Incidence of Gastric Neoplasms Arising from Autoimmune Metaplastic Atrophic Gastritis: A Systematic Review and Case Reports
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Study Selection and Eligibility Criteria
2.3. Data Extraction and Quality Assessment
2.4. Statistical Analysis
3. Results
3.1. Search Results
3.2. Quality Assessment
3.3. Characteristics of Studies
3.4. Identification of Gastric Neoplasms
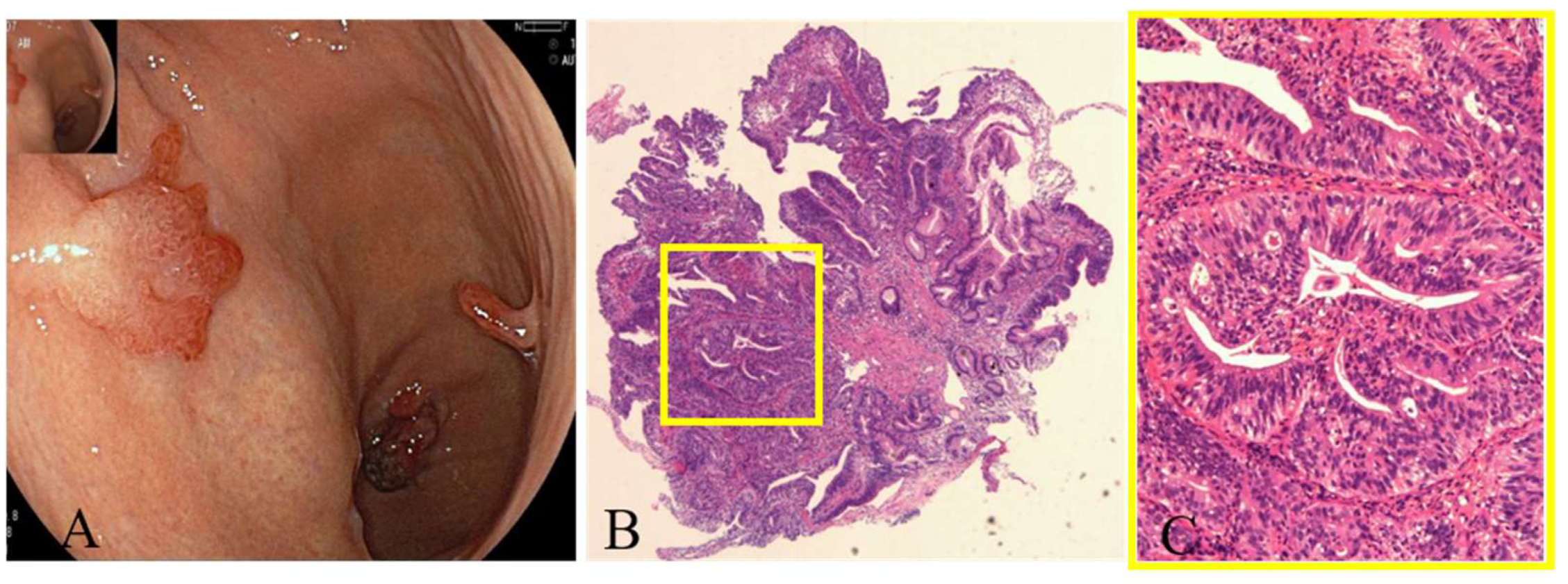
3.5. Experience from Our Center and Unusual Gastric Lesions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mårdh, S.; Song, Y.H. Characterization of antigenic structures in auto-immune atrophic gastritis with pernicious anaemia. The parietal cell H,K-ATPase and the chief cell pepsinogen are the two major antigens. Acta Physiol. Scand. 1989, 136, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Lenti, M.V.; Rugge, M.; Lahner, E.; Miceli, E.; Toh, B.H.; Genta, R.M.; De Block, C.; Hershko, C.; Di Sabatino, A. Autoimmune gastritis. Nat. Rev. Dis. Prim. 2020, 6, 56. [Google Scholar] [CrossRef] [PubMed]
- Bizzaro, N.; Antico, A. Diagnosis and classification of pernicious anemia. Autoimmun. Rev. 2014, 13, 565–568. [Google Scholar] [CrossRef] [PubMed]
- Vannella, L.; Lahner, E.; Osborn, J.; Annibale, B. Systematic review: Gastric cancer incidence in pernicious anaemia. Aliment. Pharmacol. Ther. 2013, 37, 375–382. [Google Scholar] [CrossRef]
- Kaltsas, G.; Grozinsky-Glasberg, S.; Alexandraki, K.I.; Thomas, D.; Tsolakis, A.V.; Gross, D.; Grossman, A.B. Current concepts in the diagnosis and management of type 1 gastric neuroendocrine neoplasms. Clin. Endocrinol. 2014, 81, 157–168. [Google Scholar] [CrossRef] [Green Version]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Munn, Z.; Barker, T.H.; Moola, S.; Tufanaru, C.; Stern, C.; McArthur, A.; Stephenson, M.; Aromataris, E. Methodological quality of case series studies: An introduction to the JBI critical appraisal tool. JBI Evid. Synth. 2020, 18, 2127–2133. [Google Scholar] [CrossRef]
- Globocan 2020: Estimated Cancer Incidence, Mortality and Prevalence Worldwide in 2020. Available online: https://gco.iarc.fr (accessed on 17 October 2022).
- Rugge, M.; Bricca, L.; Guzzinati, S.; Sacchi, D.; Pizzi, M.; Savarino, E.; Farinati, F.; Zorzi, M.; Fassan, M.; Dei Tos, A.P.; et al. Autoimmune gastritis: Long-term natural history in naive Helicobacter pylori-negative patients. Gut 2022, 72, 30–38. [Google Scholar] [CrossRef]
- Esposito, G.; Dilaghi, E.; Cazzato, M.; Pilozzi, E.; Conti, L.; Carabotti, M.; Di Giulio, E.; Annibale, B.; Lahner, E. Endoscopic surveillance at 3 years after diagnosis, according to European guidelines, seems safe in patients with atrophic gastritis in a low-risk region. Dig. Liver Dis. Off. J. Ital. Soc. Gastroenterol. Ital. Assoc. Study Liver 2021, 53, 467–473. [Google Scholar] [CrossRef]
- Miceli, E.; Vanoli, A.; Lenti, M.V.; Klersy, C.; Di Stefano, M.; Luinetti, O.; Caccia Dominioni, C.; Pisati, M.; Staiani, M.; Gentile, A.; et al. Natural history of autoimmune atrophic gastritis: A prospective, single centre, long-term experience. Aliment. Pharmacol. Ther. 2019, 50, 1172–1180. [Google Scholar] [CrossRef]
- Mahmud, N.; Stashek, K.; Katona, B.W.; Tondon, R.; Shroff, S.G.; Roses, R.; Furth, E.E.; Metz, D.C. The incidence of neoplasia in patients with autoimmune metaplastic atrophic gastritis: A renewed call for surveillance. Ann. Gastroenterol. 2019, 32, 67–72. [Google Scholar] [CrossRef]
- Chan, J.C.; Liu, H.S.; Kho, B.C.; Lau, T.K.; Li, V.L.; Chan, F.H.; Leong, I.S.; Pang, H.K.; Lee, C.K.; Liang, Y.S. Longitudinal study of Chinese patients with pernicious anaemia. Postgrad. Med. J. 2008, 84, 644–650. [Google Scholar] [CrossRef] [PubMed]
- Bresky, G.; Mata, A.; Llach, J.; Ginis, M.A.; Pellisi, M.; Soria, M.T.; Fernandez-Esparrach, G.; Mondelo, F.; Bordas, J.M. Endoscopic findings in a biennial follow-up program in patients with pernicious anemia. Hepato-Gastroenterol. 2003, 50, 2264–2266. [Google Scholar]
- Kokkola, A.; Sjöblom, S.M.; Haapiainen, R.; Sipponen, P.; Puolakkainen, P.; Järvinen, H. The risk of gastric carcinoma and carcinoid tumours in patients with pernicious anaemia. A prospective follow-up study. Scand. J. Gastroenterol. 1998, 33, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Borch, K. Epidemiologic, clinicopathologic, and economic aspects of gastroscopic screening of patients with pernicious anemia. Scand. J. Gastroenterol. 1986, 21, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Schafer, L.W.; Larson, D.E.; Melton, L.J., 3rd; Higgins, J.A.; Zinsmeister, A.R. Risk of development of gastric carcinoma in patients with pernicious anemia: A population-based study in Rochester, Minnesota. Mayo Clin. Proc. 1985, 60, 444–448. [Google Scholar] [CrossRef]
- Elsborg, L.; Andersen, D.; Myhere-Jensen, O.; Bastrup-Madsen, P. Gastric mucosal polyps in pernicious anaemia. Scand. J. Gastroenterol. 1977, 12, 49–52. [Google Scholar] [CrossRef] [PubMed]
- Ye, W.; Nyrén, O. Risk of cancers of the oesophagus and stomach by histology or subsite in patients hospitalised for pernicious anaemia. Gut 2003, 52, 938–941. [Google Scholar] [CrossRef] [Green Version]
- Mellemkjaer, L.; Gridley, G.; Møller, H.; Hsing, A.W.; Linet, M.S.; Brinton, L.A.; Olsen, J.H. Pernicious anaemia and cancer risk in Denmark. Br. J. Cancer 1996, 73, 998–1000. [Google Scholar] [CrossRef] [Green Version]
- Brinton, L.A.; Gridley, G.; Hrubec, Z.; Hoover, R.; Fraumeni, J.F., Jr. Cancer risk following pernicious anaemia. Br. J. Cancer 1989, 59, 810–813. [Google Scholar] [CrossRef] [Green Version]
- Hu, H.; Li, R.; Shao, L.; Zhang, Q.; Xu, R.; Zhang, S. Gastric lesions in patients with autoimmune metaplastic atrophic gastritis: A retrospective study in a single center. Scand. J. Gastroenterol. 2022, 57, 1296–1303. [Google Scholar] [CrossRef]
- Hu, H.; Zhang, Q.; Chen, G.; Pritchard, D.M.; Zhang, S. Risk factors and clinical correlates of neoplastic transformation in gastric hyperplastic polyps in Chinese patients. Sci. Rep. 2020, 10, 2582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, M.; Latorre, G.; Ivanovic-Zuvic, D.; Camargo, M.C.; Rabkin, C.S. Autoimmune Diseases and Gastric Cancer Risk: A Systematic Review and Meta-Analysis. Cancer Res. Treat. 2019, 51, 841–850. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Luo, G.; Zhang, Y.; Guo, P.; Wang, L.; Huang, Y.; Li, K. Global patterns and trends in stomach cancer incidence: Age, period and birth cohort analysis. Int. J. Cancer 2017, 141, 1333–1344. [Google Scholar] [CrossRef] [Green Version]
- Song, M.; Rabkin, C.S.; Camargo, M.C. Gastric Cancer: An Evolving Disease. Curr. Treat. Options Gastroenterol. 2018, 16, 561–569. [Google Scholar] [CrossRef] [PubMed]
- Coati, I.; Fassan, M.; Farinati, F.; Graham, D.Y.; Genta, R.M.; Rugge, M. Autoimmune gastritis: Pathologist’s viewpoint. World J. Gastroenterol. 2015, 21, 12179–12189. [Google Scholar] [CrossRef]
- Park, J.Y.; Cornish, T.C.; Lam-Himlin, D.; Shi, C.; Montgomery, E. Gastric lesions in patients with autoimmune metaplastic atrophic gastritis (AMAG) in a tertiary care setting. Am. J. Surg. Pathol. 2010, 34, 1591–1598. [Google Scholar] [CrossRef]
- Zhang, H.; Jin, Z.; Cui, R.; Ding, S.; Huang, Y.; Zhou, L. Autoimmune metaplastic atrophic gastritis in chinese: A study of 320 patients at a large tertiary medical center. Scand. J. Gastroenterol. 2017, 52, 150–156. [Google Scholar] [CrossRef]
- Terao, S.; Suzuki, S.; Yaita, H.; Kurahara, K.; Shunto, J.; Furuta, T.; Maruyama, Y.; Ito, M.; Kamada, T.; Aoki, R.; et al. Multicenter study of autoimmune gastritis in Japan: Clinical and endoscopic characteristics. Dig. Endosc. 2020, 32, 364–372. [Google Scholar] [CrossRef]
- Kitamura, S.; Muguruma, N.; Okamoto, K.; Kagemoto, K.; Kida, Y.; Mitsui, Y.; Ueda, H.; Kawaguchi, T.; Miyamoto, H.; Sato, Y.; et al. Clinicopathological characteristics of early gastric cancer associated with autoimmune gastritis. JGH Open 2021, 5, 1210–1215. [Google Scholar] [CrossRef]
- Akbari, M.; Kardeh, B.; Tabrizi, R.; Ahmadizar, F.; Lankarani, K.B. Incidence Rate of Gastric Cancer Adenocarcinoma in Patients With Gastric Dysplasia: A Systematic Review and Meta-Analysis. J. Clin. Gastroenterol. 2019, 53, 703–710. [Google Scholar] [CrossRef]
- Massironi, S.; Zilli, A.; Elvevi, A.; Invernizzi, P. The changing face of chronic autoimmune atrophic gastritis: An updated comprehensive perspective. Autoimmun. Rev. 2019, 18, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Rugge, M.; Fassan, M.; Pizzi, M.; Zorzetto, V.; Maddalo, G.; Realdon, S.; De Bernard, M.; Betterle, C.; Cappellesso, R.; Pennelli, G.; et al. Autoimmune gastritis: Histology phenotype and OLGA staging. Aliment. Pharmacol. Ther. 2012, 35, 1460–1466. [Google Scholar] [CrossRef] [PubMed]
- Murphy, G.; Dawsey, S.M.; Engels, E.A.; Ricker, W.; Parsons, R.; Etemadi, A.; Lin, S.W.; Abnet, C.C.; Freedman, N.D. Cancer Risk After Pernicious Anemia in the US Elderly Population. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2015, 13, 2282–2289.E4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campana, D.; Ravizza, D.; Ferolla, P.; Faggiano, A.; Grimaldi, F.; Albertelli, M.; Ricci, C.; Santini, D.; Brighi, N.; Fazio, N.; et al. Risk factors of type 1 gastric neuroendocrine neoplasia in patients with chronic atrophic gastritis. A retrospective, multicentre study. Endocrine 2017, 56, 633–638. [Google Scholar] [CrossRef] [Green Version]
- Delle Fave, G.; O’Toole, D.; Sundin, A.; Taal, B.; Ferolla, P.; Ramage, J.K.; Ferone, D.; Ito, T.; Weber, W.; Zheng-Pei, Z.; et al. ENETS Consensus Guidelines Update for Gastroduodenal Neuroendocrine Neoplasms. Neuroendocrinology 2016, 103, 119–124. [Google Scholar] [CrossRef] [Green Version]
- Panzuto, F.; Campana, D.; Massironi, S.; Faggiano, A.; Rinzivillo, M.; Lamberti, G.; Sciola, V.; Lahner, E.; Manuzzi, L.; Colao, A.; et al. Tumour type and size are prognostic factors in gastric neuroendocrine neoplasia: A multicentre retrospective study. Dig. Liver Dis. Off. J. Ital. Soc. Gastroenterol. Ital. Assoc. Study Liver 2019, 51, 1456–1460. [Google Scholar] [CrossRef] [PubMed]
- Massironi, S.; Zilli, A.; Fanetti, I.; Ciafardini, C.; Conte, D.; Peracchi, M. Intermittent treatment of recurrent type-1 gastric carcinoids with somatostatin analogues in patients with chronic autoimmune atrophic gastritis. Dig. Liver Dis. Off. J. Ital. Soc. Gastroenterol. Ital. Assoc. Study Liver 2015, 47, 978–983. [Google Scholar] [CrossRef] [PubMed]
- Massironi, S.; Zilli, A.; Conte, D. Somatostatin analogs for gastric carcinoids: For many, but not all. World J. Gastroenterol. 2015, 21, 6785–6793. [Google Scholar] [CrossRef] [PubMed]
- Kishino, M.; Nonaka, K. Endoscopic Features of Autoimmune Gastritis: Focus on Typical Images and Early Images. J. Clin. Med. 2022, 11, 3523. [Google Scholar] [CrossRef] [PubMed]
- Waldum, H.; Fossmark, R. Gastritis, Gastric Polyps and Gastric Cancer. Int. J. Mol. Sci. 2021, 22, 6548. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.M.; Oh, T.H.; Seo, J.Y.; Joen, T.J.; Seo, D.D.; Shin, W.C.; Choi, W.C.; Kim, J.Y. Clinical factors predicting for neoplastic transformation of gastric hyperplastic polyps. Korean J. Gastroenterol. = Taehan Sohwagi Hakhoe Chi 2011, 58, 184–189. [Google Scholar] [CrossRef] [Green Version]
- D’Elios, M.M.; Bergman, M.P.; Azzurri, A.; Amedei, A.; Benagiano, M.; De Pont, J.J.; Cianchi, F.; Vandenbroucke-Grauls, C.M.; Romagnani, S.; Appelmelk, B.J.; et al. H(+),K(+)-atpase (proton pump) is the target autoantigen of Th1-type cytotoxic T cells in autoimmune gastritis. Gastroenterology 2001, 120, 377–386. [Google Scholar] [CrossRef]
- Osaki, L.H.; Bockerstett, K.A.; Wong, C.F.; Ford, E.L.; Madison, B.B.; DiPaolo, R.J.; Mills, J.C. Interferon-gamma directly induces gastric epithelial cell death and is required for progression to metaplasia. J. Pathol. 2019, 247, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.Y.; Zhou, C.H.; Ma, J.; Jiang, C.; Ji, P. Expression of interleukin-17 and its clinical significance in gastric cancer patients. Med. Oncol. 2012, 29, 3024–3028. [Google Scholar] [CrossRef]
- Maruyama, T.; Kono, K.; Mizukami, Y.; Kawaguchi, Y.; Mimura, K.; Watanabe, M.; Izawa, S.; Fujii, H. Distribution of Th17 cells and FoxP3(+) regulatory T cells in tumor-infiltrating lymphocytes, tumor-draining lymph nodes and peripheral blood lymphocytes in patients with gastric cancer. Cancer Sci. 2010, 101, 1947–1954. [Google Scholar] [CrossRef]
- Della Bella, C.; Antico, A.; Panozzo, M.P.; Capitani, N.; Petrone, L.; Benagiano, M.; D’Elios, S.; Sparano, C.; Azzurri, A.; Pratesi, S.; et al. Gastric Th17 Cells Specific for H(+)/K(+)-ATPase and Serum IL-17 Signature in Gastric Autoimmunity. Front. Immunol. 2022, 13, 952674. [Google Scholar] [CrossRef]
- Repetto, O.; De Re, V.; Giuffrida, P.; Lenti, M.V.; Magris, R.; Venerito, M.; Steffan, A.; Di Sabatino, A.; Cannizzaro, R. Proteomics signature of autoimmune atrophic gastritis: Towards a link with gastric cancer. Gastric. Cancer 2021, 24, 666–679. [Google Scholar] [CrossRef]
- Parsons, B.N.; Ijaz, U.Z.; D’Amore, R.; Burkitt, M.D.; Eccles, R.; Lenzi, L.; Duckworth, C.A.; Moore, A.R.; Tiszlavicz, L.; Varro, A.; et al. Comparison of the human gastric microbiota in hypochlorhydric states arising as a result of Helicobacter pylori-induced atrophic gastritis, autoimmune atrophic gastritis and proton pump inhibitor use. PLoS Pathog. 2017, 13, e1006653. [Google Scholar] [CrossRef] [Green Version]
- Furuta, T.; Baba, S.; Yamade, M.; Uotani, T.; Kagami, T.; Suzuki, T.; Tani, S.; Hamaya, Y.; Iwaizumi, M.; Osawa, S.; et al. High incidence of autoimmune gastritis in patients misdiagnosed with two or more failures of H. pylori eradication. pylori eradication. Aliment. Pharmacol. Ther. 2018, 48, 370–377. [Google Scholar] [CrossRef] [PubMed]
- Tsuboi, M.; Niikura, R.; Hayakawa, Y.; Hirata, Y.; Ushiku, T.; Koike, K. Distinct Features of Autoimmune Gastritis in Patients with Open-Type Chronic Gastritis in Japan. Biomedicines 2020, 8, 419. [Google Scholar] [CrossRef] [PubMed]




| Author | Year | Country | Sources of Selection of Participants | Criteria for Diagnosis of AIG | Number of Patients (n) | Duration of Follow-Up (Years) | Type of Follow-Up | Methods of Follow-Up | Person-years | Age of Patients (Mean or Median) | Female (%) | Methods for Identification of Neoplasms | Cases of Gastric Neoplasms | Incidence of GC (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rugge et al. [9] | 2022 | Italy | Single-center | Serology, histology | 211 | 7.5 | Active | Gastroscopy | 1583 | 55.7 | 75.8 | Histology | GC 0 LGD 5 Type-1 gNET 11 | 0 |
| Esposito et al. [10] | 2021 | Italy | Single-center | Histology | 122 | 3 | Active | Gastroscopy | 366 | 68 | 73.0 | Histology | GC 3 LGD 2 Type-1 gNET 6 | 0.82 |
| Miceli et al. [11] | 2019 | Italy | Single-center | Histology | 270 | 3 | Active | Gastroscopy | 1164 | 60.3 | 70.6 | Histology | HGD/GC 3 LGD 4 Type-1 gNET 7 | 0.26 |
| Mahmud et al. [12] | 2019 | USA | Single-center | Endoscopic, histology | 59 | 1.89 | Not active | Gastroscopy | 141 | 63.5 | 80.7 | Histology | GC 2 | 1.42 |
| Chan et al. [13] | 2008 | China | Single-center | PA | 199 | 5.13 | Active | Gastroscopy performed in 46/199 | 1021 | 73.03 | 64.3 | Histology | GC 4 | 0.39 |
| Bresky et al. [14] | 2003 | Spain | Single-center | PA | 68 | - | Active | Gastroscopy | 544 | 62 | 57 | Histology | GC 0 LGC 3 | 0 |
| Kokkola et al. [15] | 1998 | Finland | Single-center | PA | 71 | 12.2 | Active | Gastroscopy | 869 | 59 | 59.2 | Histology | GC 2 Type-1 gNET 11 | 0.23 |
| Borch et al. [16] | 1986 | Sweden | Single-center | PA | 61 | 2.67 | Active | Gastroscopy | 163 | 68 | 60 | Histology | GC 0 LGD 5 | 0 |
| Schafer et al. [17] | 1985 | USA | Regional | PA | 152 | 12.5 | Non-active | NA | 1555 | 69 | 63.2 | Autopsy | GC 1 | 0.06 |
| Elsborg et al. [18] | 1977 | Denmark | Single-center | PA | 68 | 9.5 | Active | Gastroscopy | 263 | 65 | 61.8 | Histology | GC 1 | 0.38 |
| Registration studies | ||||||||||||||
| Ye et al. [19] | 2003 | Sweden | Swedish Inpatient Register | PA (ICD) | 21265 | 7.1 | Not active | Registry data | 161672 | 74.3 | 60.3 | Nationwide Register of Causes of Death | GC 230 | 0.14 |
| Mellemkjaerl et al. [20] | 1997 | Denmark | National registration study | PA (ICD) | 5072 | 5.1 | Not active | Registry | 25768 | 71-73 | 66 | Danish Cancer Registry | GC 50 | 0.19 |
| Brinton et al. [21] | 1989 | USA | Veterans Administration hospitalization records | PA (ICD) | 5161 | 6.8 | Not active | Hospitalization records | 34915 | 67.6 | 0 | Hospitalization records | GC 31 | 0.09 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.; Yang, Y.; Li, P.; Hu, H. Incidence of Gastric Neoplasms Arising from Autoimmune Metaplastic Atrophic Gastritis: A Systematic Review and Case Reports. J. Clin. Med. 2023, 12, 1062. https://doi.org/10.3390/jcm12031062
Chen C, Yang Y, Li P, Hu H. Incidence of Gastric Neoplasms Arising from Autoimmune Metaplastic Atrophic Gastritis: A Systematic Review and Case Reports. Journal of Clinical Medicine. 2023; 12(3):1062. https://doi.org/10.3390/jcm12031062
Chicago/Turabian StyleChen, Chuyan, Yi Yang, Peng Li, and Haiyi Hu. 2023. "Incidence of Gastric Neoplasms Arising from Autoimmune Metaplastic Atrophic Gastritis: A Systematic Review and Case Reports" Journal of Clinical Medicine 12, no. 3: 1062. https://doi.org/10.3390/jcm12031062
APA StyleChen, C., Yang, Y., Li, P., & Hu, H. (2023). Incidence of Gastric Neoplasms Arising from Autoimmune Metaplastic Atrophic Gastritis: A Systematic Review and Case Reports. Journal of Clinical Medicine, 12(3), 1062. https://doi.org/10.3390/jcm12031062






