The Relationship between Pulmonary Artery Pressure and Mortality in Type 2 Diabetes: A Fremantle Diabetes Study Phase II and National Echocardiographic Database of Australia Data Linkage Study
Abstract
:1. Introduction
2. Materials and Methods
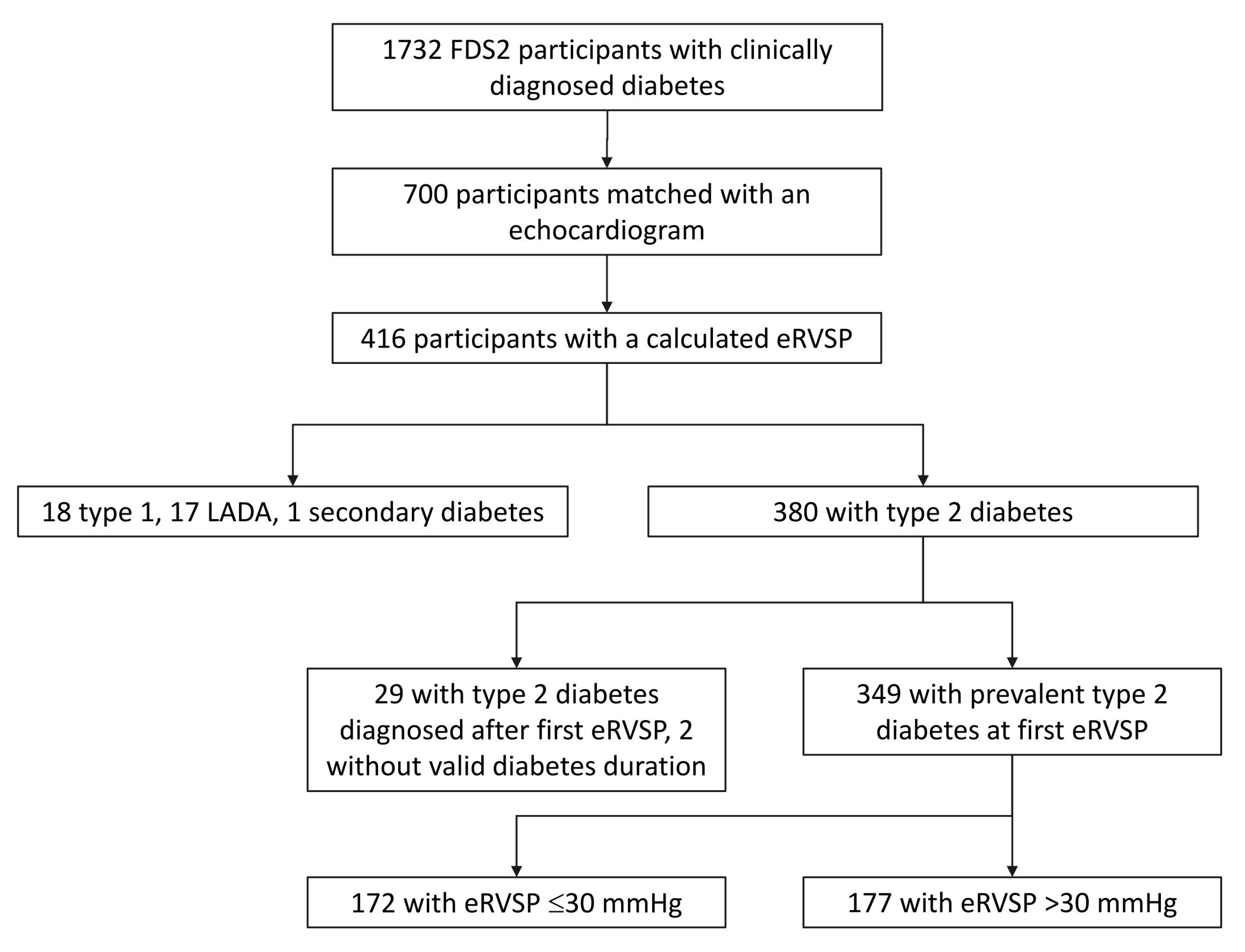
2.1. Study Site, Participants and Approvals
2.2. Clinical Assessment
2.3. Echocardiography Database and Parameters
2.4. Data Linkage
2.5. Ascertainment of Mortality and Charlson Comorbidity Index
2.6. Statistical Analysis
3. Results
3.1. Participant Characteristics
3.2. Estimated Right Ventricular Systolic Pressure All-Cause Mortality Threshold
3.3. Predictors of Mortality
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Davis, W.A.; Gregg, E.W.; Davis, T.M.E. Temporal Trends in Cardiovascular Complications in People With or Without Type 2 Diabetes: The Fremantle Diabetes Study. J. Clin. Endocrinol. Metab. 2020, 105, e2471–e2482. [Google Scholar] [CrossRef] [PubMed]
- Gregg, E.W.; Cheng, Y.J.; Srinivasan, M.; Lin, J.; Geiss, L.S.; Albright, A.L.; Imperatore, G. Trends in cause-specific mortality among adults with and without diagnosed diabetes in the USA: An epidemiological analysis of linked national survey and vital statistics data. Lancet 2018, 391, 2430–2440. [Google Scholar] [CrossRef] [PubMed]
- Harding, J.L.; Shaw, J.E.; Peeters, A.; Davidson, S.; Magliano, D.J. Age-Specific Trends From 2000–2011 in All-Cause and Cause-Specific Mortality in Type 1 and Type 2 Diabetes: A Cohort Study of More Than One Million People. Diabetes Care 2016, 39, 1018–1026. [Google Scholar] [CrossRef] [PubMed]
- Luk, A.O.; Hui, E.M.; Sin, M.-C.; Yeung, C.-Y.; Chow, W.-S.; Ho, A.Y.; Hung, H.-F.; Kan, E.; Ng, C.-M.; So, W.-Y.; et al. Declining Trends of Cardiovascular-Renal Complications and Mortality in Type 2 Diabetes: The Hong Kong Diabetes Database. Diabetes Care 2017, 40, 928–935. [Google Scholar] [CrossRef] [PubMed]
- Olafsdottir, E.; Aspelund, T.; Sigurdsson, G.; Benediktsson, R.; Thorsson, B.; Harris, T.B.; Launer, L.J.; Eiriksdottir, G.; Gudnason, V. Similar decline in mortality rate of older persons with and without type 2 diabetes between 1993 and 2004 the Icelandic population-based Reykjavik and AGES-Reykjavik cohort studies. BMC Public Health 2013, 13, 36. [Google Scholar] [CrossRef]
- Imperatore, G.; Cadwell, B.L.; Geiss, L.; Saadinne, J.B.; Williams, D.E.; Ford, E.S.; Thompson, T.J.; Narayan, K.M.V.; Gregg, E.W. Thirty-year trends in cardiovascular risk factor levels among US adults with diabetes: National Health and Nutrition Examination Surveys, 1971–2000. Am. J. Epidemiol. 2004, 160, 531–539. [Google Scholar] [CrossRef]
- Samaranayaka, S.; Gulliford, M.C. Trends in cardiovascular risk factors among people with diabetes in a population based study, Health Survey for England 1994–2009. Prim. Care Diabetes 2013, 7, 193–198. [Google Scholar] [CrossRef]
- Sun, X.; Du, T. Trends in cardiovascular risk factors among U.S. men and women with and without diabetes, 1988–2014. BMC Public Health 2017, 17, 893. [Google Scholar] [CrossRef]
- Harding, J.L.; Pavkov, M.E.; Magliano, D.J.; Shaw, J.E.; Gregg, E.W. Global trends in diabetes complications: A review of current evidence. Diabetologia 2019, 62, 3–16. [Google Scholar] [CrossRef]
- Rose-Jones, L.J.; McLaughlin, V.V. Pulmonary hypertension: Types and treatments. Curr. Cardiol. Rev. 2015, 11, 73–79. [Google Scholar] [CrossRef]
- Simonneau, G.; Montani, D.; Celermajer, D.S.; Denton, C.P.; Gatzoulis, M.A.; Krowka, M.; Williams, P.G.; Souza, R. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur. Respir. J. 2019, 53, 1801913. [Google Scholar] [CrossRef]
- Humbert, M.; Kovacs, G.; Hoeper, M.M.; Badagliacca, R.; Berger, R.M.F.; Brida, M.; Carlsen, J.; Coats, A.J.S.; Escribano-Subias, P.; Ferrari, P.; et al. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur. Heart J. 2022, 43, 3618–3731. [Google Scholar] [CrossRef]
- Galiè, N.; Humbert, M.; Vachiery, J.L.; Gibbs, S.; Lang, I.; Torbicki, A.; Simonneau, G.; Peacock, A.; Vonk Noordegraaf, A.; Beghetti, M.; et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Respir J. 2015, 46, 903–975. [Google Scholar]
- Stewart, S.; Chan, Y.-K.; Playford, D.; Strange, G.A. Mild pulmonary hypertension and premature mortality among 154,956 men and women undergoing routine echocardiography. Eur. Respir. J. 2021, 2021, 2100832. [Google Scholar] [CrossRef]
- Strange, G.; Stewart, S.; Celermajer, D.S.; Prior, D.; Scalia, G.M.; Marwick, T.H.; Gabbay, E.; Ilton, M.; Joseph, M.; Codde, J.; et al. Threshold of Pulmonary Hypertension Associated With Increased Mortality. J. Am. Coll. Cardiol. 2019, 73, 2660–2672. [Google Scholar] [CrossRef]
- Xue, L.; Yang, Y.; Sun, B.; Liu, B.; Zeng, Q.; Xiong, C. Mildly Elevated Pulmonary Arterial Pressure Is Associated With a High Risk of Progression to Pulmonary Hypertension and Increased Mortality: A Systematic Review and Meta-Analysis. J. Am. Heart Assoc. 2021, 10, e018374. [Google Scholar] [CrossRef]
- Movahed, M.R.; Hashemzadeh, M.; Jamal, M.M. The prevalence of pulmonary embolism and pulmonary hypertension in patients with type II diabetes mellitus. Chest 2005, 128, 3568–3571. [Google Scholar] [CrossRef]
- Vrigkou, E.; Vassilatou, E.; Dima, E.; Langleben, D.; Kotanidou, A.; Tzanela, M. The Role of Thyroid Disorders, Obesity, Diabetes Mellitus and Estrogen Exposure as Potential Modifiers for Pulmonary Hypertension. J. Clin. Med. 2022, 11, 921. [Google Scholar] [CrossRef]
- Trammell, A.W.; Hemnes, A.R.; Tseng, V.; Shah, A.J.; Phillips, L.S.; Hart, C.M. Influence of Body Weight and Diabetes Mellitus in Patients With Pulmonary Hypertension. Am. J. Cardiol. 2020, 134, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Trammell, A.W.; Shah, A.J.; Phillips, L.S.; Michael Hart, C. Mortality in US veterans with pulmonary hypertension: A retrospective analysis of survival by subtype and baseline factors. Pulm. Circ. 2019, 9, 2045894019825763. [Google Scholar] [CrossRef]
- Nundlall, N.; Playford, D.; Strange, G.; Davis, T.M.E.; Davis, W.A. Prevalence, Incidence and Associates of Pulmonary Hypertension Complicating Type 2 Diabetes: Insights from the Fremantle Diabetes Study Phase 2 and National Echocardiographic Database of Australia. J. Clin. Med. 2021, 10, 4503. [Google Scholar] [CrossRef]
- Davis, T.M.; Bruce, D.G.; Davis, W.A. Cohort Profile: The Fremantle Diabetes Study. Int. J. Epidemiol. 2013, 42, 412–421. [Google Scholar] [CrossRef]
- Strange, G.; Celermajer, D.S.; Marwick, T.; Prior, D.; Ilton, M.; Codde, J.; Scalia, G.M.; Stewart, S.; Bulsara, M.; Gabbay, E.; et al. The National Echocardiography Database Australia (NEDA): Rationale and methodology. Am. Heart J. 2018, 204, 186–189. [Google Scholar] [CrossRef]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gotzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. J. Clin. Epidemiol. 2008, 61, 344–349. [Google Scholar] [CrossRef]
- Holman, C.D.; Bass, A.J.; Rosman, D.L.; Smith, M.B.; Semmens, J.B.; Glasson, E.J.; Brook, E.L.; Trutwein, B.; Rouse, I.L.; Watson, C.R.; et al. A decade of data linkage in Western Australia: Strategic design, applications and benefits of the WA data linkage system. Aust. Health Rev. A Publ. Aust. Hosp. Assoc. 2008, 32, 766–777. [Google Scholar] [CrossRef]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- King, N.E.; Brittain, E. Emerging therapies: The potential roles SGLT2 inhibitors, GLP1 agonists, and ARNI therapy for ARNI pulmonary hypertension. Pulm. Circ. 2022, 12, e12028. [Google Scholar] [CrossRef]
- Kayano, H.; Koba, S.; Hirano, T.; Matsui, T.; Fukuoka, H.; Tsuijita, H.; Tsukamoto, S.; Hayashi, T.; Toshida, T.; Watanabe, N.; et al. Dapagliflozin Influences Ventricular Hemodynamics and Exercise-Induced Pulmonary Hypertension in Type 2 Diabetes Patients- A Randomized Controlled Trial. Circ. J. 2020, 84, 1807–1817. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, B.; Luu, A.Z.; Luu, V.Z.; Kabir, M.G.; Pan, Y.; Teoh, H.; Quan, A.; Sabongui, S.; Al-Omran, M.; Bhatt, D.L.; et al. The SGLT2 inhibitor empagliflozin reduces mortality and prevents progression in experimental pulmonary hypertension. Biochem. Biophys. Res. Commun. 2020, 524, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Anker, S.D.; Butler, J.; Filippatos, G.; Ferreira, J.P.; Bocchi, E.; Böhm, M.; Brunner–La Rocca, H.-P.; Choi, D.-J.; Chopra, V.; Chuquiure-Valenzuela, E.; et al. Empagliflozin in Heart Failure with a Preserved Ejection Fraction. N. Engl. J. Med. 2021, 385, 1451–1461. [Google Scholar] [CrossRef]
- Kosiborod, M.N.; Abildstrøm, S.Z.; Borlaug, B.A.; Butler, J.; Rasmussen, S.; Davies, M.; Hovingh, G.K.; Kitzman, D.W.; Lindegaard, M.L.; Møller, D.V.; et al. Semaglutide in Patients with Heart Failure with Preserved Ejection Fraction and Obesity. N. Engl. J. Med. 2023, 389, 1069–1084. [Google Scholar] [CrossRef]
- Chubb, S.A.P.; Davis, W.A.; Davis, T.M.E. Serum bicarbonate concentration and the risk of death in type 2 diabetes: The Fremantle Diabetes Study Phase II. Acta Diabetol. 2023, 60, 1333–1342. [Google Scholar] [CrossRef]
- Galasko, G.I.; Lahiri, A.; Senior, R. Portable echocardiography: An innovative tool in screening for cardiac abnormalities in the community. Eur. J. Echocardiogr. 2003, 4, 119–127. [Google Scholar] [CrossRef]
- Barry, T.; Farina, J.M.; Chao, C.-J.; Ayoub, C.; Jeong, J.; Patel, B.N.; Banerjee, I.; Arsanjani, R. The Role of Artificial Intelligence in Echocardiography. J. Imaging 2023, 9, 50. [Google Scholar] [CrossRef]

| All | ≤30 mmHg | >30 to 35 mmHg | >35 to 40 mmHg | >40 mmHg | Trend p-Value | |
|---|---|---|---|---|---|---|
| Number (%) | 349 (100) | 172 (49.3) | 62 (17.8) | 37 (10.6) | 78 (22.3) | |
| Age (years) | 71.6 ± 10.3 | 70.2 ± 10.2 | 70.5 ± 11.0 | 73.2 ± 9.4 | 74.9 ± 9.6 ** | 0.005 |
| Sex (% male) | 47.6 | 50.0 | 37.1 | 35.1 | 56.4 | 0.049 |
| APOE4 genotype (%) | 22.3 | 26.2 | 15.0 | 16.7 | 22.1 | 0.282 |
| Ethnic background (%): | ||||||
| Anglo–Celt | 57.64 | 59.3 | 61.3 | 54.1 | 52.6 | 0.671 |
| Southern European | 12.6 | 12.8 | 8.1 | 10.8 | 16.7 | 0.514 |
| Other European | 5.2 | 6.4 | 3.2 | 5.4 | 3.8 | 0.778 |
| Asian | 4.9 | 5.2 | 6.5 | 2.7 | 3.8 | 0.869 |
| Aboriginal | 7.2 | 4.1 | 8.1 | 13.5 | 10.3 | 0.082 |
| Mixed/other | 12.6 | 12.2 | 12.9 | 13.5 | 12.8 | 0.995 |
| Overseas born (%) | 42.1 | 45.3 | 41.9 | 35.1 | 38.5 | 0.601 |
| Not fluent in English (%) | 11.5 | 14.0 | 8.1 | 5.4 | 11.5 | 0.436 |
| Educated beyond primary level (%) | 86.2 | 89.4 | 85.0 | 86.1 | 79.7 | 0.238 |
| Age at diabetes diagnosis (years) | 57.2 ± 12.3 | 56.3 ± 12.4 | 58.3 ± 13.3 | 58.9 ± 10.9 | 57.2 ± 12.1 | 0.580 |
| Diabetes duration (years) | 13.6 [7.8–20.2] | 12.6 [6.5–20.1] | 12.2 [7.1–17.6] | 13.5 [8.8–18.3] | 18.8 [11.3–22.5] *,†† | 0.007 |
| BMI (kg/m²) | 29.7 ± 6.0 | 29.1 ± 5.9 | 30.2 ± 5.2 | 30.9 ± 7.4 | 29.9 ± 6.3 | 0.363 |
| CCI (%): | *** | <0.001 | ||||
| 0 | 35.0 | 41.9 | 38.7 | 27.0 | 20.5 | |
| 1 or 2 | 33.8 | 36.6 | 32.3 | 35.1 | 28.2 | |
| ≥3 | 31.2 | 21.5 | 29.0 | 37.8 | 51.3 | |
| Nonsinus rhythm ¶ (%) | 22.3 | 16.3 | 19.4 | 21.6 | 38.5 ** | 0.002 |
| LHD (%) | 48.1 | 43.0 | 38.7 | 51.4 | 65.4 *,†† | 0.004 |
| LVDD (cm; n = 248) | 4.7 ± 0.6 | 4.7 ± 0.6 | 4.7 ± 0.5 | 4.7 ± 0.7 | 4.8 ± 0.8 | 0.381 |
| LV mass index (g/m²; n = 189) | 96 (74–123) | 94 (74–119) | 92 (77–111) | 95 (72–126) | 110 (84–145) **,† | 0.002 |
| LVEF (%; n = 271) | 63 [57–67] | 64 [59–67] | 64 [57–69] | 64 [55–71] | 60 [43–66] | 0.064 |
| Mitral E inflow velocity (cm/s; n = 287) | 84 (61–116) | 74 (57–97) | 85 (65–113)* | 85 (60–120) | 111 (82–150) ***,†††,‡‡‡ | <0.001 |
| Mitral A inflow velocity (cm/s; n = 271) | 76 (51–113) | 70 (49–101) | 81 (56–117) | 80 (49–128) | 87 (57–134) ** | 0.003 |
| Mitral inflow E:A ratio (n = 268) | 0.93 (0.63–1.37) | 0.87 (0.63–1.19) | 0.94 (0.67–1.32) | 0.90 (0.63–1.28) | 1.18 (0.71–1.98) ***,†,‡ | <0.001 |
| Septal E/E’ ratio (n = 191) | 12.1 (7.8–18.7) | 10.9 (7.5–15.8) | 10.7 (8.2–14.0) | 11.5 (7.3–18.0) | 18.8 (11.8–30.2) ***,†††,‡‡‡ | <0.001 |
| LA volume index (mL/m²; n = 180) | 32 (22–47) | 30 (21–41) | 29 (21–42) | 30 (21–44) | 44 (30–65) ***,†††,‡‡ | <0.001 |
| All Diabetes Types | Type 2 Diabetes | Type 2 Diabetes with Diabetes Duration ≥0 Years at Time of First Valid eRVSP Measurement | Type 1 Diabetes (All Had Diabetes Duration ≥0 Years at Time of First Valid eRVSP Measurement) | |
|---|---|---|---|---|
| N | 416 | 380 | 349 | 18 |
| AUC (95% CI) | 0.657 (0.602–0.712) | 0.641 (0.583–0.700) | 0.646 (0.586–0.707) | 0.786 (0.569–1.000) |
| p-value | <0.001 | <0.001 | <0.001 | 0.046 |
| Youden’s index | 32.14 | 33.96 | 33.96 | 29.02 |
| Sensitivity | 0.606 | 0.544 | 0.546 | 0.857 |
| Specificity | 0.661 | 0.706 | 0.721 | 0.636 |
| Alive | Deceased | p-Value | |
|---|---|---|---|
| Number (%) | 208 (59.6) | 141 (40.4) | |
| Age (years) | 69.6 ± 10.1 | 74.7 ± 9.8 | <0.001 |
| Sex (% male) | 46.2 | 49.6 | 0.585 |
| APOE4 genotype (%) | 20.8 | 24.6 | 0.430 |
| Ethnic background (%): | |||
| Anglo–Celt | 56.7 | 58.9 | 0.741 |
| Southern European | 13.5 | 11.3 | 0.624 |
| Other European | 4.3 | 6.4 | 0.462 |
| Asian | 5.8 | 3.5 | 0.450 |
| Aboriginal | 6.3 | 8.5 | 0.526 |
| Mixed/other | 13.5 | 11.3 | 0.624 |
| Born in Australia (%) | 43.3 | 40.4 | 0.659 |
| Not fluent in English (%) | 13.0 | 9.2 | 0.308 |
| Educated beyond primary level (%) | 86.7 | 85.4 | 0.751 |
| Age at diabetes diagnosis (years) | 56.4 ± 11.8 | 58.3 ± 13.0 | 0.166 |
| Diabetes duration (years) | 12.5 [6.6–19.2] | 15.6 [10.1–21.5] | 0.002 |
| BMI (kg/m²) | 30.3 ± 6.0 | 28.7 ± 5.9 | 0.023 |
| Charlson Comorbidity Index (%): | <0.001 | ||
| 0 | 45.7 | 19.1 | |
| 1 or 2 | 35.6 | 31.2 | |
| ≥3 | 18.8 | 49.6 | |
| Nonsinus rhythm ¶ (%) | 17.3 | 29.8 | 0.009 |
| LHD (%) | 40.4 | 59.6 | <0.001 |
| eRVSP category (%): | <0.001 | ||
| ≤30 mmHg | 58.7 | 35.5 | |
| >30 to ≤35 mmHg | 17.8 | 17.7 | |
| >35 to ≤40 mmHg | 8.2 | 14.2 | |
| >40 mmHg | 15.4 | 32.6 | |
| LVEDD (cm; n = 249) | 4.6 ± 0.6 | 4.8 ± 0.7 | 0.029 |
| LV mass index (g/m²; n = 189) | 91 (71–117) | 105 (83–133) | <0.001 |
| LVEF (%; n = 272) | 64 [59–68] | 61 [47–66] | 0.005 |
| Mitral E inflow velocity (cm/s; n = 288) | 82 (61–111) | 86 (60–123) | 0.252 |
| Mitral A inflow velocity (cm/s; n = 272) | 73 (50–107) | 81 (54–123) | 0.026 |
| Mitral inflow E/A ratio (n = 269) | 0.93 (0.66–1.32) | 0.93 (0.59–1.47) | 0.999 |
| Septal E/E1 ratio (n = 191) | 11.2 (7.8–16.0) | 14.0 (8.2–24.0) | 0.004 |
| LA volume index (mL/m²; n = 180) | 30 (21–43) | 37 (26–54) | <0.001 |
| Hazard Ratio (95% Confidence Interval) | p-Value | |
|---|---|---|
| Age (increase of 1 year) | 1.07 (1.05, 1.09) | <0.001 |
| Aboriginal descent | 2.35 (1.17, 4.69) | 0.016 |
| CCI ≥ 3 | 3.66 (2.57, 5.21) | <0.001 |
| LHD | 1.93 (1.35, 2.76) | <0.001 |
| eRVSP category: | ||
| <30 mmHg (reference) | 1.00 | |
| >30 to ≤35 mmHg | 1.86 (1.14, 3.03) | 0.013 |
| >35 to ≤40 mmHg | 1.89 (1.10, 3.24) | 0.021 |
| >40 mmHg | 1.98 (1.30, 3.03) | 0.002 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nundlall, N.; Playford, D.; Strange, G.; Davis, T.M.E.; Davis, W.A. The Relationship between Pulmonary Artery Pressure and Mortality in Type 2 Diabetes: A Fremantle Diabetes Study Phase II and National Echocardiographic Database of Australia Data Linkage Study. J. Clin. Med. 2023, 12, 7685. https://doi.org/10.3390/jcm12247685
Nundlall N, Playford D, Strange G, Davis TME, Davis WA. The Relationship between Pulmonary Artery Pressure and Mortality in Type 2 Diabetes: A Fremantle Diabetes Study Phase II and National Echocardiographic Database of Australia Data Linkage Study. Journal of Clinical Medicine. 2023; 12(24):7685. https://doi.org/10.3390/jcm12247685
Chicago/Turabian StyleNundlall, Nishant, David Playford, Geoff Strange, Timothy M. E. Davis, and Wendy A. Davis. 2023. "The Relationship between Pulmonary Artery Pressure and Mortality in Type 2 Diabetes: A Fremantle Diabetes Study Phase II and National Echocardiographic Database of Australia Data Linkage Study" Journal of Clinical Medicine 12, no. 24: 7685. https://doi.org/10.3390/jcm12247685
APA StyleNundlall, N., Playford, D., Strange, G., Davis, T. M. E., & Davis, W. A. (2023). The Relationship between Pulmonary Artery Pressure and Mortality in Type 2 Diabetes: A Fremantle Diabetes Study Phase II and National Echocardiographic Database of Australia Data Linkage Study. Journal of Clinical Medicine, 12(24), 7685. https://doi.org/10.3390/jcm12247685







