Impact of SARS-CoV-2 Positivity on Delivery Outcomes for Pregnant Women between 2020 and 2021: A Single-Center Population-Based Analysis
Abstract
:1. Introduction
2. Methods
2.1. Study Design and Settings
2.2. Study Variables
2.3. Statistical Analysis
2.4. Sensitivity Analysis
3. Results
Sensitivity Analysis
4. Discussion
5. Strength and Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xie, F.; Zhou, J.; Lee, J.W.; Tan, M.; Li, S.; Rajnthern, L.S.; Chee, M.L.; Chakraborty, B.; Wong, A.-K.I.; Dagan, A.; et al. Benchmarking emergency department prediction models with machine learning and public electronic health records. Sci. Data 2022, 9, 658. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, D.A.; Hyg, M. An Analysis of 38 Pregnant Women with COVID-19, Their Newborn Infants, and Maternal-Fetal Transmission of SARS-CoV-2 Maternal Coronavirus Infections and Pregnancy Outcomes. Arch. Pathol. Lab. Med. 2020, 144, 799–805. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Tiwari, S.; Deb, M.K.; Marty, J.L. Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2): A global pandemic and treatment strategies. Int. J. Antimicrob. Agents 2020, 56, 106054. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Guo, H.; Zhou, P.; Shi, Z.L. Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol. 2020, 19, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Wastnedge, E.A.N.; Reynolds, R.M.; van Boeckel, S.R.; Stock, S.J.; Denison, F.C.; Maybin, J.A.; Critchley, H.O.D. Pregnancy and COVID-19. Physiol. Rev. 2021, 101, 303–318. [Google Scholar] [CrossRef] [PubMed]
- Allotey, J.; Stallings, E.; Bonet, M.; Yap, M.; Chatterjee, S.; Kew, T.; Debenham, L.; Llavall, A.C.; Dixit, A.; Zhou, D.; et al. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: Living systematic review and meta-analysis. BMJ 2020, 370, m3320. [Google Scholar] [CrossRef]
- Zambrano, L.D.; Ellington, S.; Strid, P.; Galang, R.R.; Oduyebo, T.; Tong, V.T.; Woodworth, K.R.; Nahabedian, I.I.I.J.F.; Azziz-Baumgartner, E.; Gilboa, S.M.; et al. Update: Characteristics of Symptomatic Women of Reproductive Age with Laboratory-Confirmed SARS-CoV-2 Infection by Pregnancy Status — United States, January 22–October 3, 2020. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 1641–1647. [Google Scholar] [CrossRef]
- de Medeiros, K.S.; Sarmento, A.C.A.; Costa, A.P.F.; Macêdo, L.T.d.A.; da Silva, L.A.S.; de Freitas, C.L.; Simões, A.C.Z.; Gonçalves, A.K. Consequences and implications of the coronavirus disease (COVID-19) on pregnancy and newborns: A comprehensive systematic review and meta-analysis. Int. J. Gynecol. Obstet. 2021, 156, 394–405. [Google Scholar] [CrossRef]
- Al Hashmi, I.; Khalaf, A.; Seshan, V.; Alsabti, H.; Al Omari, O.; Yehia, D.; Baqer, M.; Al Khadhuri, J. Maternal and Neonatal Outcomes of Healthy Pregnant Women With COVID-19 Versus High-risk Pregnant Women: A Multi-Center Case-Control Comparison Study. Clin. Nurs. Res. 2022, 31, 702–712. [Google Scholar] [CrossRef]
- Metz, T.D.; Clifton, R.G.; Hughes, B.L.; Sandoval, G.J.; Grobman, W.A.; Saade, G.R.; Manuck, T.A.; Longo, M.; Sowles, A.; Clark, K.; et al. Association of SARS-CoV-2 infection with serious maternal morbidity and mortality from obstetric complications. JAMA 2022, 327, 748–759. [Google Scholar] [CrossRef]
- Martins, I.; Louwen, F.; Ayres-de-Campos, D.; Mahmood, T. EBCOG position statement on COVID-19 vaccination for pregnant and breastfeeding women. Eur. J. Obstet. Gynecol. Reprod. Biol. 2021, 262, 256–258. [Google Scholar] [CrossRef]
- Kiefer, M.K.; Mehl, R.; Costantine, M.M.; Johnson, A.; Cohen, J.; Summerfield, T.L.; Landon, M.B.; Rood, K.M.; Venkatesh, K.K. Characteristics and perceptions associated with COVID-19 vaccination hesitancy among pregnant and postpartum individuals: A cross-sectional study. BJOG Int. J. Obstet. Gynaecol. 2022, 129, 1342–1351. [Google Scholar] [CrossRef]
- Sutton, D.; D’Alton, M.; Zhang, Y.; Kahe, K.; Cepin, A.; Goffman, D.; Staniczenko, A.; Yates, H.; Burgansky, A.; Coletta, J.; et al. COVID-19 vaccine acceptance among pregnant, breastfeeding, and nonpregnant reproductive-aged women. Am. J. Obstet. Gynecol. MFM 2021, 3, 100403. [Google Scholar] [CrossRef]
- Mirzadeh, M.; Khedmat, L. Pregnant women in the exposure to COVID-19 infection outbreak: The unseen risk factors and preventive healthcare patterns. J. Matern. Fetal Neonatal Med. 2022, 35, 1377–1378. [Google Scholar] [CrossRef]
- Trocado, V.; Silvestre-Machado, J.; Azevedo, L.; Miranda, A.; Nogueira-Silva, C. Pregnancy and COVID-19: A systematic review of maternal, obstetric and neonatal outcomes. J. Matern. Fetal Neonatal Med. 2022, 35, 2362–2374. [Google Scholar] [CrossRef]
- Marchand, G.; Patil, A.S.; Masoud, A.T.; Ware, K.; King, A.; Ruther, S.; Brazil, G.; Calteux, N.; Ulibarri, H.; Parise, J.; et al. Systematic review and meta-analysis of COVID-19 maternal and neonatal clinical features and pregnancy outcomes up to 3 June 2021. AJOG Glob. Rep. 2022, 2, 100049. [Google Scholar] [CrossRef]
- Di Toro, F.; Gjoka, M.; Di Lorenzo, G.; De Santo, D.; De Seta, F.; Maso, G.; Risso, F.M.; Romano, F.; Wiesenfeld, U.; Levi-D’Ancona, R.; et al. Impact of COVID-19 on maternal and neonatal outcomes: A systematic review and meta-analysis. Clin. Microbiol. Infect. 2021, 27, 36–46. [Google Scholar] [CrossRef]
- de Curtis, M.; Villani, L.; Polo, A. Increase of stillbirth and decrease of late preterm infants during the COVID-19 pandemic lockdown. Arch. Dis. Child. Fetal Neonatal Ed. 2021, 106, 456. [Google Scholar] [CrossRef]
- Rusconi, F.; Puglia, M.; Pacifici, M.; Brescianini, S.; Gagliardi, L.; Nannavecchia, A.M.; Buono, P.; Cantoira, S.; Farchi, S.; Gobbato, M.; et al. Pregnancy outcomes in Italy during COVID-19 pandemic: A population-based cohort study. BJOG Int. J. Obstet. Gynaecol. 2022, 130, 276–284. [Google Scholar] [CrossRef]
- Istituto Superiore di Sanitá, Rome. Prevalenza e Distribuzione Delle Varianti di SARS-CoV-2 di Interesse per la Sanità Pubblica in Italia. 2022. Available online: https://www.epicentro.iss.it/coronavirus/pdf/sars-cov-2-monitoraggio-varianti-rapporti-periodici-1-luglio-2022.pdf (accessed on 12 December 2023).
- Istituto Superiore di Sanitá, Rome. Prevalenza e Distribuzione Delle Varianti di SARS-CoV-2 di Interesse per la Sanità Pubblica in Italia Rapporto n. 5 del 23 luglio 2021. Available online: https://www.iss.it/documents/20126/0/versione+h++17+BOLLETTINO+VARIANTI+n.5+23+luglio.pdf/ (accessed on 12 December 2023).
- Stima della Prevalenza delle Varianti VOC (Variants of Concern) in Italia: Beta, Gamma, Delta, Omicron e Altre Varianti di SARS-CoV-2. Available online: https://www.epicentro.iss.it/coronavirus/pdf/sars-cov-2-monitoraggio-varianti-indagini-rapide-6-dicembre-2021.pdf (accessed on 12 June 2021).
- Bateman, B.T.M.; Mhyre, J.M.; Hernandez-Diaz, S.M.; Huybrechts, K.F.; Fischer, M.A.; Creanga, A.A.; Callaghan, W.M.; Gagne, J.J.P. Development of a Comorbidity Index for Use in Obstetric Patients. Obstet. Gynecol. 2013, 122, 957–965. [Google Scholar] [CrossRef]
- Easter, S.R.; Bateman, B.T.; Sweeney, V.H.; Manganaro, K.; Lassey, S.C.; Gagne, J.J.; Robinson, J.N. A comorbidity-based screening tool to predict severe maternal morbidity at the time of delivery. Am. J. Obstet. Gynecol. 2019, 221, 271.e1–271.e10. [Google Scholar] [CrossRef]
- Choi, E.-Y.; Jeong, H.E.; Noh, Y.; Choi, A.; Yon, D.K.; Han, J.Y.; Sung, J.-H.; Choe, S.-A.; Shin, J.-Y. Neonatal and maternal adverse outcomes and exposure to nonsteroidal anti-inflammatory drugs during early pregnancy in South Korea: A nationwide cohort study. PLoS Med. 2023, 20, e1004183. [Google Scholar] [CrossRef]
- Padilla, C.; Markwei, M.; Easter, S.R.; Fox, K.A.; Shamshirsaz, A.A.; Foley, M.R. Critical care in obstetrics: A strategy for addressing maternal mortality. Am. J. Obstet. Gynecol. 2021, 224, 567–573. [Google Scholar] [CrossRef]
- Fernández-De-Las-Peñas, C.; Notarte, K.I.; Peligro, P.J.; Velasco, J.V.; Ocampo, M.J.; Henry, B.M.; Arendt-Nielsen, L.; Torres-Macho, J.; Plaza-Manzano, G. Long-COVID Symptoms in Individuals Infected with Different SARS-CoV-2 Variants of Concern: A Systematic Review of the Literature. Viruses 2022, 14, 2629. [Google Scholar] [CrossRef] [PubMed]
- Ho, M.; Levy, T.J.; Koulas, I.; Founta, K.; Coppa, K.; Hirsch, J.S.; Davidson, K.W.; Spyropoulos, A.C.; Zanos, T.P. Longitudinal dynamic clinical phenotypes of in-hospital COVID-19 patients across three dominant virus variants in New York. Int. J. Med. Inform. 2024, 181, 105286. [Google Scholar] [CrossRef] [PubMed]
- Westerhof, I.; de Hoog, M.; Ieven, M.; Lammens, C.; van Beek, J.; Rozhnova, G.; Eggink, D.; Euser, S.; Wildenbeest, J.; Duijts, L.; et al. The impact of variant and vaccination on SARS-CoV-2 symptomatology; three prospective household cohorts. Int. J. Infect. Dis. 2023, 128, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Caesarean Section Rates Continue to Rise, Amid Growing Inequalities in Access. Available online: https://www.who.int/news/item/16-06-2021-caesarean-section-rates-continue-to-rise-amid-growing-inequalities-in-access (accessed on 2 December 2023).
- Certificato di Assistenza al Parto (CeDAP). Analisi Dell’evento Nascita—Anno 2020. Available online: https://www.salute.gov.it/portale/documentazione/p6_2_2_1.jsp?lingua=italiano&id=3149 (accessed on 2 December 2023).
- OECD. Tackling Wasteful Spending on Health; OECD: Paris, France, 2017. [Google Scholar] [CrossRef]
- Colais, P.; Fantini, M.P.; Fusco, D.; Carretta, E.; Stivanello, E.; Lenzi, J.; Pieri, G.; A Perucci, C. Risk adjustment models for interhospital comparison of CS rates using Robson’s ten group classification system and other socio-demographic and clinical variables. BMC Pregnancy Childbirth 2012, 12, 54. [Google Scholar] [CrossRef]
- Longo, V.L.; Odjidja, E.N.; Beia, T.K.; Neri, M.; Kielmann, K.; Gittardi, I.; Di Rosa, A.I.; Boldrini, M.; Melis, G.B.; Scambia, G.; et al. ‘An unnecessary cut?’ multilevel health systems analysis of drivers of caesarean sections rates in Italy: A systematic review. BMC Pregnancy Childbirth 2020, 20, 770. [Google Scholar] [CrossRef]
- Favre, G.; Pomar, L.; Qi, X.; Nielsen-Saines, K.; Musso, D.; Baud, D. Guidelines for pregnant women with suspected SARS-CoV-2 infection. Lancet Infect. Dis. 2020, 20, 652–653. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Peng, L.; Siddique, R.; Nabi, G.; Nawsherwan; Xue, M.; Liu, J.; Han, G. Impact of COVID-19 infection on pregnancy outcomes and the risk of maternal-to-neonatal intrapartum transmission of COVID-19 during natural birth. Infect. Control Hosp. Epidemiol. 2020, 41, 748–750. [Google Scholar] [CrossRef]
- Sorveglianza Integrata COVID-19: I Principali dati Nazionali. Available online: https://www.epicentro.iss.it/coronavirus/sars-cov-2-sorveglianza-dati (accessed on 21 July 2023).
- Chmielewska, B.; Barratt, I.; Townsend, R.; Kalafat, E.; van der Meulen, J.; Gurol-Urganci, I.; O’Brien, P.; Morris, E.; Draycott, T.; Thangaratinam, S.; et al. Effects of the COVID-19 pandemic on maternal and perinatal outcomes: A systematic review and meta-analysis. Lancet Glob. Health 2021, 9, e759–e772. [Google Scholar] [CrossRef]
- Been, J.V.; Ochoa, L.B.; Bertens, L.C.M.; Schoenmakers, S.; Steegers, E.A.P.; Reiss, I.K.M. Impact of COVID-19 mitigation measures on the incidence of preterm birth: A national quasi-experimental study. Lancet Public Health 2020, 5, e604–e611. [Google Scholar] [CrossRef]
- Sun, Z.; Yang, L.; Bai, X.; Du, W.; Shen, G.; Fei, J.; Wang, Y.; Chen, A.; Chen, Y.; Zhao, M. Maternal ambient air pollution exposure with spatial-temporal variations and preterm birth risk assessment during 2013–2017 in Zhejiang Province, China. Environ. Int. 2019, 133, 105242. [Google Scholar] [CrossRef]
- Kramer, M.S.; Lydon, J.; Séguin, L.; Goulet, L.; Kahn, S.R.; McNamara, H.; Genest, J.; Dassa, C.; Chen, M.F.; Sharma, S.; et al. Stress Pathways to Spontaneous Preterm Birth: The Role of Stressors, Psychological Distress, and Stress Hormones. Am. J. Epidemiol. 2009, 169, 1319–1326. [Google Scholar] [CrossRef]
- Dubey, P.; Thakur, B.; Reddy, S.; Martinez, C.A.; Nurunnabi; Manuel, S.L.; Chheda, S.; Bracamontes, C.; Dwivedi, A.K. Current trends and geographical differences in therapeutic profile and outcomes of COVID-19 among pregnant women—A systematic review and meta-analysis. BMC Pregnancy Childbirth 2021, 21, 247. [Google Scholar] [CrossRef]
- Rasmussen, S.A.; Smulian, J.C.; Lednicky, J.A.; Wen, T.S.; Jamieson, D.J. Coronavirus Disease 2019 (COVID-19) and pregnancy: What obstetricians need to know. Am. J. Obstet. Gynecol. 2020, 222, 415–426. [Google Scholar] [CrossRef]
- Alserehi, H.; Wali, G.; Alshukairi, A.; Alraddadi, B. Impact of Middle East Respiratory Syndrome coronavirus (MERS-CoV) on pregnancy and perinatal outcome. BMC Infect. Dis. 2016, 16, 105. [Google Scholar] [CrossRef]
| 2020 | 2021 | Total | |||||||
|---|---|---|---|---|---|---|---|---|---|
| SARS-CoV-2 + | SARS-CoV-2 − | p-Value | SARS-CoV-2 + | SARS-CoV-2 − | p-Value | SARS-CoV-2 + | SARS-CoV-2 − | p-Value | |
| N | 149 | 2533 | 204 | 2350 | 353 | 4883 | |||
| Average age of mothers (mean, SD) | 30.1 (SD 5.3) | 32.3 (SD 5.3) | <0.01 | 30.5 (SD 5.8) | 32.7 (SD 5.5) | <0.01 | 30.3 (SD 5.6) | 32.5 (SD 5.4) | <0.01 |
| Prevalent SARS-CoV-2 variant | |||||||||
| Before the outbreak—Jan ‘20 to Feb ’20 | - | 446 | <0.01 | - | - | - | 446 | <0.01 | |
| Alpha—March to Sept ‘20 + March to June ‘21 | 2 (1%) | 1444 | 73 (36%) | 717 | 0.241 | 75 (21%) | 2161 | ||
| Beta—Oct ‘20 to Feb ‘21 | 147 (99%) | 643 | 36 (18%) | 372 | 183 (52%) | 1015 | |||
| Delta—July to Nov ‘21 | 0 | 0 | 81 (40%) | 1103 | 81 (23%) | 1103 | |||
| Omicron—Dec ‘21 | 0 | 0 | 14 (7%) | 158 | 14 (4%) | 158 | |||
| Vaccine availability | - | - | 100 | - | 100 | - | |||
| Complete vaccine cycle | - | - | 4 | - | 4 | - | |||
| Obstetric Comorbidity Index | |||||||||
| 0 | 109 | 1456 | 0.008 | 149 | 1284 | 0.000 | 258 | 2740 | <0.01 |
| 1–2 | 38 | 892 | 46 | 924 | 84 | 1816 | |||
| 3–4 | 0 | 75 | 3 | 70 | 3 | 145 | |||
| 5–6 | 1 | 74 | 6 | 52 | 7 | 126 | |||
| 7–8 | 1 | 28 | 0 | 16 | 1 | 44 | |||
| 9–10 | 0 | 3 | 0 | 2 | 0 | 5 | |||
| >10 | 0 | 5 | 0 | 2 | 0 | 7 | |||
| Delivery mode | |||||||||
| Vaginal | 55 | 1363 | 0.000 | 93 | 1232 | 0.061 | 148 | 2595 | <0.01 |
| Caesarean section | 94 | 1170 | 111 | 1118 | 205 | 2288 | |||
| Pregnancy | |||||||||
| Full term | 144 | 2404 | 0.344 | 190 | 2185 | 0.932 | 334 | 4589 | 0.625 |
| Preterm birth | 5 | 129 | 14 | 2185 | 19 | 294 | |||
| Length of stay (median, IQR) | 5 (4 to 8) | 5 (4 to 8) | 0.834 | 5 (4 to 8) | 4 (4 to 6) | 0.000 | 5 (4 to 8) | 5 (4 to 7) | 0.014 |
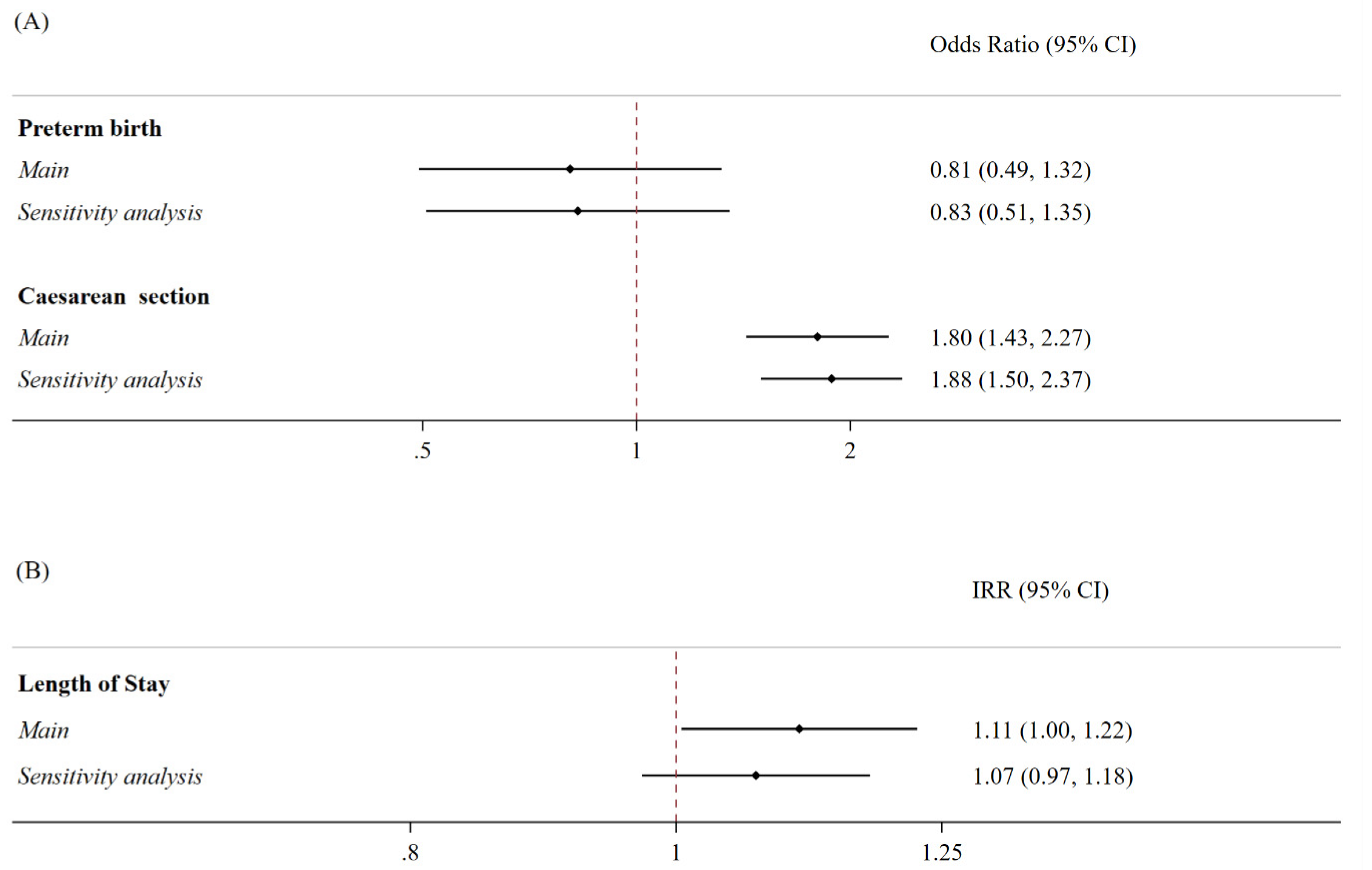
| Preterm BirTH | Caesarean Section | Length of Stay | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Main Analysis | OR | 95% CI | OR | 95% CI | IRR | 95% CI | |||
| IV study period (ref) (October–December 2020) | |||||||||
| V study period (January–March 2021) | 2.10 | 0.53 | 8.25 | 0.60 | 0.32 | 1.12 | 1.18 | 0.93 | 1.50 |
| VI study period (April–June 2021) | 1.94 | 0.42 | 8.90 | 0.79 | 0.39 | 1.57 | 1.21 | 0.98 | 1.48 |
| VII study period (July–September 2021) | 3.70 | 0.99 | 13.79 | 0.39 | 0.19 | 0.79 | 1.00 | 0.77 | 1.29 |
| VIII study period (October–December 2021) | 0.57 | 0.06 | 5.09 | 1.00 | 0.52 | 1.93 | 1.01 | 0.78 | 1.32 |
| Sensitive Analysis | OR | 95% CI | OR | 95% CI | IRR | 95% CI | |||
| Alpha (ref) | |||||||||
| Beta | 0.50 | 0.15 | 1.68 | 0.95 | 0.54 | 1.66 | 0.83 | 0.66 | 1.03 |
| Delta | 1.07 | 0.31 | 3.72 | 0.67 | 0.35 | 1.28 | 0.80 | 0.61 | 1.06 |
| Omicron | - | - | - | 1.57 | 0.48 | 5.15 | 0.77 | 0.48 | 1.23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palladino, R.; Balsamo, F.; Mercogliano, M.; Sorrentino, M.; Monzani, M.; Egidio, R.; Piscitelli, A.; Borrelli, A.; Bifulco, G.; Triassi, M. Impact of SARS-CoV-2 Positivity on Delivery Outcomes for Pregnant Women between 2020 and 2021: A Single-Center Population-Based Analysis. J. Clin. Med. 2023, 12, 7709. https://doi.org/10.3390/jcm12247709
Palladino R, Balsamo F, Mercogliano M, Sorrentino M, Monzani M, Egidio R, Piscitelli A, Borrelli A, Bifulco G, Triassi M. Impact of SARS-CoV-2 Positivity on Delivery Outcomes for Pregnant Women between 2020 and 2021: A Single-Center Population-Based Analysis. Journal of Clinical Medicine. 2023; 12(24):7709. https://doi.org/10.3390/jcm12247709
Chicago/Turabian StylePalladino, Raffaele, Federica Balsamo, Michelangelo Mercogliano, Michele Sorrentino, Marco Monzani, Rosanna Egidio, Antonella Piscitelli, Anna Borrelli, Giuseppe Bifulco, and Maria Triassi. 2023. "Impact of SARS-CoV-2 Positivity on Delivery Outcomes for Pregnant Women between 2020 and 2021: A Single-Center Population-Based Analysis" Journal of Clinical Medicine 12, no. 24: 7709. https://doi.org/10.3390/jcm12247709
APA StylePalladino, R., Balsamo, F., Mercogliano, M., Sorrentino, M., Monzani, M., Egidio, R., Piscitelli, A., Borrelli, A., Bifulco, G., & Triassi, M. (2023). Impact of SARS-CoV-2 Positivity on Delivery Outcomes for Pregnant Women between 2020 and 2021: A Single-Center Population-Based Analysis. Journal of Clinical Medicine, 12(24), 7709. https://doi.org/10.3390/jcm12247709





