group (F = 8.27, p = 0.00) and CONT group (F = 6.51, p = 0.00); INR in the OCC-COO group (F = 3.28, p = 0.01), OCC group (F = 4.22, p = 0.00) and CONT group (F = 2.67, p = 0.05 ); and TT in the OCC-COO group (F = 4.31, p = 0.00).
| Parameters | Group | I | II | III | IV | V | VI |
|---|---|---|---|---|---|---|---|
| HGH [ng/mL] | OCC-COO | 1.23 ± 0.79 | 6.64 ± 14.62 | 6.83 ± 10.51 | 1.18 ± 1.01 | 1.41 ± 1.27 | 1.21 ± 0.78 |
| OCC | 3.64 ± 5.53 | 4.66 ± 9.64 | 3.45 ± 4.86 | 7.02 ± 12.90 | 2.05 ± 3.29 | 3.53 ± 7.93 | |
| CONT | 2.06 ± 1.25 | 1.27 ± 0.78 | 1.15 ± 0.88 | 1.42 ± 1.13 | 2.46 ± 2.70 | 2.04 ± 2.82 | |
| IGF-1 [ng/mL] | OCC-COO | 84.27 ± 17.11 | 83.49 ± 20.48 | 88.97 ± 22.16 | 95.18 ± 49.15 | 147.50 ± 75.62 | 117.90 ± 43.82 |
| OCC | 85.35 ± 11.71 | 96.27 ± 28.76 | 94.96 ± 37.09 | 116.05 ± 62.60 | 117.54 ± 42.13 | 131.81 ± 60.50 | |
| CONT | 120.76 ± 50.52 | 92.89 ± 39.38 | 105.85 ± 29.30 | 147.61 ± 49.20 | 99.78 ± 51.50 | 115.35 ± 65.21 | |
| MDA [nmol/mL] | OCC-COO | 4.30 ± 1.81 | 4.06 ± 1.83 | 3.45 ± 2.27 | 2.75 ± 1.13 | 3.60 ± 1.92 | 2.73 ± 1.80 |
| OCC | 3.84 ± 1.16 | 3.43 ± 1.56 | 3.32 ± 1.01 | 2.45 ± 1.04 | 3.26 ± 1.42 | 2.89 ± 1.24 | |
| CONT | 4.55 ± 1.86 | 3.57 ± 1.12 | 3.24 ± 0.95 | 3.44 ± 1.08 | 2.86 ± 1.85 | 2.85 ± 1.04 | |
| T-AOC [U/mL] | OCC-COO | 6.91 ± 3.98 | 6.88 ± 2.25 | 7.57 ± 3.34 | 3.98 ± 1.88 | 4.00 ± 1.25 | 5.91 ± 3.17 |
| OCC | 5.87 ± 3.48 | 7.61 ± 2.37 | 6.07 ± 2.90 | 3.61 ± 1.78 | 4.62 ± 2.76 | 3.15 ± 2.20 | |
| CONT | 6.14 ± 1.51 | 8.84 ± 5.60 | 7.35 ± 5.39 | 3.55 ± 1.33 | 4.67 ± 2.25 | 3.33 ± 1.61 | |
| TOS [U/mL] | OCC-COO | 25.97 ± 9.03 | 30.07 ± 17.30 | 31.71 ± 13.43 | 25.96 ± 6.51 | 36.36 ± 20.72 | 37.56 ± 17.75 |
| OCC | 24.38 ± 13.95 | 33.41 ± 18.83 | 39.92 ± 20.17 | 63.33 ± 101.49 | 68.12 ± 102.31 | 25.53 ± 6.66 | |
| CONT | 40.51 ± 14.44 | 31.35 ± 9.56 | 28.39 ± 11.78 | 31.97 ± 8.43 | 33.84 ± 5.00 | 81.06 ± 142.98 | |
| VEGF [ng/L] | OCC-COO | 857.52 ± 258.86 | 837.71 ± 281.89 | 745.30 ± 285.13 | 953.71 ± 395.19 | 911.27 ± 424.97 | 834.30 ± 239.77 |
| OCC | 891.03 ± 265.45 | 997.31 ± 349.36 | 744.82 ± 268.71 | 1183.08 ± 681.44 | 976.39 ± 442.41 | 900.97 ± 436.82 | |
| CONT | 753.57 ± 162.34 | 906.42 ± 380.74 | 830.65 ± 223.52 | 861.13 ± 280.11 | 800.30 ± 116.45 | 985.13 ± 375.70 | |
| XOD [ng/mL] | OCC-COO | 17.18 ± 3.82 | 13.06 ± 3.53 | 14.16 ± 1.93 | 25.96 ± 10.02 | 21.21 ± 5.63 | 20.07 ± 3.50 |
| OCC | 15.82 ± 3.08 | 16.50 ± 5.08 | 12.98 ± 1.86 | 21.94 ± 9.05 | 24.93 ± 11.62 | 21.79 ± 8.77 | |
| CONT | 16.23 ± 3.87 | 14.73 ± 4.19 | 13.76 ± 2.55 | 19.18 ± 4.87 | 24.22 ± 5.96 | 16.70 ± 4.61 | |
| CORT [μg/dL] | OCC-COO | 18.10 ± 4.71 | 18.61 ± 2.40 | 16.32 ± 2.31 | 17.52 ± 1.79 | 17.63 ± 1.44 | 18.19 ± 1.65 |
| OCC | 19.72 ± 4.19 | 19.04 ± 5.49 | 18.38 ± 2.30 | 18.09 ± 2.69 | 18.37 ± 2.81 | 17.57 ± 2.49 | |
| CONT | 19.42 ± 4.78 | 20.05 ± 3.21 | 18.08 ± 4.13 | 21.12 ± 2.98 | 20.75 ± 5.14 | 19.72 ± 4.10 | |
| MIO [μg/L] | OCC-COO | 40.13 ± 29.22 | 31.79 ± 8.43 | 37.01 ± 18.04 | 34.76 ± 13.06 | 34.07 ± 14.92 | 32.71 ± 8.48 |
| OCC | 40.99 ± 19.57 | 36.98 ± 14.65 | 37.22 ± 17.62 | 34.01 ± 13.41 | 28.53 ± 9.47 | 60.69 ± 86.90 | |
| CONT | 25.67 ± 6.12 | 29.63 ± 12.63 | 28.83 ± 12.27 | 36.40 ± 23.88 | 27.87 ± 5.72 | 33.45 ± 18.23 | |
| PT Quick [%] | OCC-COO | 99.41 ± 9.63 | 100.87 ± 7.18 | 95.25 ± 8.20 | 90.96 ± 7.70 | 90.94 ± 8.83 | 89.03 ± 7.30 |
| OCC | 104.45 ± 10.80 | 104.60 ± 14.94 | 99.58 ± 16.48 | 91.73 ± 16.70 | 95.70 ± 14.19 | 95.81 ± 16.20 | |
| CONT | 101.20 ± 8.82 | 101.37 ± 11.00 | 97.80 ± 6.09 | 90.38 ± 6.91 | 93.97 ± 8.59 | 91.58 ± 9.39 | |
| INR | OCC-COO | 1.12 ± 0.07 | 1.08 ± 0.05 | 1.09 ± 0.05 | 1.12 ± 0.05 | 1.12 ± 0.06 | 1.10 ± 0.05 |
| OCC | 1.09 ± 0.07 | 1.06 ± 0.08 | 1.07 ± 0.11 | 1.13 ± 0.12 | 1.10 ± 0.09 | 1.06 ± 0.08 | |
| CONT | 1.11 ± 0.06 | 1.08 ± 0.06 | 1.08 ± 0.04 | 1.13 ± 0.05 | 1.10 ± 0.06 | 1.08 ± 0.06 | |
| aPTT [s] | OCC-COO | 29.40 ± 1.72 | 29.80 ± 1.80 | 29.88 ± 2.45 | 29.42 ± 1.32 | 29.87 ± 1.75 | 29.39 ± 1.77 |
| OCC | 28.17 ± 2.87 | 28.50 ± 3.13 | 29.12 ± 3.68 | 28.73 ± 3.62 | 28.73 ± 4.00 | 28.42 ± 3.21 | |
| CONT | 27.98 ± 1.11 | 28.07 ± 1.89 | 28.53 ± 1.03 | 28.37 ± 0.70 | 27.65 ± 1.48 | 27.62 ± 1.69 | |
| Fibrinogen [g/L] | OCC-COO | 2.32 ± 0.27 | 2.30 ± 0.51 | 2.15 ± 0.32 | 2.78 ± 0.83 | 2.39 ± 0.29 | 2.29 ± 0.20 |
| OCC | 2.78 ± 0.66 | 2.55 ± 0.41 | 2.43 ± 0.27 | 2.44 ± 0.53 | 2.51 ± 0.69 | 2.71 ± 0.82 | |
| CONT | 2.76 ± 0.69 | 2.69 ± 0.84 | 2.57 ± 0.69 | 2.85 ± 0.95 | 2.77 ± 0.76 | 2.63 ± 0.68 | |
| D-Dimer [mg/L] | OCC-COO | 0.20 ± 0.04 | 0.23 ± 0.08 | 0.28 ± 0.18 | 0.20 ± 0.06 | 0.38 ± 0.24 | 0.28 ± 0.21 |
| OCC | 0.28 ± 0.17 | 0.28 ± 0.19 | 0.24 ± 0.13 | 0.24 ± 0.13 | 0.29 ± 0.20 | 0.25 ± 0.13 | |
| CONT | 0.27 ± 0.10 | 0.30 ± 0.14 | 0.27 ± 0.11 | 0.26 ± 0.11 | 0.34 ± 0.17 | 0.29 ± 0.18 | |
| TT [s] | OCC-COO | 18.12 ± 0.79 | 18.39 ± 1.14 | 18.80 ± 0.86 | 17.22 ± 1.21 | 18.79 ± 0.77 | 18.07 ± 0.99 |
| OCC | 17.62 ± 1.23 | 18.08 ± 1.21 | 18.20 ± 1.07 | 18.29 ± 1.44 | 18.50 ± 1.43 | 17.73 ± 1.07 | |
| CONT | 17.30 ± 1.39 | 17.77 ± 1.16 | 17.90 ± 0.88 | 17.45 ± 1.58 | 18.42 ± 1.21 | 17.85 ± 0.95 |
| Parameters | Groups | F Test Value | Significance Level p |
|---|---|---|---|
| HGH [ng/mL] | OCC-COO | 1.58 | 0.19 |
| OCC | 0.84 | 0.53 | |
| CONT | 0.65 | 0.66 | |
| IGF-1 [ng/mL] | OCC-COO | 2.85 | 0.03 |
| OCC | 1.44 | 0.23 | |
| CONT | 0.96 | 0.46 | |
| MDA [nmol/mL] | OCC-COO | 3.74 | 0.01 |
| OCC | 2.30 | 0.06 | |
| CONT | 3.12 | 0.03 | |
| T-AOC [U/mL] | OCC-COO | 3.96 | 0.01 |
| OCC | 7.24 | 0.00 | |
| CONT | 2.76 | 0.04 | |
| TOS [U/mL] | OCC-COO | 1.30 | 0.28 |
| OCC | 1.11 | 0.37 | |
| CONT | 0.70 | 0.63 | |
| VEGF [ng/L] | OCC-COO | 0.57 | 0.72 |
| OCC | 1.17 | 0.34 | |
| CONT | 0.78 | 0.57 | |
| XOD [ng/mL] | OCC-COO | 10.69 | 0.00 |
| OCC | 4.42 | 0.00 | |
| CONT | 6.48 | 0.00 | |
| CORT [μg/dL] | OCC-COO | 0.94 | 0.46 |
| OCC | 0.94 | 0.46 | |
| CONT | 2.11 | 0.10 | |
| MIO [μg/L] | OCC-COO | 0.40 | 0.84 |
| OCC | 0.85 | 0.53 | |
| CONT | 1.03 | 0.42 | |
| PT Quick [%] | OCC-COO | 17.45 | 0.00 |
| OCC | 8.27 | 0.00 | |
| CONT | 6.51 | 0.00 | |
| INR | OCC-COO | 3.28 | 0.01 |
| OCC | 4.22 | 0.00 | |
| CONT | 2.67 | 0.05 | |
| aPTT [s] | OCC-COO | 0.59 | 0.71 |
| OCC | 1.36 | 0.26 | |
| CONT | 0.85 | 0.53 | |
| Fibrinogen [g/L] | OCC-COO | 2.81 | 0.03 |
| OCC | 0.97 | 0.45 | |
| CONT | 0.41 | 0.84 | |
| D-Dimer [mg/L] | OCC-COO | 2.66 | 0.04 |
| OCC | 0.57 | 0.72 | |
| CONT | 1.26 | 0.31 | |
| TT [s] | OCC-COO | 4.31 | 0.00 |
| OCC | 1.77 | 0.14 | |
| CONT | 0.73 | 0.61 |
| Parameters | Study | I | II | III | IV | V | VI |
|---|---|---|---|---|---|---|---|
| IGF-1 [ng/mL] OCC-COO | I | 0.97 | 0.83 | 0.61 | 0.00 | 0.12 | |
| II | 0.97 | 0.80 | 0.58 | 0.00 | 0.11 | ||
| III | 0.83 | 0.80 | 0.77 | 0.01 | 0.18 | ||
| IV | 0.61 | 0.58 | 0.77 | 0.02 | 0.29 | ||
| V | 0.00 | 0.00 | 0.01 | 0.02 | 0.17 | ||
| VI | 0.12 | 0.11 | 0.18 | 0.29 | 0.17 | ||
| MDA [nmol/mL] OCC-COO | I | 0.62 | 0.08 | 0.00 | 0.15 | 0.00 | |
| II | 0.62 | 0.21 | 0.01 | 0.33 | 0.01 | ||
| III | 0.08 | 0.21 | 0.15 | 0.76 | 0.14 | ||
| IV | 0.00 | 0.01 | 0.15 | 0.08 | 0.98 | ||
| V | 0.15 | 0.33 | 0.76 | 0.08 | 0.08 | ||
| VI | 0.00 | 0.01 | 0.14 | 0.98 | 0.08 | ||
| MDA [nmol/mL] CONT | I | 0.06 | 0.02 | 0.04 | 0.00 | 0.00 | |
| II | 0.06 | 0.51 | 0.79 | 0.17 | 0.16 | ||
| III | 0.02 | 0.51 | 0.69 | 0.46 | 0.44 | ||
| IV | 0.04 | 0.79 | 0.69 | 0.26 | 0.25 | ||
| V | 0.00 | 0.17 | 0.46 | 0.26 | 0.98 | ||
| VI | 0.00 | 0.16 | 0.44 | 0.25 | 0.98 | ||
| T-AOC [U/mL] OCC-COO | I | 0.98 | 0.55 | 0.01 | 0.01 | 0.37 | |
| II | 0.98 | 0.53 | 0.01 | 0.01 | 0.38 | ||
| III | 0.55 | 0.53 | 0.00 | 0.00 | 0.14 | ||
| IV | 0.01 | 0.01 | 0.00 | 0.99 | 0.09 | ||
| V | 0.01 | 0.01 | 0.00 | 0.99 | 0.09 | ||
| VI | 0.37 | 0.38 | 0.14 | 0.09 | 0.09 | ||
| T-AOC [U/mL] OCC | I | 0.06 | 0.83 | 0.01 | 0.16 | 0.00 | |
| II | 0.06 | 0.09 | 0.00 | 0.00 | 0.00 | ||
| III | 0.83 | 0.09 | 0.01 | 0.11 | 0.00 | ||
| IV | 0.01 | 0.00 | 0.01 | 0.26 | 0.61 | ||
| V | 0.16 | 0.00 | 0.11 | 0.26 | 0.10 | ||
| VI | 0.00 | 0.00 | 0.00 | 0.61 | 0.10 | ||
| T-AOC [U/mL] CONT | I | 0.16 | 0.52 | 0.18 | 0.44 | 0.14 | |
| II | 0.16 | 0.43 | 0.01 | 0.04 | 0.01 | ||
| III | 0.52 | 0.43 | 0.05 | 0.16 | 0.04 | ||
| IV | 0.18 | 0.01 | 0.05 | 0.55 | 0.90 | ||
| V | 0.44 | 0.04 | 0.16 | 0.55 | 0.48 | ||
| VI | 0.14 | 0.01 | 0.04 | 0.90 | 0.48 | ||
| XOD [ng/mL] OCC-COO | I | 0.05 | 0.15 | 0.00 | 0.06 | 0.17 | |
| II | 0.05 | 0.60 | 0.00 | 0.00 | 0.00 | ||
| III | 0.15 | 0.60 | 0.00 | 0.00 | 0.01 | ||
| IV | 0.00 | 0.00 | 0.00 | 0.03 | 0.01 | ||
| V | 0.06 | 0.00 | 0.00 | 0.03 | 0.59 | ||
| VI | 0.17 | 0.00 | 0.01 | 0.01 | 0.59 | ||
| XOD [ng/mL] OCC | I | 0.83 | 0.36 | 0.05 | 0.01 | 0.06 | |
| II | 0.83 | 0.26 | 0.08 | 0.01 | 0.09 | ||
| III | 0.36 | 0.26 | 0.01 | 0.00 | 0.01 | ||
| IV | 0.05 | 0.08 | 0.01 | 0.34 | 0.96 | ||
| V | 0.01 | 0.01 | 0.00 | 0.34 | 0.31 | ||
| VI | 0.06 | 0.09 | 0.01 | 0.96 | 0.31 | ||
| XOD [ng/mL] CONT | I | 0.48 | 0.25 | 0.17 | 0.00 | 0.82 | |
| II | 0.48 | 0.65 | 0.05 | 0.00 | 0.36 | ||
| III | 0.25 | 0.65 | 0.02 | 0.00 | 0.17 | ||
| IV | 0.17 | 0.05 | 0.02 | 0.02 | 0.25 | ||
| V | 0.00 | 0.00 | 0.00 | 0.02 | 0.00 | ||
| VI | 0.82 | 0.36 | 0.17 | 0.25 | 0.00 | ||
| PT Quick [%] OCC-COO | I | 0.38 | 0.02 | 0.00 | 0.00 | 0.00 | |
| II | 0.38 | 0.00 | 0.00 | 0.00 | 0.00 | ||
| III | 0.02 | 0.00 | 0.01 | 0.01 | 0.00 | ||
| IV | 0.00 | 0.00 | 0.01 | 0.99 | 0.25 | ||
| V | 0.00 | 0.00 | 0.01 | 0.99 | 0.26 | ||
| VI | 0.00 | 0.00 | 0.00 | 0.25 | 0.26 | ||
| PT Quick [%] OCC | I | 0.95 | 0.06 | 0.00 | 0.00 | 0.00 | |
| II | 0.95 | 0.06 | 0.00 | 0.00 | 0.00 | ||
| III | 0.06 | 0.06 | 0.00 | 0.14 | 0.15 | ||
| IV | 0.00 | 0.00 | 0.00 | 0.13 | 0.12 | ||
| V | 0.00 | 0.00 | 0.14 | 0.13 | 0.96 | ||
| VI | 0.00 | 0.00 | 0.15 | 0.12 | 0.96 | ||
| PT Quick [%] CONT | I | 0.95 | 0.21 | 0.00 | 0.01 | 0.00 | |
| II | 0.95 | 0.19 | 0.00 | 0.01 | 0.00 | ||
| III | 0.21 | 0.19 | 0.01 | 0.16 | 0.03 | ||
| IV | 0.00 | 0.00 | 0.01 | 0.19 | 0.65 | ||
| V | 0.01 | 0.01 | 0.16 | 0.19 | 0.38 | ||
| VI | 0.00 | 0.00 | 0.03 | 0.65 | 0.38 | ||
| INR OCC-COO | I | 0.01 | 0.06 | 1.00 | 1.00 | 0.08 | |
| II | 0.01 | 0.32 | 0.01 | 0.01 | 0.25 | ||
| III | 0.06 | 0.32 | 0.06 | 0.06 | 0.88 | ||
| IV | 1.00 | 0.01 | 0.06 | 1.00 | 0.08 | ||
| V | 1.00 | 0.01 | 0.06 | 1.00 | 0.08 | ||
| VI | 0.08 | 0.25 | 0.88 | 0.08 | 0.08 | ||
| INR OCC | I | 0.11 | 0.42 | 0.04 | 0.66 | 0.11 | |
| II | 0.11 | 0.42 | 0.00 | 0.04 | 1.00 | ||
| III | 0.42 | 0.42 | 0.00 | 0.21 | 0.42 | ||
| IV | 0.04 | 0.00 | 0.00 | 0.10 | 0.00 | ||
| V | 0.66 | 0.04 | 0.21 | 0.10 | 0.04 | ||
| VI | 0.11 | 1.00 | 0.42 | 0.00 | 0.04 | ||
| INR CONT | I | 0.09 | 0.06 | 0.34 | 0.70 | 0.13 | |
| II | 0.09 | 0.85 | 0.01 | 0.19 | 0.85 | ||
| III | 0.06 | 0.85 | 0.01 | 0.13 | 0.70 | ||
| IV | 0.34 | 0.01 | 0.01 | 0.19 | 0.02 | ||
| V | 0.70 | 0.19 | 0.13 | 0.19 | 0.26 | ||
| VI | 0.13 | 0.85 | 0.70 | 0.02 | 0.26 | ||
| Fibrinogen [g/L] OCC-COO | I | 0.94 | 0.36 | 0.01 | 0.69 | 0.88 | |
| II | 0.94 | 0.40 | 0.01 | 0.64 | 0.94 | ||
| III | 0.36 | 0.40 | 0.00 | 0.19 | 0.45 | ||
| IV | 0.01 | 0.01 | 0.00 | 0.04 | 0.01 | ||
| V | 0.69 | 0.64 | 0.19 | 0.04 | 0.58 | ||
| VI | 0.88 | 0.94 | 0.45 | 0.01 | 0.58 | ||
| D-Dimer [mg/L] OCC-COO | I | 0.60 | 0.18 | 0.99 | 0.00 | 0.19 | |
| II | 0.60 | 0.40 | 0.59 | 0.02 | 0.43 | ||
| III | 0.18 | 0.40 | 0.17 | 0.10 | 0.97 | ||
| IV | 0.99 | 0.59 | 0.17 | 0.00 | 0.18 | ||
| V | 0.00 | 0.02 | 0.10 | 0.00 | 0.10 | ||
| VI | 0.19 | 0.43 | 0.97 | 0.18 | 0.10 | ||
| TT [s] OCC-COO | I | 0.51 | 0.10 | 0.03 | 0.10 | 0.89 | |
| II | 0.51 | 0.31 | 0.01 | 0.32 | 0.42 | ||
| III | 0.10 | 0.31 | 0.00 | 0.98 | 0.07 | ||
| IV | 0.03 | 0.01 | 0.00 | 0.00 | 0.04 | ||
| V | 0.10 | 0.32 | 0.98 | 0.00 | 0.08 | ||
| VI | 0.89 | 0.42 | 0.07 | 0.04 | 0.08 |
| Parameters | Factor | F Test Value | Significance Level p |
|---|---|---|---|
| HGH [ng/mL] | S | 0.66 | 0.66 |
| I | 0.45 | 0.65 | |
| S*I | 1.20 | 0.30 | |
| IGF-1 [ng/mL] | S | 2.37 | 0.04 |
| I | 0.69 | 0.51 | |
| S*I | 1.22 | 0.29 | |
| MDA [nmol/mL] | S | 7.14 | 0.00 |
| I | 0.12 | 0.88 | |
| S*I | 0.81 | 0.62 | |
| T-AOC [U/mL] | S | 11.17 | 0.00 |
| I | 0.34 | 0.72 | |
| S*I | 1.01 | 0.44 | |
| TOS [U/mL] | S | 0.69 | 0.63 |
| I | 0.50 | 0.62 | |
| S*I | 1.14 | 0.34 | |
| VEGF [ng/L] | S | 1.30 | 0.27 |
| I | 0.59 | 0.57 | |
| S*I | 0.53 | 0.87 | |
| XOD [ng/mL] | S | 14.26 | 0.00 |
| I | 0.29 | 0.75 | |
| S*I | 1.32 | 0.23 | |
| CORT [μg/dL] | S | 1.60 | 0.17 |
| I | 1.15 | 0.34 | |
| S*I | 0.94 | 0.50 | |
| MIO [μg/L] | S | 0.64 | 0.67 |
| I | 0.75 | 0.48 | |
| S*I | 0.69 | 0.73 | |
| PT Quick [%] | S | 26.37 | 0.00 |
| I | 0.37 | 0.70 | |
| S*I | 0.49 | 0.89 | |
| INR | S | 8.79 | 0.00 |
| I | 0.29 | 0.75 | |
| S*I | 0.54 | 0.86 | |
| aPTT [s] | S | 1.75 | 0.13 |
| I | 0.90 | 0.42 | |
| S*I | 0.48 | 0.90 | |
| Fibrinogen [g/L] | S | 1.51 | 0.19 |
| I | 1.00 | 0.38 | |
| S*I | 1.20 | 0.30 | |
| D-Dimer [mg/L] | S | 2.90 | 0.02 |
| I | 0.09 | 0.91 | |
| S*I | 1.06 | 0.40 | |
| TT [s] | S | 3.81 | 0.00 |
| I | 0.56 | 0.58 | |
| S*I | 1.20 | 0.30 |
| Parameters | Study | I | II | III | IV | V | VI |
|---|---|---|---|---|---|---|---|
| IGF-1 [ng/mL] | I | 0.81 | 0.90 | 0.09 | 0.02 | 0.03 | |
| II | 0.81 | 0.71 | 0.05 | 0.01 | 0.02 | ||
| III | 0.90 | 0.71 | 0.12 | 0.03 | 0.04 | ||
| IV | 0.09 | 0.05 | 0.12 | 0.53 | 0.63 | ||
| V | 0.02 | 0.01 | 0.03 | 0.53 | 0.89 | ||
| VI | 0.03 | 0.02 | 0.04 | 0.63 | 0.89 | ||
| MDA [nmol/mL] | I | 0.08 | 0.00 | 0.00 | 0.00 | 0.00 | |
| II | 0.08 | 0.20 | 0.00 | 0.13 | 0.00 | ||
| III | 0.00 | 0.20 | 0.05 | 0.82 | 0.06 | ||
| IV | 0.00 | 0.00 | 0.05 | 0.09 | 0.97 | ||
| V | 0.00 | 0.13 | 0.82 | 0.09 | 0.09 | ||
| VI | 0.00 | 0.00 | 0.06 | 0.97 | 0.09 | ||
| T-AOC [U/mL] | I | 0.06 | 0.37 | 0.00 | 0.01 | 0.00 | |
| II | 0.06 | 0.33 | 0.00 | 0.00 | 0.00 | ||
| III | 0.37 | 0.33 | 0.00 | 0.00 | 0.00 | ||
| IV | 0.00 | 0.00 | 0.00 | 0.35 | 0.48 | ||
| V | 0.01 | 0.00 | 0.00 | 0.35 | 0.81 | ||
| VI | 0.00 | 0.00 | 0.00 | 0.48 | 0.81 | ||
| XOD [ng/mL] | I | 0.27 | 0.06 | 0.00 | 0.00 | 0.02 | |
| II | 0.27 | 0.44 | 0.00 | 0.00 | 0.00 | ||
| III | 0.06 | 0.44 | 0.00 | 0.00 | 0.00 | ||
| IV | 0.00 | 0.00 | 0.00 | 0.69 | 0.06 | ||
| V | 0.00 | 0.00 | 0.00 | 0.69 | 0.02 | ||
| VI | 0.02 | 0.00 | 0.00 | 0.06 | 0.02 | ||
| PT Quick [%] | I | 0.62 | 0.00 | 0.00 | 0.00 | 0.00 | |
| II | 0.62 | 0.00 | 0.00 | 0.00 | 0.00 | ||
| III | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | ||
| IV | 0.00 | 0.00 | 0.00 | 0.07 | 0.40 | ||
| V | 0.00 | 0.00 | 0.00 | 0.07 | 0.34 | ||
| VI | 0.00 | 0.00 | 0.00 | 0.40 | 0.34 | ||
| INR | I | 0.00 | 0.01 | 0.06 | 0.90 | 0.01 | |
| II | 0.00 | 0.30 | 0.00 | 0.00 | 0.46 | ||
| III | 0.01 | 0.30 | 0.00 | 0.01 | 0.76 | ||
| IV | 0.06 | 0.00 | 0.00 | 0.08 | 0.00 | ||
| V | 0.90 | 0.00 | 0.01 | 0.08 | 0.00 | ||
| VI | 0.01 | 0.46 | 0.76 | 0.00 | 0.00 | ||
| D-Dimer [mg/L] | I | 0.50 | 0.56 | 0.53 | 0.00 | 0.42 | |
| II | 0.50 | 0.93 | 0.20 | 0.02 | 0.88 | ||
| III | 0.56 | 0.93 | 0.23 | 0.01 | 0.82 | ||
| IV | 0.53 | 0.20 | 0.23 | 0.00 | 0.15 | ||
| V | 0.00 | 0.02 | 0.01 | 0.00 | 0.03 | ||
| VI | 0.42 | 0.88 | 0.82 | 0.15 | 0.03 | ||
| TT [s] | I | 0.13 | 0.02 | 0.85 | 0.00 | 0.54 | |
| II | 0.13 | 0.37 | 0.09 | 0.07 | 0.37 | ||
| III | 0.02 | 0.37 | 0.01 | 0.36 | 0.07 | ||
| IV | 0.85 | 0.09 | 0.01 | 0.00 | 0.42 | ||
| V | 0.00 | 0.07 | 0.36 | 0.00 | 0.01 | ||
| VI | 0.54 | 0.37 | 0.07 | 0.42 | 0.01 |
4. Discussion
5. Conclusions
- Changes in the examined indicators (IGF-1, MDA, T-AOC, XOD, PT Quick, INR, Fibrinogen, D-Dimer, and TT) were observed after a series of training sessions, not after a single training unit.
- Both interval training without and with the modifications used in the study influence coagulation (PT Quick, INR, Fibrinogen, D-Dimer, and TT) and oxidative stress (MDA, T-AOC, and XOD) parameters and, to a small extent, muscle metabolism (IGF-1).
- It seems reasonable to use occlusion and local cryotherapy in combination with occlusion.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jawień, A.; Grzela, T.; Ciecierski, M.; Piotrowicz, R.; Szotkiewicz, A.; Migdalski, A. Buflomedil associated with pentoxiflylline in the treatment of patients with intermittent claudication. Opened, randomised, one-center-based study. Acta Angiol. 2003, 3, 109–122. [Google Scholar]
- Conte, M.S.; Bradbury, A.W.; Kolh, P.; White, J.V.; Dick, F.; Fitridge, R.; Mills, J.L.; Ricco, J.B.; Suresh, K.R.; Murad, M.H.; et al. Global vascular guidelines on the management of chronic limb-threatening ischemia. J. Vasc. Surg. 2019, 58, S1–S109. [Google Scholar]
- Frank, U.; Nikol, S.; Belch, J.; Boc, V.; Brodmann, M.; Carpentier, P.H.; Chraim, A.; Canning, C.; Dimakakos, E.; Gottsäter, A.; et al. ESVM Guideline on peripheral arterial disease. Vasa 2019, 48, 1–79. [Google Scholar] [CrossRef] [PubMed]
- Manini, T.M.; Clark, B.C. Blood Flow Restricted Exercise and Skeletal Muscle Health. Exerc. Sport Sci. Rev. 2009, 37, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Hylden, C.; Burns, T.; Stinner, D.J.; Owens, J. Blood flow restriction rehabilitation for extremity weakness: A case series. J. Spec. Oper. Med. 2015, 15, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Loenneke, J.; Fahs, C.; Rossow, L.; Abe, T.; Bemben, M. The anabolic benefits of venous blood flow restriction training may be induced by muscle cell swelling. Med. Hypothes. 2012, 78, 151–154. [Google Scholar] [CrossRef]
- Loenneke, J.; Fahs, C.; Wilson, J.; Bemben, M. Blood flow restriction: The metabolite/volume threshold theory. Med. Hypothes. 2011, 77, 748–752. [Google Scholar] [CrossRef]
- Loenneke, J.P.; Pujol, T.J. The Use of Occlusion Training to Produce Muscle Hypertrophy. Strength Cond. J. 2009, 31, 77–84. [Google Scholar] [CrossRef]
- Yasuda, T.; Fukumura, K.; Fukuda, T.; Iida, H.; Imuta, H.; Sato, Y.; Yamasoba, T.; Nakajima, T. Effects of low-intensity, elastic band resistance exercise combined with blood flow restriction on muscle activation. Scand. J. Med. Sci. Sports 2014, 24, 55–61. [Google Scholar] [CrossRef]
- Kang, D.Y.; Kim, H.S.; Lee, K.S.; Kim, Y.M. The effects of bodyweight-based exercise with blood flow restriction on isokinetic knee muscular function and thigh circumference in college students. J. Phys. Ther. Sci. 2015, 27, 2709–2712. [Google Scholar] [CrossRef]
- Hackney, K.J.; Everett, M.; Scott, J.M.; Ploutz-Snyder, L. Blood flow-restricted exercise in space. Extreme Physiol. Med. 2012, 1, 12. [Google Scholar] [CrossRef]
- Patterson, S.D.; Leggate, M.; Nimmo, M.A.; Ferguson, R.A. Circulating hormone and cytokine response to low-load resistance training with blood flow restriction in older men. Eur. J. Appl. Physiol. 2013, 113, 713–719. [Google Scholar] [CrossRef] [PubMed]
- Tanimoto, M.; Madarame, H.; Ishii, N. Muscle oxygenation and plasma growth hormone concentration during and after re-sistance exercise: Comparison between “KAATSU” and other types of regimen. Int. J. KAATSU Train. Res. 2005, 1, 51–56. [Google Scholar] [CrossRef]
- Takarada, Y.; Nakamura, Y.; Aruga, S.; Onda, T.; Miyazaki, S.; Ishii, N.; D’souza, R.F.; Woodhead, J.S.T.; Zeng, N.; Blenkiron, C.; et al. Rapid increase in plasma growth hormone after low-intensity resistance exercise with vascular occlusion. J. Appl. Physiol. 2000, 88, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Yoshitomi, A.; Abe, T. Acute growth hormone response to low-intensity KAATSU resistance exercise: Comparison between arm and leg. Int. J. KAATSU Train. Res. 2005, 1, 45–50. [Google Scholar] [CrossRef]
- Victor, R.G.; Seals, D.R.; Kaur, J.; Senador, D.; Krishnan, A.C.; Hanna, H.W.; Alvarez, A.; Machado, T.M.; O’Leary, D.S.; Altamimi, Y.H.; et al. Reflex stimulation of sympathetic outflow during rhythmic exercise in humans. Am. J. Physiol. Circ. Physiol. 1989, 257, H2017–H2024. [Google Scholar] [CrossRef]
- Gosselink, K.L.; Zhong, H.; Bigbee, A.J.; Grossman, E.J.; Pierce, J.R.; Clark, B.C.; Ploutz-Snyder, L.L.; Kanaley, J.A.; Arnaud, S.; Nemet, D.; et al. Skeletal muscle afferent regulation of bioassayable growth hormone in the rat pituitary. J. Appl. Physiol. 1998, 84, 1425–1430. [Google Scholar] [CrossRef]
- Fry, C.S.; Glynn, E.L.; Drummond, M.J.; Timmerman, K.L.; Fujita, S.; Abe, T.; Dhanani, S.; Volpi, E.; Rasmussen, B.B.; Pignanelli, C.; et al. Blood flow restriction exercise stimulates mTORC1 signaling and muscle protein synthesis in older men. J. Appl. Physiol. 2010, 108, 1199–1209. [Google Scholar] [CrossRef]
- Lacka, K.; Czyzyk, A. Hormony a układ sercowo-naczyniowy [Hormones and the cardiovascular system]. Endokrynol. Pol. 2008, 59, 420–432. [Google Scholar]
- Patterson, S.D.; Ferguson, R.A. Increase in calf post-occlusive blood flow and strength following short-term resistance exercise training with blood flow restriction in young women. Eur. J. Appl. Physiol. 2010, 108, 1025–1033. [Google Scholar] [CrossRef]
- Marino, F.E. Methods, advantages, and limitations of body cooling for exercise performance. Br. J. Sports Med. 2002, 36, 89–94. [Google Scholar] [CrossRef]
- Arngrïmsson, S.Á.; Petitt, D.S.; Stueck, M.G.; Jorgensen, D.K.; Cureton, K.J. Cooling vest worn during active warm-up improves 5-km run performance in the heat. J. Appl. Physiol. 2004, 96, 1867–1874. [Google Scholar] [CrossRef]
- Prior, B.M.; Lloyd, P.G.; Yang, H.T.; Terjung, R.L. Exercise-Induced Vascular Remodeling. Exerc. Sport Sci. Rev. 2003, 31, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Gavin, T.P.; Drew, J.L.; Kubik, C.J.; Pofahl, W.E.; Hickner, R.C. Acute resistance exercise increases skeletal muscle angiogenic growth factor expression. Acta Physiol. 2007, 191, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Drummond, M.J.; Fujita, S.; Takashi, A.; Dreyer, H.C.; Volpi, E.; Rasmussen, B.B. Human muscle gene expression following resistance exercise and blood flow restriction. Med. Sci. Sports Exerc. 2008, 40, 691–698. [Google Scholar] [CrossRef]
- Gerber, H.-P.; Hillan, K.J.; Ryan, A.M.; Kowalski, J.; Keller, G.-A.; Rangell, L.; Wright, B.D.; Radtke, F.; Aguet, M.; Ferrara, N. VEGF is required for growth and survival in neonatal mice. Development 1999, 126, 1149–1159. [Google Scholar] [CrossRef] [PubMed]
- Gerber, H.-P.; Vu, T.H.; Ryan, A.M.; Kowalski, J.; Werb, Z.; Ferrara, N. VEGF couples hypertrophic cartilage remodeling, ossification and angiogenesis during endochondral bone formation. Nat. Med. 1999, 5, 623–628. [Google Scholar] [CrossRef] [PubMed]
- Semsarian, C.; Sutrave, P.; Richmond, D.R.; Graham, R.M. Insulin-like growth factor (IGF-I) induces myotube hypertrophy associated with an increase in anaerobic glycolysis in a clonal skeletal-muscle cell model. Biochem. J. 1999, 339, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Hunt, T.K.; Aslam, R.; Hussain, Z.; Beckert, S. Lactate, with oxygen, incites angiogenesis. Adv. Exp. Med. Biol. 2008, 614, 73–80. [Google Scholar] [PubMed]
- Larkin, K.A.; Macneil, R.G.; Dirain, M.; Sandesara, B.; Manini, T.M.; Buford, T.W. Blood flow restriction enhances post–resistance exercise angiogenic gene expression. Med. Sci. Sports Exerc. 2012, 44, 2077–2083. [Google Scholar] [CrossRef]
- Mackintosh, S.F.H.; Goldie, P.; Hill, K. Falls incidence and factors associated with falling in older, community-dwelling, chronic stroke survivors (>1 year after stroke) and matched controls. Aging Clin. Exp. Res. 2005, 17, 74–81. [Google Scholar] [CrossRef]
- Mackintosh, S.F.; Hill, K.; Dodd, K.J.; Goldie, P.; Culham, E. Falls and injury prevention should be part of every stroke rehabilitation plan. Clin. Rehabil. 2005, 19, 441–451. [Google Scholar] [CrossRef]
- Häkkinen, K. Neuromuscular fatigue and recovery in male and female athletes during heavy resistance exercise. Int. J. Sports Med. 1993, 14, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Häkkinen, K.; Pakarinen, A.; Suga, T.; Okita, K.; Morita, N.; Yokota, T.; Hirabayashi, K.; Horiuchi, M.; Takada, S.; Omokawa, M.; et al. Acute hormonal responses to two different fatiguing heavy-resistance protocols in male athletes. J. Appl. Physiol. 1993, 74, 882–887. [Google Scholar] [CrossRef]
- Kraemer, R.R.; Hollander, D.B.; Reeves, G.V.; Francois, M.; Ramadan, Z.G.; Meeker, B.; Tryniecki, J.L.; Hebert, E.P.; Castracane, V.D. Similar hormonal responses to concentric and eccentric muscle actions using relative loading. Eur. J. Appl. Physiol. 2006, 96, 551–557. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.A.; Rutherford, O.M. Human muscle strength training: The effects of three different regimens and the nature of the resultant changes. J. Physiol. 1987, 391, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Takano, H.; Morita, T.; Iida, H.; Asada, K.-I.; Kato, M.; Uno, K.; Hirose, K.; Matsumoto, A.; Takenaka, K.; Hirata, Y.; et al. Hemodynamic and hormonal responses to a short-term low-intensity resistance exercise with the reduction of muscle blood flow. Eur. J. Appl. Physiol. 2005, 95, 65–73. [Google Scholar] [CrossRef]
- Kraemer, W.J.; Ratamess, N.A. Hormonal Responses and Adaptations to Resistance Exercise and Training. Sports Med. 2005, 35, 339–361. [Google Scholar] [CrossRef]
- Pierce, J.D.; Hall, S.; Clancy, R.L.; Goodyear-Bruch, C. Effect of dopamine on rat diaphragm apoptosis and muscle performance. Exp. Physiol. 2006, 91, 731–740. [Google Scholar] [CrossRef]
- Takarada, Y.; Takazawa, H.; Sato, Y.; Takebayashi, S.; Tanaka, Y.; Ishii, N.; Mitchell, E.A.; Martin, N.R.W.; Turner, M.C.; Taylor, C.W.; et al. Effects of resistance exercise combined with moderate vascular occlusion on muscular function in humans. J. Appl. Physiol. 2000, 88, 2097–2106. [Google Scholar] [CrossRef]
- Takarada, Y.; Sato, Y.; Ishii, N. Effects of resistance exercise combined with vascular occlusion on muscle function in athletes. Eur. J. Appl. Physiol. 2002, 86, 308–314. [Google Scholar] [CrossRef]
- Takarada, Y.; Takazawa, H.; Ishii, N. Applications of vascular occlusion diminish disuse atrophy of knee extensor muscles. Med. Sci. Sports Exerc. 2000, 32, 2035–2039. [Google Scholar] [CrossRef]
- Ploutz, L.L.; Tesch, P.A.; Biro, R.L.; Barrett-O’Keefe, Z.; Helgerud, J.; Wagner, P.D.; Richardson, R.S.; Giesebrecht, S.; van Duinen, H.; Todd, G.; et al. Effect of resistance training on muscle use during exercise. J. Appl. Physiol. 1994, 76, 1675–1681. [Google Scholar] [CrossRef]
- Godfrey, R.J.; Whyte, G.P.; Buckley, J.; Quinlivan, R. The role of lactate in the exercise-induced human growth hormone response: Evidence from McArdle disease. Br. J. Sports Med. 2009, 43, 521–525. [Google Scholar] [CrossRef] [PubMed]
- Reeves, G.V.; Kraemer, R.R.; Hollander, D.B.; Clavier, J.; Thomas, C.; Francois, M.; Castracane, V.D. Comparison of hormone responses following light resistance exercise with partial vascular occlusion and moderately difficult resistance exercise without occlusion. J. Appl. Physiol. 2006, 101, 1616–1622. [Google Scholar] [CrossRef]
- Hawke, T.J.; Garry, D.J. Myogenic satellite cells: Physiology to molecular biology. J. Appl. Physiol. 2001, 91, 534–551. [Google Scholar] [CrossRef] [PubMed]
- Barton, E.R. Viral expression of insulin-like growth factor-I isoforms promotes different responses in skeletal muscle. J. Appl. Physiol. 2006, 100, 1778–1784. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wernbom, M.; Augustsson, J.; Raastad, T. Ischemic strength training: A low-load alternative to heavy resistance exercise? Scand. J. Med. Sci. Sports 2008, 18, 401–416. [Google Scholar] [CrossRef]
- Abe, T.; Yasuda, T.; Midorikawa, T.; Sato, Y.; Kearns, C.F.; Inoue, K.; Koizumi, K.; Ishii, N. Skeletal muscle size and circulating IGF-1 are increased after two weeks of twice daily “KAATSU” resistance training. Int. J. KAATSU Train. Res. 2005, 1, 6–12. [Google Scholar] [CrossRef]
- West, D.W.; Kujbida, G.W.; Moore, D.R.; Atherton, P.; Burd, N.A.; Padzik, J.P.; De Lisio, M.; Tang, J.E.; Parise, G.; Rennie, M.J.; et al. Resistance exercise-induced increases in putative anabolic hormones do not enhance muscle protein synthesis or intracellular signalling in young men. J. Physiol. 2009, 587, 5239–5247. [Google Scholar] [CrossRef]
- Fujita, S.; Abe, T.; Drummond, M.J.; Cadenas, J.G.; Dreyer, H.C.; Sato, Y.; Volpi, E.; Rasmussen, B.B. Blood flow restriction during lowintensity resistance exercise increases S6K1 phos-phorylation and muscle protein synthesis. J. Appl. Physiol. 2007, 103, 903–910. [Google Scholar] [CrossRef]
- Wernbom, M.; Paulsen, G.; Bjørnsen, T.M.; Cumming, K.; Raastad, T. Risk of muscle damage with blood flow-restricted exercise should not be overlooked. Clin. J. Sport Med. 2021, 31, 223–224. [Google Scholar] [CrossRef] [PubMed]
- Renzi, C.P.; Tanaka, H.; Sugawara, J. Effects of leg blood flow restriction during walking on cardiovascular function. Med. Sci. Sports Exerc. 2010, 42, 726–732. [Google Scholar] [CrossRef] [PubMed]
- Pearson, S.J.; Hussain, S.R. A review on the mechanisms of blood-flow restriction resistance training-induced muscle hyper-trophy. Sports Med. 2015, 45, 187–200. [Google Scholar] [CrossRef] [PubMed]
- Kawada, S.; Ishii, N. Skeletal muscle hypertrophy after chronic restriction of venous blood flow in rats. Med. Sci. Sports Exerc. 2005, 37, 1144–1150. [Google Scholar] [CrossRef] [PubMed]
- Pope, Z.K.; Willardson, J.M.; Schoenfeld, B.J. Exercise and blood flow restriction. J. Strength Cond. Res. 2013, 27, 2914–2926. [Google Scholar] [CrossRef]
- Yasuda, T.; Brechue, W.F.; Fujita, T.; Shirakawa, J.; Sato, Y.; Abe, T. Muscle activation during low-intensity muscle contractions with restricted blood flow. J. Sports Sci. 2009, 27, 479–489. [Google Scholar] [CrossRef]
- Yasuda, T.; Loenneke, J.; Ogasawara, R.; Abe, T. Influence of continuous or intermittent blood flow restriction on muscle activation during low-intensity multiple sets of resistance exercise. Acta Physiol. Hung. 2013, 100, 419–426. [Google Scholar] [CrossRef]
- Laurentino, G.C.; Ugrinowitsch, C.; Roschel, H.; Aoki, M.S.; Soares, A.G.; Neves, M.; Aihara, A.Y.; Fernandes, A.D.R.C.; Tricoli, V. Strength training with blood flow restriction diminishes myostatin gene expression. Med. Sci. Sports Exerc. 2012, 44, 406–412. [Google Scholar] [CrossRef]
- Goldfarb, A.H.; Garten, R.S.; Chee, P.D.M.; Cho, C.; Reeves, G.V.; Hollander, D.B.; Thomas, C.; Aboudehen, K.S.; Francois, M.; Kraemer, R.R. Resistance exercise effects on blood glutathione status and plasma protein carbonyls: Influence of partial vascular occlusion. Eur. J. Appl. Physiol. 2008, 104, 813–819. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ptaszek, B.; Podsiadło, S.; Czerwińska-Ledwig, O.; Zając, B.; Niżankowski, R.; Mika, P.; Teległów, A. The Influence of Interval Training Combined with Occlusion and Cooling on Selected Indicators of Blood, Muscle Metabolism and Oxidative Stress. J. Clin. Med. 2023, 12, 7636. https://doi.org/10.3390/jcm12247636
Ptaszek B, Podsiadło S, Czerwińska-Ledwig O, Zając B, Niżankowski R, Mika P, Teległów A. The Influence of Interval Training Combined with Occlusion and Cooling on Selected Indicators of Blood, Muscle Metabolism and Oxidative Stress. Journal of Clinical Medicine. 2023; 12(24):7636. https://doi.org/10.3390/jcm12247636
Chicago/Turabian StylePtaszek, Bartłomiej, Szymon Podsiadło, Olga Czerwińska-Ledwig, Bartosz Zając, Rafał Niżankowski, Piotr Mika, and Aneta Teległów. 2023. "The Influence of Interval Training Combined with Occlusion and Cooling on Selected Indicators of Blood, Muscle Metabolism and Oxidative Stress" Journal of Clinical Medicine 12, no. 24: 7636. https://doi.org/10.3390/jcm12247636
APA StylePtaszek, B., Podsiadło, S., Czerwińska-Ledwig, O., Zając, B., Niżankowski, R., Mika, P., & Teległów, A. (2023). The Influence of Interval Training Combined with Occlusion and Cooling on Selected Indicators of Blood, Muscle Metabolism and Oxidative Stress. Journal of Clinical Medicine, 12(24), 7636. https://doi.org/10.3390/jcm12247636









