Cancer Risk in Patients with Gaucher Disease Using Real-World Data
Abstract
:1. Introduction
2. Methods
2.1. Study Cohort
2.2. Data Extraction
2.3. Statistical Analyses
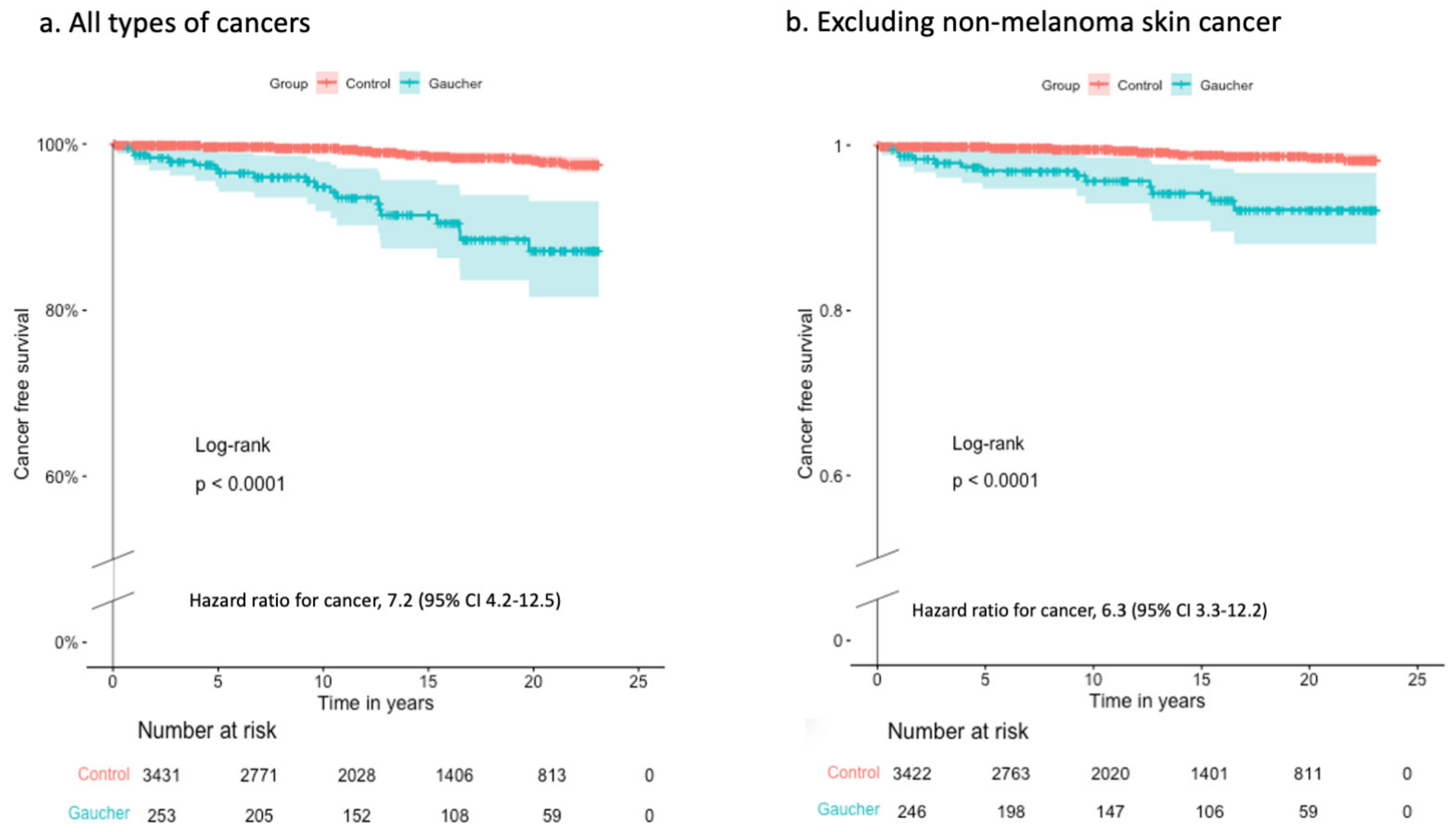
3. Results
3.1. Cancer Incidence before Index Date in Patients with GD Compared to Controls
3.2. Cancer Incidence after Index Date in Patients with GD Compared to Controls
3.3. Cancer-Associated Risk Factors and Screening Tests in Patients with GD Compared to Controls
3.4. Risk Factors for Cancer in Patients with GD
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Revel-Vilk, S.; Szer, J.; Zimran, A. Gaucher disease and related lysosomal storage diseases. In Williams Hematology, 10th ed.; Kaushansky, K., Lichtman, M., Prchal, J., Levi, M., Press, O., Burns, L., Caligiuri, M., Eds.; McGraw-Hill: New York, NY, USA, 2021; pp. 1189–1202. [Google Scholar]
- Castillon, G.; Chang, S.C.; Moride, Y. Global Incidence and Prevalence of Gaucher Disease: A Targeted Literature Review. J. Clin. Med. 2022, 12, 85. [Google Scholar] [CrossRef] [PubMed]
- Schiffmann, R.; Sevigny, J.; Rolfs, A.; Davies, E.H.; Goker-Alpan, O.; Abdelwahab, M.; Vellodi, A.; Mengel, E.; Lukina, E.; Yoo, H.W.; et al. The definition of neuronopathic Gaucher disease. J. Inherit. Metab. Dis. 2020, 43, 1056–1059. [Google Scholar] [CrossRef] [PubMed]
- De Fost, M.; Vom Dahl, S.; Weverling, G.J.; Brill, N.; Brett, S.; Haussinger, D.; Hollak, C.E. Increased incidence of cancer in adult Gaucher disease in Western Europe. Blood Cells Mol. Dis. 2006, 36, 53–58. [Google Scholar] [CrossRef]
- Taddei, T.H.; Kacena, K.A.; Yang, M.; Yang, R.; Malhotra, A.; Boxer, M.; Aleck, K.A.; Rennert, G.; Pastores, G.M.; Mistry, P.K. The underrecognized progressive nature of N370S Gaucher disease and assessment of cancer risk in 403 patients. Am. J. Hematol. 2009, 84, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Zimran, A.; Liphshitz, I.; Barchana, M.; Abrahamov, A.; Elstein, D. Incidence of malignancies among patients with type I Gaucher disease from a single referral clinic. Blood Cells Mol. Dis. 2005, 34, 197–200. [Google Scholar] [CrossRef] [PubMed]
- Choy, F.Y.; Campbell, T.N. Gaucher disease and cancer: Concept and controversy. Int. J. Cell Biol. 2011, 2011, 150450. [Google Scholar] [CrossRef]
- Rosenbloom, B.E.; Weinreb, N.J.; Zimran, A.; Kacena, K.A.; Charrow, J.; Ward, E. Gaucher disease and cancer incidence: A study from the Gaucher Registry. Blood 2005, 105, 4569–4572. [Google Scholar] [CrossRef]
- Landgren, O.; Turesson, I.; Gridley, G.; Caporaso, N.E. Risk of malignant disease among 1525 adult male US Veterans with Gaucher disease. Arch. Intern. Med. 2007, 167, 1189–1194. [Google Scholar] [CrossRef]
- Rosenbloom, B.E.; Cappellini, M.D.; Weinreb, N.J.; Dragosky, M.; Revel-Vilk, S.; Batista, J.L.; Sekulic, D.; Mistry, P.K. Cancer risk and gammopathies in 2123 adults with Gaucher disease type 1 in the International Gaucher Group Gaucher Registry. Am. J. Hematol. 2022, 97, 1337–1347. [Google Scholar] [CrossRef]
- Revel-Vilk, S.; Chodick, G.; Shalev, V.; Lotan, R.; Zarakowska, K.; Gadir, N. Using the Gaucher Earlier Diagnosis Consensus (GED-C) Delphi Score in a Real-World Dataset. Int. J. Transl. Med. 2022, 2, 506–514. [Google Scholar] [CrossRef]
- Anton-Culver, H.; Chang, J.; Bray, F.; Znaor, A.; Stevens, L.; Eser, S.; Silverman, B.; Nimri, O.; Pavlou, P.; Charalambous, H.; et al. Cancer burden in four countries of the Middle East Cancer Consortium (Cyprus; Jordan; Israel; Izmir (Turkey)) with comparison to the United States surveillance; epidemiology and end results program. Cancer Epidemiol. 2016, 44, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Tancredi, S.; Cullati, S.; Chiolero, A. Screening and Surveillance Bias in Cancer. Epidemiologia 2023, 4, 117–120. [Google Scholar] [CrossRef] [PubMed]
- Drucker, A.M.; Li, W.Q.; Savitz, D.A.; Weinstock, M.A.; Han, J.; Li, T.; Qureshi, A.A.; Cho, E. Association Between Health Maintenance Practices and Skin Cancer Risk as a Possible Source of Detection Bias. JAMA Dermatol. 2019, 155, 353–357. [Google Scholar] [CrossRef]
- Levin, T.R.; Corley, D.A.; Jensen, C.D.; Schottinger, J.E.; Quinn, V.P.; Zauber, A.G.; Lee, J.K.; Zhao, W.K.; Udaltsova, N.; Ghai, N.R.; et al. Effects of Organized Colorectal Cancer Screening on Cancer Incidence and Mortality in a Large Community-Based Population. Gastroenterology 2018, 155, 1383–1391.e5. [Google Scholar] [CrossRef] [PubMed]
- Goshen, R.; Mizrahi, B.; Akiva, P.; Kinar, Y.; Choman, E.; Shalev, V.; Sopik, V.; Kariv, R.; Narod, S.A. Predicting the presence of colon cancer in members of a health maintenance organisation by evaluating analytes from standard laboratory records. Br. J. Cancer 2017, 116, 944–950. [Google Scholar] [CrossRef] [PubMed]
- Revel-Vilk, S.; Szer, J.; Zimran, A. Hematological manifestations and complications of Gaucher disease. Expert Rev. Hematol. 2021, 14, 347–354. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, A.; Bransfield, A.; O’Halloran, F.; Mykytiv, V. Calculated Globulin as a potential screening tool for paraproteinemia to aid in the early diagnosis of Multiple Myeloma. Clin. Biochem. 2023, 116, 113–119. [Google Scholar] [CrossRef]
- Nguyen, Y.; Stirnemann, J.; Lautredoux, F.; Cador, B.; Bengherbia, M.; Yousfi, K.; Hamroun, D.; Astudillo, L.; Billette de Villemeur, T.; Brassier, A.; et al. Immunoglobulin Abnormalities in Gaucher Disease: An Analysis of 278 Patients Included in the French Gaucher Disease Registry. Int. J. Mol. Sci. 2020, 21, 1247. [Google Scholar] [CrossRef]
- Andrade-Campos, M.M.; de Frutos, L.L.; Cebolla, J.J.; Serrano-Gonzalo, I.; Medrano-Engay, B.; Roca-Espiau, M.; Gomez-Barrera, B.; Perez-Heredia, J.; Iniguez, D.; Giraldo, P. Identification of risk features for complication in Gaucher’s disease patients: A machine learning analysis of the Spanish registry of Gaucher disease. Orphanet J. Rare Dis. 2020, 15, 256. [Google Scholar] [CrossRef]
- Shiran, A.; Brenner, B.; Laor, A.; Tatarsky, I. Increased risk of cancer in patients with Gaucher disease. Cancer 1993, 72, 219–224. [Google Scholar] [CrossRef]
- Lo, S.M.; Stein, P.; Mullaly, S.; Bar, M.; Jain, D.; Pastores, G.M.; Mistry, P.K. Expanding spectrum of the association between Type 1 Gaucher disease and cancers: A series of patients with up to 3 sequential cancers of multiple types--Correlation with genotype and phenotype. Am. J. Hematol. 2010, 85, 340–345. [Google Scholar] [CrossRef]
- Dubot, P.; Astudillo, L.; Therville, N.; Carrie, L.; Pettazzoni, M.; Cheillan, D.; Stirnemann, J.; Levade, T.; Andrieu-Abadie, N.; Sabourdy, F. Potential Role of Sphingolipidoses-Associated Lysosphingolipids in Cancer. Cancers 2022, 14, 4858. [Google Scholar] [CrossRef] [PubMed]
- Regenboog, M.; van Dussen, L.; Verheij, J.; Weinreb, N.J.; Santosa, D.; Vom Dahl, S.; Haussinger, D.; Muller, M.N.; Canbay, A.; Rigoldi, M.; et al. Hepatocellular carcinoma in Gaucher disease: An international case series. J. Inherit. Metab. Dis. 2018, 41, 819–827. [Google Scholar] [CrossRef] [PubMed]
- Dubot, P.; Astudillo, L.; Therville, N.; Sabourdy, F.; Stirnemann, J.; Levade, T.; Andrieu-Abadie, N. Are Glucosylceramide-Related Sphingolipids Involved in the Increased Risk for Cancer in Gaucher Disease Patients? Review and Hypotheses. Cancers 2020, 12, 475. [Google Scholar] [CrossRef] [PubMed]
- Arends, M.; van Dussen, L.; Biegstraaten, M.; Hollak, C.E. Malignancies and monoclonal gammopathy in Gaucher disease; a systematic review of the literature. Br. J. Haematol. 2013, 161, 832–842. [Google Scholar] [CrossRef] [PubMed]
- Harel, R.; Gavish, I.; Aviv, A.; Greenman Maravi, N.; Trougouboff, P.; Zimran, A.; Revel-Vilk, S. Enzyme replacement therapy leading to improvement in myeloma indices in a patient with concomitant Gaucher disease. Intern. Med. J. 2022, 52, 872–875. [Google Scholar] [CrossRef] [PubMed]
- Watek, M.; Piktel, E.; Wollny, T.; Durnas, B.; Fiedoruk, K.; Lech-Maranda, E.; Bucki, R. Defective Sphingolipids Metabolism and Tumor Associated Macrophages as the Possible Links Between Gaucher Disease and Blood Cancer Development. Int. J. Mol. Sci. 2019, 20, 843. [Google Scholar] [CrossRef]
- Stahl-Meyer, K.; Bilgin, M.; Holland, L.K.K.; Stahl-Meyer, J.; Kirkegaard, T.; Petersen, N.H.T.; Maeda, K.; Jaattela, M. Galactosyl- and glucosylsphingosine induce lysosomal membrane permeabilization and cell death in cancer cells. PLoS ONE 2022, 17, e0277058. [Google Scholar] [CrossRef]
- Cox, T.M.; Rosenbloom, B.E.; Barker, R.A. Gaucher disease and comorbidities: B-cell malignancy and parkinsonism. Am. J. Hematol. 2015, 90 (Suppl. 1), S25–S28. [Google Scholar] [CrossRef]
| Patients with GD | Controls | |
|---|---|---|
| Number | 264 | 3440 |
| Male | 130 (49%) | 1689 (49%) |
| Age at index date *, median (range) | 31 (0–88) | 31 (0–88) |
| Age > 60 years at index date | 26 (10%) | 338 (10%) |
| Age at the end of the follow-up, median (range) | 43 (4–94) | 43 (2–96) |
| Age > 60 years at last follow-up | 60 (23%) | 816 (24%) |
| Patients with GD | Controls | OR (95% CI) | p-Value | |
|---|---|---|---|---|
| Total | 11/264 (4.2%) | 7/3441 (0.2%) | 21 (8.2–55.5) | p < 0.001 |
| Cervical | 0 | 5 | - | NS |
| Hematology ^ | 3 | 0 | 91 (4.8–1763) | <0.01 |
| NMSC | 4 | 0 | 118 (6.3–2214) | <0.01 |
| Prostate | 1 | 0 | 39 (1.6–958) | 0.02 |
| Respiratory | 1 | 0 | 39 (1.6–958) | 0.02 |
| Connective tissue | 1 | 0 | 39 (1.6–958) | 0.02 |
| Unspecified | 1 | 0 | 39 (1.6–958) | 0.02 |
| Other | 0 | 2 | - | NS |
| Patients with GD, n = 253 | Controls, n = 3431 | ||||
|---|---|---|---|---|---|
| Cancer cases | 20 (7.9%) | 37 (1.1%) | |||
| Median follow-up, years | 12.8 | 12.9 | |||
| Patient-years (PY) | 3220.9 | 43,237.2 | |||
| n | Rate per 1000 PY | n | Rate per 1000 PY | IRR (95% CI) | |
| Cervical | 1 | 0.31 (0.01 to 1.73) | 21 | 0.49 (0.3 to 0.74) | 0.64 (95% CI 0.02–3.97) |
| NMSC | 7 | 2.17 (0.87 to 4.48) | 9 | 0.21 (0.20–0.56) | 10.4 (95% CI 3.3–32) |
| Colon | 2 | 0.62 (0.08 to 2.24) | 1 | 0.02 (0 to 0.13) | 26.8 (95% CI 2.5–298) |
| Brain | 2 | 0.62 (0.08 to 2.24) | 1 | 0.02 (0 to 0.13) | 26.8 (95% CI 2.5–298) |
| Multiple myeloma | 2 | 0.62 (0.08–2.24) | 0 | 0 | 67.2 (95% CI 1.8–1000) ^ |
| Melanoma | 1 | 0.31 (0.01 to 1.73) | 2 | 0.05 (0.01 to 0.17) | 6.7 (95% CI 0.11–128) |
| Bladder | 1 | 0.31 (0.01 to 1.73) | 2 | 0.05 (0.01 to 0.17) | 6.7 (95% CI 0.11–128) |
| Prostate | 1 | 0.31 (0.01 to 1.73) | 1 | 0.02 (0 to 0.13) | 13.6 (95% CI 0.85–216) |
| Other | 3 | 0.93 (0.02 to 2.72) | 0 | 0 | - |
| Patients with GD | Controls | p-Value | |
|---|---|---|---|
| Male/Female | 130/134 | 1689/1751 | |
| Colonoscopy | 79 (29.9%) | 662 (19.2%) | <0.001 |
| Occult blood in stool | 86 (32.6%) | 983 (28.6%) | 0.19 |
| Mammography $ | 64 (47.8%) | 605 (42.5%) | 0.58 |
| PAP smear $ | 19 (14.2%) | 152 (8.7%) | 0.048 |
| PSA test ^ | 55 (42.3%) | 554 (32.5%) | 0.024 |
| Skin screen | 20 (7.6%) | 86 (2.5%) | <0.001 |
| Cancer n = 18 | No Cancer n = 158 | Univariate, HR (95% CI) | p-Value | Multivariate, HR (95% CI) | p-Value | |
|---|---|---|---|---|---|---|
| Age at index date, years * | 52 (22–72) | 35.5 (20–88) | 1.06 (1.03–1.1) | 0.0004 | 1.06 (1.02–1.1) | 0.002 |
| Male | 17 (54.8%) | 8 (18.2%) | 0.79 (0.3–2.06) | 0.62 | 0.68 (0.26–1.8) | 0.4 |
| SES status * | 6.5 (3–9) | 7 (1–10) | 0.89 (0.74–1.08) | 0.24 | 0.92 (0.75–1.12) | 0.4 |
| Splenectomy | 1 (5.6%) | 6 (3.8%) | 2.27 (0.29–17.2) | 0.43 | 1.35 (0.17–10.3) | 0.77 |
| GD medication | 6 (33%) | 93 (58.8%) | 0.36 (0.13–0.96) | 0.04 | 0.42 (0.15–1.15) | 0.09 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Revel-Vilk, S.; Zimran, A.; Istaiti, M.; Azani, L.; Shalev, V.; Chodick, G.; Manor, O.; Paltiel, O. Cancer Risk in Patients with Gaucher Disease Using Real-World Data. J. Clin. Med. 2023, 12, 7707. https://doi.org/10.3390/jcm12247707
Revel-Vilk S, Zimran A, Istaiti M, Azani L, Shalev V, Chodick G, Manor O, Paltiel O. Cancer Risk in Patients with Gaucher Disease Using Real-World Data. Journal of Clinical Medicine. 2023; 12(24):7707. https://doi.org/10.3390/jcm12247707
Chicago/Turabian StyleRevel-Vilk, Shoshana, Ari Zimran, Majdolen Istaiti, Liat Azani, Varda Shalev, Gabriel Chodick, Orly Manor, and Ora Paltiel. 2023. "Cancer Risk in Patients with Gaucher Disease Using Real-World Data" Journal of Clinical Medicine 12, no. 24: 7707. https://doi.org/10.3390/jcm12247707
APA StyleRevel-Vilk, S., Zimran, A., Istaiti, M., Azani, L., Shalev, V., Chodick, G., Manor, O., & Paltiel, O. (2023). Cancer Risk in Patients with Gaucher Disease Using Real-World Data. Journal of Clinical Medicine, 12(24), 7707. https://doi.org/10.3390/jcm12247707








