Use of Virtual Reality in Patients with Acquired Brain Injury: A Systematic Review
Abstract
:1. Introduction
VR Rehabilitation
2. Materials and Methods
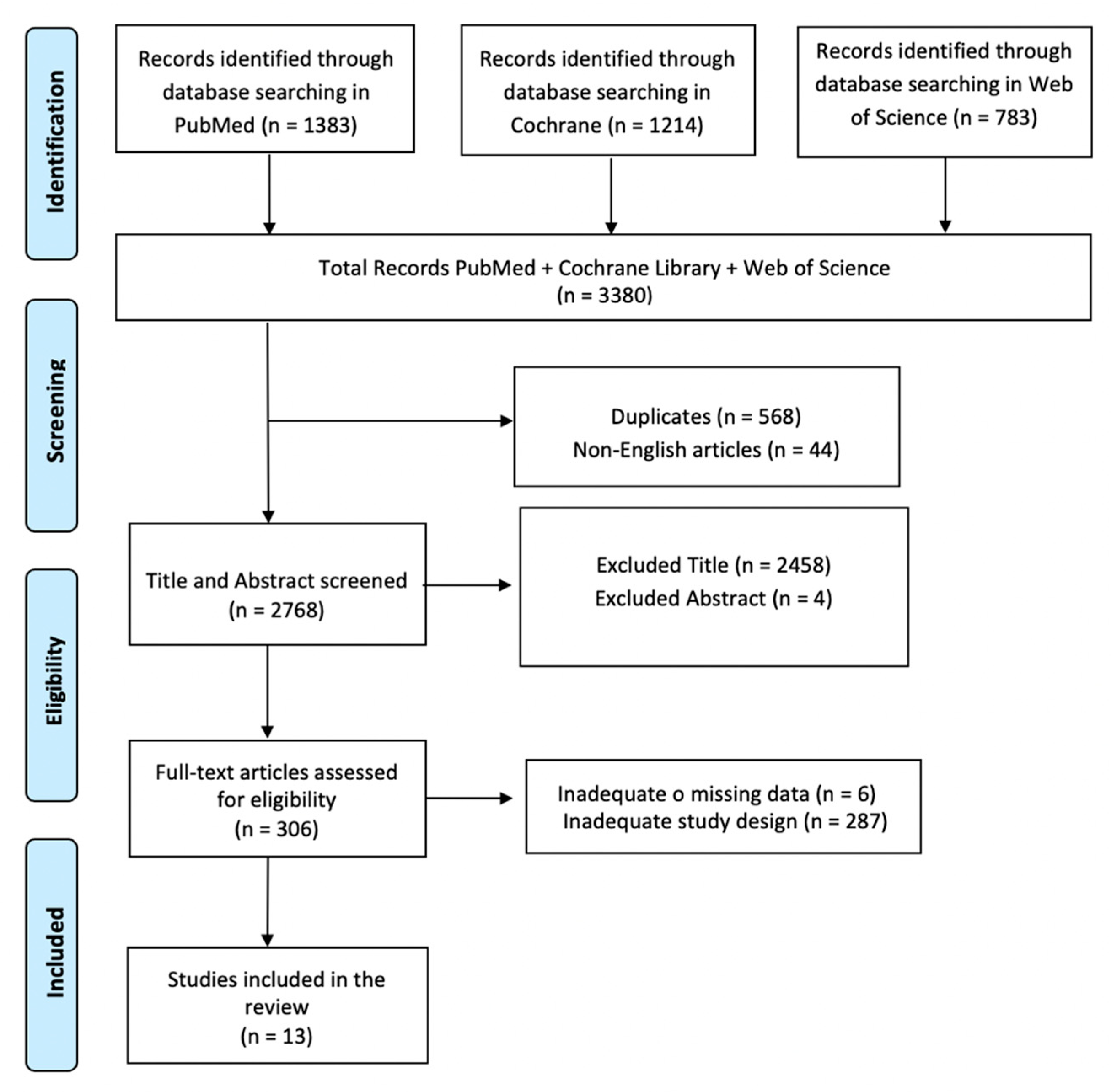
2.1. Search Strategy
2.2. Inclusion Criteria
2.3. Exclusion Criteria
3. Results
VR Rehabilitation in ABI Patients: The Use of Virtual Environments
4. Discussion
Neurorehabilitation with VR
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Neurological Disorders: Public Health Challenges; World Health Organization: Geneva, Switzerland, 2006.
- Tagliaferri, F.; Compagnone, C.; Korsic, M.; Servadei, F.; Kraus, J. A systematic review of brain injury epidemiology in Europe. Acta Neurochir. 2006, 148, 255–268. [Google Scholar] [CrossRef] [PubMed]
- Hayes, R.L.; Jenkins, L.W.; Lyeth, B.G. Neurotransmitter-mediated mechanisms of traumatic brain injury: Acetylcholine and excitatory amino acids. J. Neurotrauma 1992, 9 (Suppl. 1), S173–S187. [Google Scholar] [PubMed]
- Faden, A.I. Pharmacologic treatment of acute traumatic brain injury. JAMA 1996, 276, 569–570. [Google Scholar] [CrossRef] [PubMed]
- Najem, D.; Rennie, K.; Ribecco-Lutkiewicz, M.; Ly, D.; Haukenfrers, J.; Liu, Q.; Nzau, M.; Fraser, D.D.; Bani-Yaghoub, M. Traumatic brain injury: Classification, models, and markers. Biochem. Cell Biol. 2018, 96, 391–406. [Google Scholar] [CrossRef] [PubMed]
- Reis, C.; Wang, Y.; Akyol, O.; Ho, W.M.; Ii, R.A.; Stier, G.; Martin, R.; Zhang, J.H. What’s New in Traumatic Brain Injury: Update on Tracking, Monitoring and Treatment. Int. J. Mol. Sci. 2015, 16, 11903–11965. [Google Scholar] [CrossRef] [PubMed]
- McIntosh, T.K.; Saatman, K.E.; Raghupathi, R.; Graham, D.I.; Smith, D.H.; Lee, V.M.; Trojanowski, J.Q. The Dorothy Russell Memorial Lecture. The molecular and cellular sequelae of experimental traumatic brain injury: Pathogenetic mechanisms. Neuropathol. Appl. Neurobiol. 1998, 24, 251–267. [Google Scholar] [CrossRef] [PubMed]
- Selassie, A.W.; Zaloshnja, E.; Langlois, J.A.; Miller, T.; Jones, P.; Steiner, C. Incidence of long-term disability following traumatic brain injury hospitalization, United States, 2003. J. Head Trauma Rehabil. 2008, 23, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Vespa, P. Traumatic brain injury is a longitudinal disease process. Curr. Opin. Neurol. 2017, 30, 563–564. [Google Scholar] [CrossRef]
- Teasdale, G.; Jennett, B. Assessment of coma and impaired consciousness. A practical scale. Lancet 1974, 2, 81–84. [Google Scholar] [CrossRef]
- Weiss, P.L.; Kizony, R.; Feintuch, U.; Katz, N. Virtual reality in neurorehabilitation. In Textbook of Neural Repair and Rehabilitation; Cambridge University Press: Cambridge, UK, 2006; Volume 51, pp. 182–197. [Google Scholar]
- Kwakkel, G.; van Peppen, R.; Wagenaar, R.C.; Wood Dauphinee, S.; Richards, C.; Ashburn, A.; Miller, K.; Lincoln, N.; Partridge, C.; Wellwood, I.; et al. Effects of augmented exercise therapy time after stroke: A meta-analysis. Stroke 2004, 35, 2529–2539. [Google Scholar] [CrossRef]
- Rizzo, A.A.; Schultheis, M.; Kerns, K.A.; Mateer, C. Analysis of assets for virtual reality applications in neuropsychology. Neuropsychol. Rehabil. 2004, 14, 207–239. [Google Scholar] [CrossRef]
- Knight, R.; Titov, N. Use of Virtual Reality Tasks to Assess Prospective Memory: Applicability and Evidence. Brain Impair. 2009, 10, 3–13. [Google Scholar] [CrossRef]
- Riva, G.; Davide, F.; IJsselsteijn, W.A. Being There: Concepts, Effects and Measurements of User Presence in Synthetic Environments; IOS Press: Amsterdam, The Netherlands, 2003. [Google Scholar]
- Maggio, M.G.; De Luca, R.; Molonia, F.; Porcari, B.; Destro, M.; Casella, C.; Salvati, R.; Bramanti, P.; Calabro, R.S. Cognitive rehabilitation in patients with traumatic brain injury: A narrative review on the emerging use of virtual reality. J. Clin. Neurosci. 2019, 61, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Zanier, E.R.; Zoerle, T.; Di Lernia, D.; Riva, G. Virtual Reality for Traumatic Brain Injury. Front. Neurol. 2018, 9, 345. [Google Scholar] [CrossRef] [PubMed]
- Tate, R.; Kennedy, M.; Ponsford, J.; Douglas, J.; Velikonja, D.; Bayley, M.; Stergiou-Kita, M. INCOG recommendations for management of cognition following traumatic brain injury, part III: Executive function and self-awareness. J. Head Trauma Rehabil. 2014, 29, 338–352. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Abreu, B.C.; Seale, G.S.; Masel, B.; Christiansen, C.H.; Ottenbacher, K.J. A virtual reality environment for evaluation of a daily living skill in brain injury rehabilitation: Reliability and validity. Arch. Phys. Med. Rehabil. 2003, 84, 1118–1124. [Google Scholar] [CrossRef]
- Hanten, G.; Cook, L.; Orsten, K.; Chapman, S.B.; Li, X.; Wilde, E.A.; Schnelle, K.P.; Levin, H.S. Effects of traumatic brain injury on a virtual reality social problem solving task and relations to cortical thickness in adolescence. Neuropsychologia 2011, 49, 486–497. [Google Scholar] [CrossRef]
- Bruschetta, R.; Maggio, M.G.; Naro, A.; Ciancarelli, I.; Morone, G.; Arcuri, F.; Tonin, P.; Tartarisco, G.; Pioggia, G.; Cerasa, A.; et al. Gender Influences Virtual Reality-Based Recovery of Cognitive Functions in Patients with Traumatic Brain Injury: A Secondary Analysis of a Randomized Clinical Trial. Brain Sci. 2022, 12, 491. [Google Scholar] [CrossRef]
- Grealy, M.A.; Johnson, D.A.; Rushton, S.K. Improving cognitive function after brain injury: The use of exercise and virtual reality. Arch. Phys. Med. Rehabil. 1999, 80, 661–667. [Google Scholar] [CrossRef]
- Zhang, L.; Abreu, B.C.; Masel, B.; Scheibel, R.S.; Christiansen, C.H.; Huddleston, N.; Ottenbacher, K.J. Virtual reality in the assessment of selected cognitive function after brain injury. Am. J. Phys. Med. Rehabil. 2001, 80, 597–604. [Google Scholar] [CrossRef]
- Calabrò, R.S.; Bonanno, M.; Torregrossa, W.; Cacciante, L.; Celesti, A.; Rifici, C.; Tonin, P.; De Luca, R.; Quartarone, A. Benefits of Telerehabilitation for Patients with Severe Acquired Brain Injury: Promising Results from a Multicenter Randomized Controlled Trial Using Nonimmersive Virtual Reality. J. Med. Internet Res. 2023, 25, e45458. [Google Scholar] [CrossRef] [PubMed]
- Canty, A.L.; Fleming, J.; Patterson, F.; Green, H.J.; Man, D.; Shum, D.H. Evaluation of a virtual reality prospective memory task for use with individuals with severe traumatic brain injury. Neuropsychol. Rehabil. 2014, 24, 238–265. [Google Scholar] [CrossRef] [PubMed]
- De Luca, R.; Maggio, M.G.; Maresca, G.; Latella, D.; Cannavò, A.; Sciarrone, F.; Lo Voi, E.; Accorinti, M.; Bramanti, P.; Calabrò, R.S. Improving Cognitive Function after Traumatic Brain Injury: A Clinical Trial on the Potential Use of the Semi-Immersive Virtual Reality. Behav. Neurol. 2019, 2019, 9268179. [Google Scholar] [CrossRef] [PubMed]
- Man, D.W.; Poon, W.S.; Lam, C. The effectiveness of artificial intelligent 3-D virtual reality vocational problem-solving training in enhancing employment opportunities for people with traumatic brain injury. Brain Inj. 2013, 27, 1016–1025. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.Y.; Yi, S.H.; Ao, L.; Tang, X.; Xu, X.; Shim, D.; Yoo, B.; Park, E.S.; Rha, D.W. Virtual reality rehabilitation in children with brain injury: A randomized controlled trial. Dev. Med. Child. Neurol. 2021, 63, 480–487. [Google Scholar] [CrossRef] [PubMed]
- Yip, B.C.; Man, D.W. Virtual reality-based prospective memory training program for people with acquired brain injury. NeuroRehabilitation 2013, 32, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, A.B.; Passos, J.O.; Brito, D.P.; Campos, T.F. Comparison of the immediate effect of the training with a virtual reality game in stroke patients according side brain injury. NeuroRehabilitation 2014, 35, 39–45. [Google Scholar] [CrossRef]
- Besnard, J.; Richard, P.; Banville, F.; Nolin, P.; Aubin, G.; Le Gall, D.; Richard, I.; Allain, P. Virtual reality and neuropsychological assessment: The reliability of a virtual kitchen to assess daily-life activities in victims of traumatic brain injury. Appl. Neuropsychol. Adult. 2016, 23, 223–235. [Google Scholar] [CrossRef]
- Pugnetti, L.; Mendozzi, L.; Motta, A.; Cattaneo, A.; Barbieri, E.; Brancotti, A. Evaluation and retraining of adults’ cognitive impairment: Which role for virtual reality technology? Comput. Biol. Med. 1995, 25, 213–227. [Google Scholar] [CrossRef]
- Rose, F.D.; Attree, E.A.; Johnson, D.A. Virtual reality: An assistive technology in neurological rehabilitation. Curr. Opin. Neurol. 1996, 9, 461–467. [Google Scholar] [CrossRef]
- Christiansen, C.; Abreu, B.; Ottenbacher, K.; Huffman, K.; Masel, B.; Culpepper, R. Task performance in virtual environments used for cognitive rehabilitation after traumatic brain injury. Arch. Phys. Med. Rehabil. 1998, 79, 888–892. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Ku, J.; Cho, W.; Hahn, W.Y.; Kim, I.Y.; Lee, S.M.; Kang, Y.; Kim, D.Y.; Yu, T.; Wiederhold, B.K.; et al. A virtual reality system for the assessment and rehabilitation of the activities of daily living. Cyberpsychol. Behav. 2003, 6, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Dawson, D.R.; Chipman, M. The disablement experienced by traumatically brain-injured adults living in the community. Brain Inj. 1995, 9, 339–353. [Google Scholar] [CrossRef] [PubMed]
- Giovannetti, T.; Schwartz, M.F.; Buxbaum, L.J. The Coffee Challenge: A new method for the study of everyday action errors. J. Clin. Exp. Neuropsychol. 2007, 7, 690–705. [Google Scholar] [CrossRef] [PubMed]
- Corregidor-Sánchez, A.I.; Segura-Fragoso, A.; Criado-Álvarez, J.J.; Rodríguez-Hernández, M.; Mohedano-Moriano, A.; Polonio-López, B. Effectiveness of Virtual Reality Systems to Improve the Activities of Daily Life in Older People. Int. J. Environ. Res. Public Health 2020, 17, 6283. [Google Scholar] [CrossRef]
- Maas, A.I.R.; Menon, D.K.; Adelson, P.D.; Andelic, N.; Bell, M.J.; Belli, A.; Bragge, P.; Brazinova, A.; Büki, A.; Chesnut, R.M.; et al. Traumatic brain injury: Integrated approaches to improve prevention, clinical care, and research. Lancet Neurol. 2017, 16, 987–1048. [Google Scholar] [CrossRef] [PubMed]
- Parsons, T.D. Virtual Reality for Enhanced Ecological Validity and Experimental Control in the Clinical, Affective and Social Neurosciences. Front. Hum. Neurosci. 2015, 9, 660. [Google Scholar] [CrossRef] [PubMed]
- Orihuela-Espina, F.; Fernández del Castillo, I.; Palafox, L.; Pasaye, E.; Sánchez-Villavicencio, I.; Leder, R.; Franco, J.H.; Sucar, L.E. Neural reorganization accompanying upper limb motor rehabilitation from stroke with virtual reality-based gesture therapy. Top. Stroke Rehabil. 2013, 20, 197–209. [Google Scholar] [CrossRef]
- Newcombe, V.F.; Outtrim, J.G.; Chatfield, D.A.; Manktelow, A.; Hutchinson, P.J.; Coles, J.P.; Williams, G.B.; Sahakian, B.J.; Menon, D.K. Parcellating the neuroanatomical basis of impaired decision-making in traumatic brain injury. Brain 2011, 134, 759–768. [Google Scholar] [CrossRef]
- Kinnunen, K.M.; Greenwood, R.; Powell, J.H.; Leech, R.; Hawkins, P.C.; Bonnelle, V.; Patel, M.C.; Counsell, S.J.; Sharp, D.J. White matter damage and cognitive impairment after traumatic brain injury. Brain 2011, 134, 449–463. [Google Scholar] [CrossRef]
- Knight, C.; Alderman, N.; Burgess, P.W. Development of a simplifiedversion of the multiple errands test for use in hospital settings. Neuropsychol. Rehabil. 2002, 12, 231–255. [Google Scholar] [CrossRef]
- Morone, G.; Paolucci, S.; Cherubini, A.; De Angelis, D.; Venturiero, V.; Coiro, P.; Iosa, M. Robot-assisted gait training for stroke patients: Current state of the art and perspectives of robotics. Neuropsychiatr. Dis. Treat. 2017, 13, 1303–1311. [Google Scholar] [CrossRef] [PubMed]
- Mukamel, R.; Ekstrom, A.D.; Kaplan, J.; Iacoboni, M.; Fried, I. Single-neuron responses in humans during execution and observation of actions. Curr. Biol. 2010, 20, 750–756. [Google Scholar] [CrossRef] [PubMed]
- Ustinova, K.I.; Perkins, J.; Leonard, W.A.; Hausbeck, C.J. Virtual reality game-based therapy for treatment of postural and co-ordination abnormalities secondary to TBI: A pilot study. Brain Inj. 2014, 28, 486–495. [Google Scholar] [CrossRef] [PubMed]
- Glueck, A.C.; Han, D.Y. Improvement potentials in balance and visuo-motor reaction time after mixed reality action game play: A pilot study. Virtual Real. 2019, 24, 223–229. [Google Scholar] [CrossRef]
- Fusco, A.; Giovannini, S.; Castelli, L.; Coraci, D.; Gatto, D.M.; Reale, G.; Pastorino, R.; Padua, L. Virtual Reality and Lower Limb Rehabilitation: Effects on Motor and Cognitive Outcome-A Crossover Pilot Study. J. Clin. Med. 2022, 11, 2300. [Google Scholar] [CrossRef]
- Cano Porras, D.; Siemonsma, P.; Inzelberg, R.; Zeilig, G.; Plotnik, M. Advantages of virtual reality in the rehabilitation of balance and gait: Systematic review. Neurology 2018, 90, 1017–1025. [Google Scholar] [CrossRef]
- Kleim, J.A.; Jones, T.A. Principles of experience-dependent neural plasticity: Implications for rehabilitation after brain damage. J. Speech Lang. Hear. Res. 2008, 51, S225–S239. [Google Scholar] [CrossRef]
- Kelley, M.S.; Steward, O. Injury-induced physiological events that may modulate gene expression in neurons and glia. Rev. Neurosci. 1997, 8, 147–177. [Google Scholar] [CrossRef]
| Author | Aim | Study Design/Intervention | Treatment Period | Sample Size | Outcomes Measures | Main Findings |
|---|---|---|---|---|---|---|
| Zang et al., 2022 [19] | To establish the stability and validity of information collected from persons with TBI in a virtual reality environment. | Prospective correlation design to examine 3-week test-retest results for equivalence reliability between computer-simulated and natural environments. | 3 weeks. | 54 patients with TBI who received rehabilitation services and who were at different stages of recovery. | Time and error in task completion using a virtual reality assessment, actual kitchen performance (analogous to the virtual reality environment), an OT assessment, and neuropsychological testing | The VR system displays adequate reliability and validity as an assessment method for individuals with brain injury. |
| Hanten et al., 2011 [20] | To determine the effects of TBI on virtual reality social problem-solving tasks and relations to cortical thickness in adolescence. | A comparative study. | Not specified. | 28 youths ages 12–19 years (15 with moderate to severe TBI, 13 uninjured). | Naturalistic, computerized VR version of the Interpersonal Negotiations Strategy interview. | Adolescents with TBI were significantly impaired in the summarized VR-SPS score. Significant group differences were strongest and most consistent in problem definition and outcome scores. |
| Bruschetta et al., 2022 [21] | To assess whether demographic and clinical variables might be associated with recovery of cognitive function in TBI patients after well-validated VR training. | A secondary analysis of data from a prospective randomized controlled trial. | From January 2016 to December 2018. | 100 patients with TBI. | All patients were assessed before and after the end of training with a complete neuropsychological battery. | VR-induced improvement in mood, cognitive flexibility, and selective attention was influenced by sex. Women who had participated in VR training showed better cognitive recovery. |
| Grealy et al., 1999 [22] | Evaluation of the impact of exercise and VR on cognitive rehabilitation of individuals with TBI. | Comparative study. | 4-week program. | A consecutive sample of 13 suitable TBI adults. | Tests on attention, information processing, learning, and memory. Reaction and movement times. | Training in a virtual environment offers the potential for a significant improvement in cognitive functions. |
| Zhang et al., 2001 [23] | Assessment of selected cognitive functions of individuals with TBI using a computer-simulated virtual reality environment. | A clinical trial. | Ranged from 14 to 5061 days. | 30 patients with brain injury and 30 volunteers without brain injury. | The assessment was based on the number of correct answers and the time taken to complete everyday tasks. | A computer-generated VR environment provides a reproducible tool for assessing selected cognitive functions and can be used as an adjunct to traditional rehabilitation assessment in individuals with ABI. |
| Calabrò et al., 2023 [24] | Testing the efficacy of advanced training with a non-immersive VRRS to improve functional outcomes in patients with SABI. | Multicenter randomized controlled trial. | From October 2018 to August 2022. | 40 patients with SABI and their 40 caregivers visiting 2 Italian rehabilitation centers were enrolled in the study protocol and randomized into 2 groups. | BI, TS, MAS, MoCa, FAB, BDI-II, SF-36, and PGWBI. | The VRRS is a suitable alternative or complementary tool, or both, to improve motor and cognitive outcomes and reduce behavioral changes in patients with SABI, which also positively impacts managing caregiver burden by alleviating stress and promoting positive aspects of care. |
| Canty et al., 2014 [25] | Evaluation of the sensitivity, convergent validity, and ecological validity of a newly developed PM task in virtual reality for use with individuals with TBI. | A comparative study. | The total duration of administration was about two hours, with some participants being tested in two sessions if they were prone to fatigue. | 30 individuals with TBI and 24 uninjured adults matched on age, sex, and education level were administered. | The VRST, a lexical decision PM task, an index of task-friendliness, and a cognitive assessment battery. | Performance on the VRST significantly predicted caregiver ratings of patients’ occupational activities and independent living skills. |
| De Luca et al., 2019 [26] | Evaluation of the effects of VR training BTs-N on the recovery of cognitive functions in subjects with TBI using the interactive semi-immersive program. | A randomized controlled trial. | 8 weeks. | 100 patients with TBI. | MoCA, HRS-D, HRS-A, FAB, WT, VS, TMT. | VRTG and TCRG showed significant improvement in cognitive function and mood, but only VRTG showed significant improvement in cognitive flexibility and shifting abilities as well as selective attention. |
| Man et al., 2013 [27] | Investigating the effectiveness of an artificial intelligence-based VR vocational problem-solving skills training program to improve employment opportunities for people with TBI. | A randomized controlled trial. | 12 sessions of 20–25 min, with 3 follow-ups (1 month, 3 months, 6 months). | 40 patients with brain injury. | WCST, TOL, and VCRS. | An improvement in selective memory processes and the perception of memory function was observed. A cross-group comparison showed that the VR group performed better on objective and subjective outcome measures than the therapist-led group and achieved better occupational outcomes. |
| Choi et al., 2021 [28] | Investigating the efficacy of a VR rehabilitation system with wearable multi-inertial sensors to improve upper limb function in children with brain injury. | Randomized controlled trial. | 4 weeks. | 80 children (39 boys, 41 girls) with brain injury including cerebral palsy aged 3 to 16 years. | Both functional and kinematic assessments were performed for all patients at baseline (within 72 h before intervention), at the end of the fourth week intervention. | Both virtual reality rehabilitation and conventional occupational therapy were effective in upper limb training. VRT was superior in improving dexterity, performance of activities of daily living, and active supination movement of the forearm. The effect of VRT was significant in children with more severe motor impairments. |
| Yip et al., 2013 [29] | Determining the effectiveness of the VRPM training program for everyday PM compared to controls. | Randomized controlled trial. | 12 sessions VRPM training program. It was conducted twice a week and each session lasted about 30 to 45 min (depending on the difficulty of the tasks and the abilities of the individual participants). | 37 subjects. | MMSE-CV was used to screen out those with severe cognitive impairment. TONI-3 was developed to be a non-verbal abstract problem-solving test. and SADI-CV. | Both VR-based and real PM results showed significantly better changes in cognitive attributes such as frontal lobe functions and semantic fluency. VR-based training can be well accepted by ABI patients. |
| Fernandes et al., 2014 [30] | Comparison of the immediate effect of training with a virtual reality game in stroke patients depending on the side of the brain injury. | Comparative study. | Two series of 10 tries of 45 s, with 15 min rest between them, a total of 30 min. | The participants included 20 patients (10 right brain injury), mean age of 50.6 ± 9.2 years, and 20 healthy subjects of 50.9 ± 8.8 years. | All participants performed a kinematic evaluation of drinking a cup of water before and after training with the XBOX 360 Kinect® (Microsoft, Redmond, WA, USA) table tennis game, in two series of 10 trials of 45 s, with 15 min rest in between, for a total of 30 min. | Patients with a right hemisphere injury responded better to the VR game, indicating the introduction of new treatment techniques to promote neurorehabilitation. |
| Besnard et al., 2016 [31] | To examine the use of the NI-VCT for IADL assessment in patients with TBI. | A quasi-experimental research design. | Two training sessions on using the virtual environment before the test session. The training sessions lasted until the participants were familiar with the devices (maximum 10 min). | 19 Patients with TBI and 24 healthy controls. | TMT, MCST, TOL, and ST. | The virtual kitchen is a valid tool for the assessment of IADL in patients who have suffered from TBI. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calderone, A.; Carta, D.; Cardile, D.; Quartarone, A.; Rifici, C.; Calabrò, R.S.; Corallo, F. Use of Virtual Reality in Patients with Acquired Brain Injury: A Systematic Review. J. Clin. Med. 2023, 12, 7680. https://doi.org/10.3390/jcm12247680
Calderone A, Carta D, Cardile D, Quartarone A, Rifici C, Calabrò RS, Corallo F. Use of Virtual Reality in Patients with Acquired Brain Injury: A Systematic Review. Journal of Clinical Medicine. 2023; 12(24):7680. https://doi.org/10.3390/jcm12247680
Chicago/Turabian StyleCalderone, Andrea, Diamante Carta, Davide Cardile, Angelo Quartarone, Carmela Rifici, Rocco Salvatore Calabrò, and Francesco Corallo. 2023. "Use of Virtual Reality in Patients with Acquired Brain Injury: A Systematic Review" Journal of Clinical Medicine 12, no. 24: 7680. https://doi.org/10.3390/jcm12247680





