Co-Culture of Cryopreserved Healthy Sertoli Cells with Testicular Tissue of Non-Obstructive Azoospermia (NOA) Patients in Culture Media Containing Follicle-Stimulating Hormone (FSH)/Testosterone Has No Advantage in Germ Cell Maturation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.1.1. Healthy Sertoli Cell Isolation and Culture
2.1.2. Characterization of Sertoli Cells
Flow Cytometry
Immunofluorescence
2.1.3. Studying the Viability (Apoptosis) and Proliferation of SCs
FITC-Conjugated Annexin V/PI Staining
MTT Assay
2.2. Preparation of Testicular Tissue for Co-Culture
2.3. Co-Culture Studies
2.4. Measuring Germ Cell Markers by Flow Cytometry
2.5. Statistical Analysis
3. Results
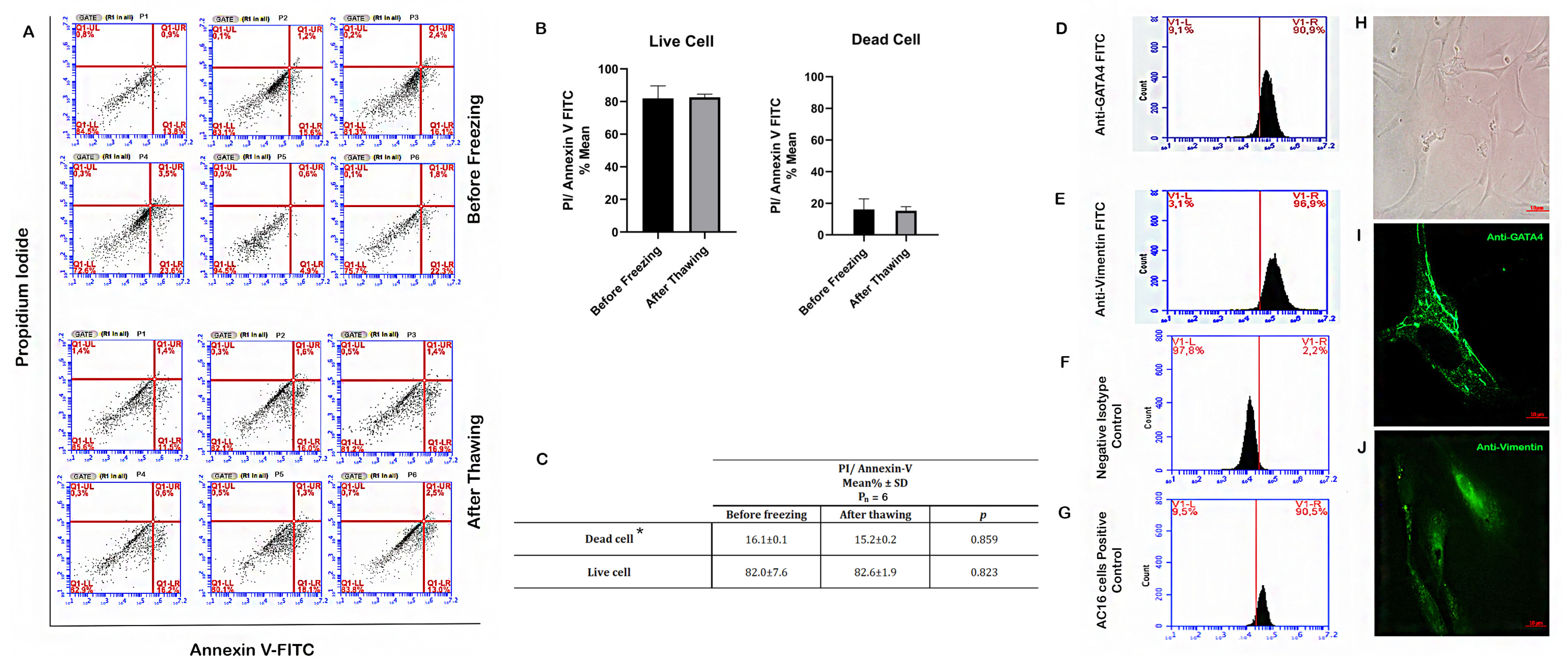
3.1. Viability of SCs before Freezing and Post-Thawing
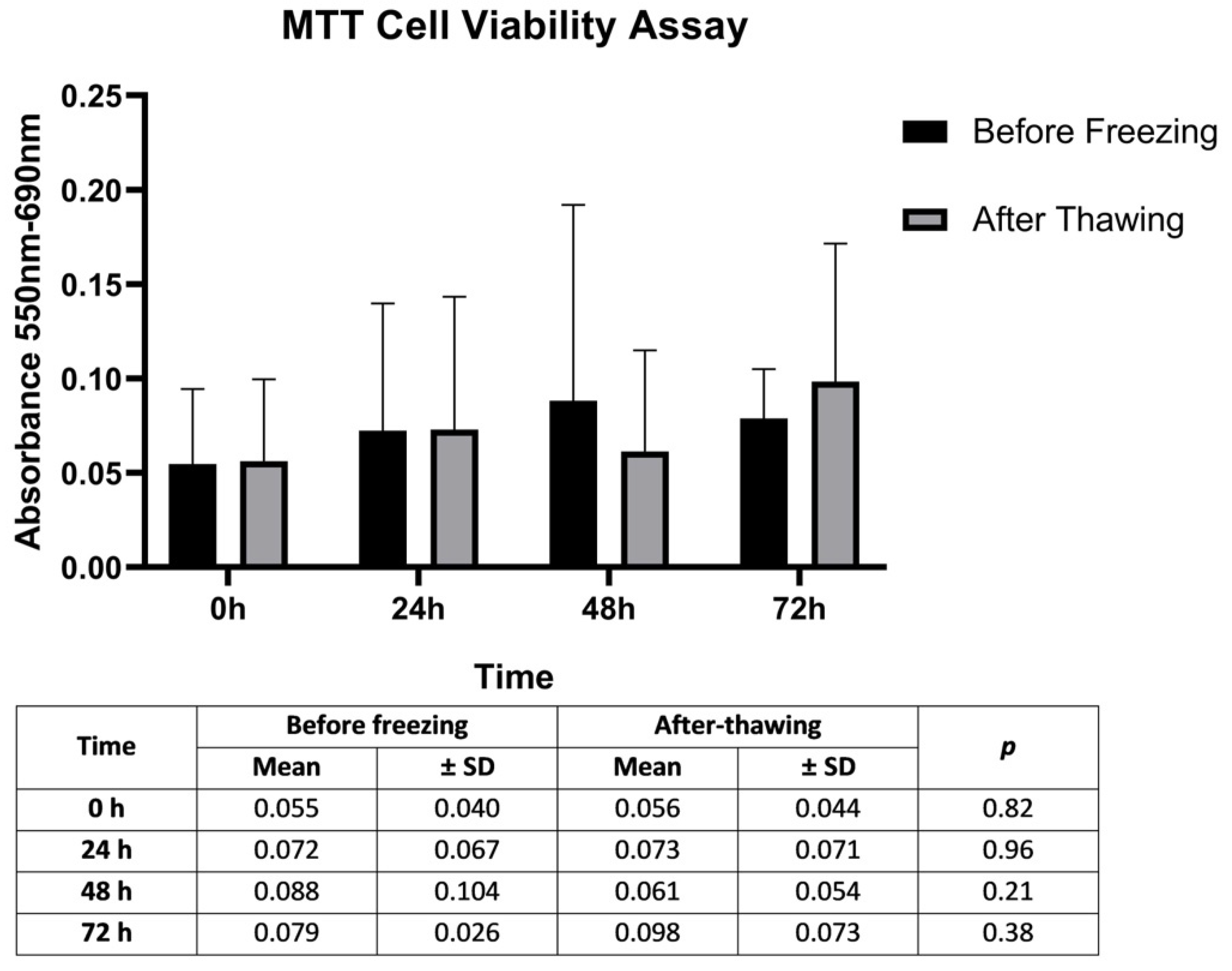
3.2. Proliferation Rates of SCs before Freezing and Post-Thawing
3.3. Characterization of SCs
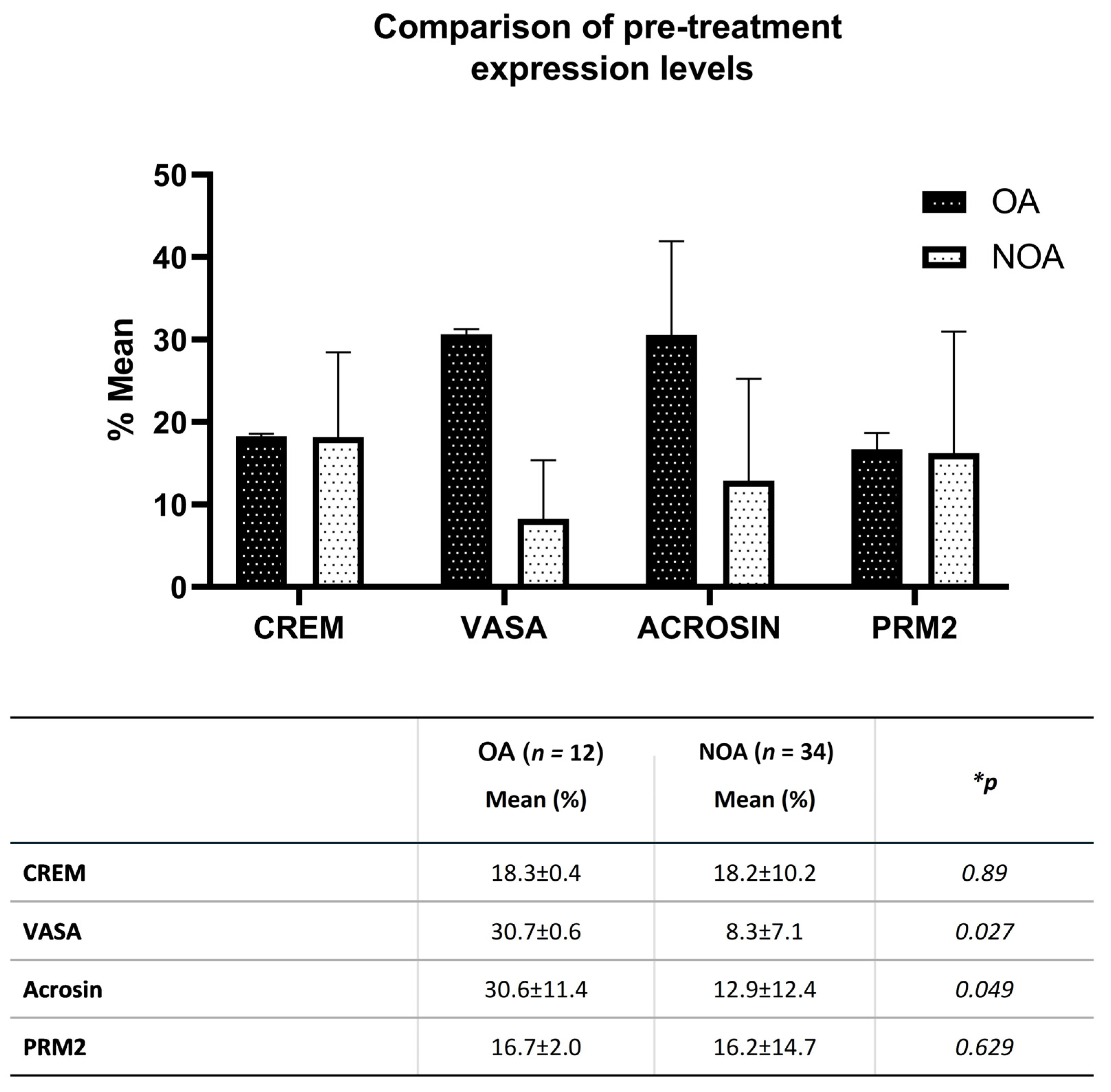
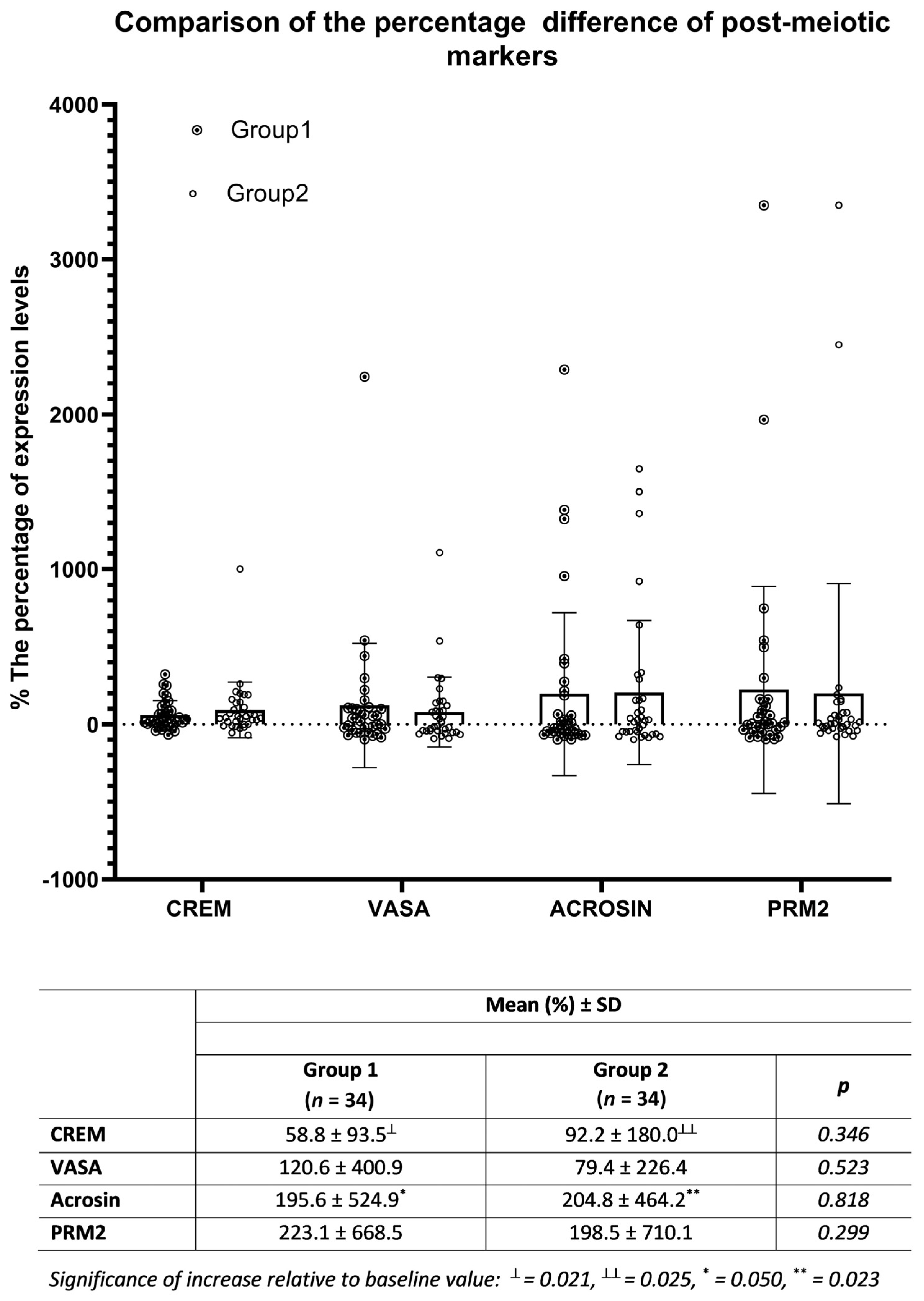
3.4. Co-Culture Results of SCs with Testicular Tissue Suspension
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alkandari, M.H.; Zini, A. Medical management of non-obstructive azoospermia: A systematic review. Arab. J. Urol. 2021, 19, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Bernie, A.M.; Mata, D.A.; Ramasamy, R.; Schlegel, P.N. Comparison of microdissection testicular sperm extraction, conventional testicular sperm extraction, and testicular sperm aspiration for nonobstructive azoospermia: A systematic review and meta-analysis. Fertil. Steril. 2015, 104, 1099–1103.e3. [Google Scholar] [CrossRef] [PubMed]
- Corona, G.; Minhas, S.; Giwercman, A.; Bettocchi, C.; Dinkelman-Smit, M.; Dohle, G.; Fusco, F.; Kadioglou, A.; Kliesch, S.; Kopa, Z.; et al. Sperm recovery and ICSI outcomes in men with non-obstructive azoospermia: A systematic review and meta-analysis. Hum. Reprod. Updat. 2019, 25, 733–757. [Google Scholar] [CrossRef]
- Tanaka, A.; Nagayoshi, M.; Takemoto, Y.; Tanaka, I.; Kusunoki, H.; Watanabe, S.; Kuroda, K.; Takeda, S.; Ito, M.; Yanagimachi, R. Fourteen babies born after round spermatid injection into human oocytes. Proc. Natl. Acad. Sci. USA 2015, 112, 14629–14634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cremades, N.; Bernabeu, R.; Barros, A.; Sousa, M. In-vitro maturation of round spermatids using co-culture on Vero cells. Hum. Reprod. 1999, 14, 1287–1293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanaka, A.; Nagayoshi, M.; Awata, S.; Mawatari, Y.; Tanaka, I.; Kusunoki, H. Completion of meiosis in human primary spermatocytes through in vitro coculture with Vero cells. Fertil. Steril. 2003, 79 (Suppl. S1), 795–801. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Ping, P.; Ma, M.; Li, P.; Tian, R.; Yang, H.; Liu, Y.; Gong, Y.; Zhang, Z.; Li, Z.; et al. Generation of Haploid Spermatids with Fertilization and Development Capacity from Human Spermatogonial Stem Cells of Cryptorchid Patients. Stem Cell Rep. 2014, 3, 663–675. [Google Scholar] [CrossRef]
- Qian, C.; Meng, Q.; Lu, J.; Zhang, L.; Li, H.; Huang, B. Human amnion mesenchymal stem cells restore spermatogenesis in mice with busulfan-induced testis toxicity by inhibiting apoptosis and oxidative stress. Stem Cell Res. Ther. 2020, 11, 290. [Google Scholar] [CrossRef]
- Mobarak, H.; Heidarpour, M.; Rahbarghazi, R.; Nouri, M.; Mahdipour, M. Amniotic fluid-derived exosomes improved spermatogenesis in a rat model of azoospermia. Life Sci. 2021, 274, 119336. [Google Scholar] [CrossRef]
- Liang, J.; Wang, N.; He, J.; Du, J.; Guo, Y.; Li, L.; Wu, W.; Yao, C.; Li, Z.; Kee, K. Induction of Sertoli-like cells from human fibroblasts by NR5A1 and GATA4. eLife 2019, 8, e48767. [Google Scholar] [CrossRef]
- Gholami, K.; Pourmand, G.; Koruji, M.; Sadighigilani, M.; Navid, S.; Izadyar, F.; Abbasi, M. Efficiency of colony formation and differentiation of human spermatogenic cells in two different culture systems. Reprod. Biol. 2018, 18, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Gye, M.C.; Choi, K.W.; Hong, J.Y.; Lee, Y.B.; Park, D.-W.; Lee, S.J.; Min, C.K. In vitro differentiation of germ cells from nonobstructive azoospermic patients using three-dimensional culture in a collagen gel matrix. Fertil. Steril. 2007, 87, 824–833. [Google Scholar] [CrossRef] [PubMed]
- Yi, H.; Xiao, S.; Zhang, Y. Stage-specific approaches promote in vitro induction for spermatogenesis. Vitr. Cell. Dev. Biol. Anim. 2018, 54, 217–230. [Google Scholar] [CrossRef] [PubMed]
- Mirzapour, T.; Movahedin, M.; Ibrahim, T.A.T.; Koruji, M.; Haron, A.W.; Nowroozi, M.R.; Rafieian, S.H. Effects of basic fibroblast growth factor and leukaemia inhibitory factor on proliferation and short-term culture of human spermatogonial stem cells. Andrologia 2012, 44, 41–55. [Google Scholar] [CrossRef]
- Medrano, J.V.; Vilanova-Pérez, T.; Fornés-Ferrer, V.; Navarro-Gomezlechon, A.; Martínez-Triguero, M.L.; García, S.; Gómez-Chacón, J.; Povo, I.; Pellicer, A.; Andrés, M.M.; et al. Influence of temperature, serum, and gonadotropin supplementation in short- and long-term organotypic culture of human immature testicular tissue. Fertil. Steril. 2018, 110, 1045–1057.e3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, M.; Yang, S.; Zhang, Z.; Li, P.; Gong, Y.; Liu, L.; Zhu, Y.; Tian, R.; Liu, Y.; Wang, X.; et al. Sertoli cells from non-obstructive azoospermia and obstructive azoospermia patients show distinct morphology, Raman spectrum and biochemical phenotype. Hum. Reprod. 2013, 28, 1863–1873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azizi, H.; Karoii, D.H.; Skutella, T. Whole Exome Sequencing and In Silico Analysis of Human Sertoli in Patients with Non-Obstructive Azoospermia. Int. J. Mol. Sci. 2022, 23, 12570. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.-H.; Gu, S.-J.; Tian, W.-J.; Song, W.-P.; Gu, Y.-Y.; Yuan, Y.-M.; Li, X.-S.; Xin, Z.-C.; Kim, S.W.; Guan, R.-L.; et al. Extracellular vesicles derived from human Sertoli cells: Characterizations, proteomic analysis, and miRNA profiling. Mol. Biol. Rep. 2022, 49, 4673–4681. [Google Scholar] [CrossRef]
- Oduwole, O.O.; Peltoketo, H.; Huhtaniemi, I.T. Role of Follicle-Stimulating Hormone in Spermatogenesis. Front. Endocrinol. 2018, 9, 763. [Google Scholar] [CrossRef] [Green Version]
- Gnessi, L.; Fabbri, A.; Spera, G. Gonadal Peptides as Mediators of Development and Functional Control of the Testis: An Integrated System with Hormones and Local Environment*. Endocr. Rev. 1997, 18, 541–609. [Google Scholar] [CrossRef]
- Arato, I.; Grande, G.; Barrachina, F.; Bellucci, C.; Lilli, C.; Jodar, M.; Aglietti, M.C.; Mancini, F.; Vincenzoni, F.; Pontecorvi, A.; et al. “In vitro” Effect of Different Follicle—Stimulating Hormone Preparations on Sertoli Cells: Toward a Personalized Treatment for Male Infertility. Front. Endocrinol. 2020, 11, 401. [Google Scholar] [CrossRef]
- Sousa, M.; Cremades, N.; Alves, C.; Silva, J.; Barros, A. Developmental potential of human spermatogenic cells co-cultured with Sertoli cells. Hum. Reprod. 2002, 17, 161–172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boitani, C.; Politi, M.G.; Menna, T. Spermatogonial Cell Proliferation in Organ Culture of Immature Rat Testis1. Biol. Reprod. 1993, 48, 761–767. [Google Scholar] [CrossRef] [PubMed]
- Stanton, P.G.; Sluka, P.; Foo, C.F.H.; Stephens, A.N.; Smith, I.; McLachlan, R.I.; O’Donnell, L. Proteomic Changes in Rat Spermatogenesis in Response to In Vivo Androgen Manipulation; Impact on Meiotic Cells. PLoS ONE 2012, 7, e41718. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, A.; Nagayoshi, M.; Awata, S.; Tanaka, I.; Kusunoki, H. Differentiation of human round spermatids into motile spermatozoa through in vitro coculture with Vero cells. Reprod. Med. Biol. 2009, 8, 169–175. [Google Scholar] [CrossRef] [PubMed]
- McLachlan, R.I.; Wreford, N.G.; O’Donnell, L.; de Kretser, D.M.; Robertson, D.M. The endocrine regulation of spermatogenesis: Independent roles for testosterone and FSH. J. Endocrinol. 1996, 148, 1–9. [Google Scholar] [CrossRef]
- Tesarik, J.; Nagy, P.; Abdelmassih, R.; Greco, E.; Mendoza, C. Pharmacological concentrations of follicle-stimulating hormone and testosterone improve the efficacy of in vitro germ cell differentiation in men with maturation arrest. Fertil. Steril. 2002, 77, 245–251. [Google Scholar] [CrossRef]
- Solomon, R.; AbuMadighem, A.; Kapelushnik, J.; Amano, B.-C.; Lunenfeld, E.; Huleihel, M. Involvement of Cytokines and Hormones in the Development of Spermatogenesis In Vitro from Spermatogonial Cells of Cyclophosphamide-Treated Immature Mice. Int. J. Mol. Sci. 2021, 22, 1672. [Google Scholar] [CrossRef]
- Cooper, T.G.; Noonan, E.; Von Eckardstein, S.; Auger, J.; Gordon Baker, H.W.; Behre, H.M.; Haugen, T.B.; Kruger, T.; Wang, C.; Mbizvo, M.T.; et al. World Health Organization reference values for human semen characteristics. Hum. Reprod. Update 2010, 16, 231–245. [Google Scholar] [CrossRef]
- Ozkavukcu, S.; Aydos, K.; Ibis, E.; Isbacar, S.; Kizil, S. A laboratory modification to testicular sperm preparation technique improves spermatogenic cell yield. Asian J. Androl. 2014, 16, 852–857. [Google Scholar] [CrossRef]
- Caroppo, E.; Colpi, E.M.; D’Amato, G.; Gazzano, G.; Colpi, G.M. Prediction model for testis histology in men with non-obstructive azoospermia: Evidence for a limited predictive role of serum follicle-stimulating hormone. J. Assist. Reprod. Genet. 2019, 36, 2575–2582. [Google Scholar] [CrossRef] [PubMed]
- Aydos, K.; Demirel, L.C.; Baltacı, V.; Ünlü, C. Enzymatic digestion plus mechanical searching improves testicular sperm retrieval in non-obstructive azoospermia cases. Eur. J. Obstet. Gynecol. Reprod. Biol. 2005, 120, 80–86. [Google Scholar] [CrossRef]
- Lakpour, M.R.; Koruji, M.; Shahverdi, A.; Aghajanpour, S.; Naghandar, M.R.; Gilani, M.A.S.; Sabbaghian, M.; Aflatoonian, R. The Expression of TLR2 and TLR3 in Sertoli Cells of Azoospermic Patients. Cell J. 2017, 19, 375–385. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Yang, D.; Jiang, Y.; Cai, N.; Yang, R.; Zhang, X. Effective isolation of Sertoli cells from New Zealand rabbit testis. J. Adv. Vet. Anim. Res. 2021, 8, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Kabiri, D.; Safrai, M.; Gropp, M.; Hidas, G.; Mordechai-Daniel, T.; Meir, K.; Revel, A.; Imbar, T.; Reubinoff, B. Establishment of a controlled slow freezing-based approach for experimental clinical cryopreservation of human prepubertal testicular tissues. F&S Rep. 2021, 3, 47–56. [Google Scholar] [CrossRef]
- Heidargholizadeh, S.; Aydos, S.E.; Yukselten, Y.; Ozkavukcu, S.; Sunguroglu, A.; Aydos, K. A differential cytokine expression profile before and after rFSH treatment in Sertoli cell cultures of men with nonobstructive azoospermia. Andrologia 2017, 49, e12647. [Google Scholar] [CrossRef]
- Yukselten, Y.; Aydos, O.S.E.; Sunguroglu, A.; Aydos, K. Investigation of CD133 and CD24 as candidate azoospermia markers and their relationship with spermatogenesis defects. Gene 2019, 706, 211–221. [Google Scholar] [CrossRef]
- Sluka, P.; O’Donnell, L.; Bartles, J.R.; Stanton, P. FSH regulates the formation of adherens junctions and ectoplasmic specialisations between rat Sertoli cells in vitro and in vivo. J. Endocrinol. 2006, 189, 381–395. [Google Scholar] [CrossRef]
- Fix, C.; Jordan, C.; Cano, P.; Walker, W.H. Testosterone activates mitogen-activated protein kinase and the cAMP response element binding protein transcription factor in Sertoli cells. Proc. Natl. Acad. Sci. USA 2004, 101, 10919–10924. [Google Scholar] [CrossRef] [Green Version]
- Riboldi, M.; Rubio, C.; Pellicer, A.; Gil-Salom, M.; Simón, C. In vitro production of haploid cells after coculture of CD49f+ with Sertoli cells from testicular sperm extraction in nonobstructive azoospermic patients. Fertil. Steril. 2012, 98, 580–590.e4. [Google Scholar] [CrossRef]
- Cao, B.; Qin, J.; Pan, B.; Qazi, I.H.; Ye, J.; Fang, Y.; Zhou, G. Oxidative Stress and Oocyte Cryopreservation: Recent Advances in Mitigation Strategies Involving Antioxidants. Cells 2022, 11, 3573. [Google Scholar] [CrossRef] [PubMed]
- Tatone, C.; Di Emidio, G.; Vento, M.; Ciriminna, R.; Artini, P.G. Cryopreservation and oxidative stress in reproductive cells. Gynecol. Endocrinol. 2010, 26, 563–567. [Google Scholar] [CrossRef] [PubMed]
- Kupcho, K.R.; Niles, A.L. A Real-Time, Bioluminescent Apoptosis Assay. Methods Mol. Biol. 2021, 2255, 55–67. [Google Scholar] [CrossRef] [PubMed]
- Baert, Y.; Braye, A.; Struijk, R.B.; van Pelt, A.M.; Goossens, E. Cryopreservation of testicular tissue before long-term testicular cell culture does not alter in vitro cell dynamics. Fertil. Steril. 2015, 104, 1244–1252.e4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chui, K.; Trivedi, A.; Cheng, C.Y.; Cherbavaz, D.B.; Dazin, P.F.; Huynh, A.L.T.; Mitchell, J.B.; Rabinovich, G.A.; Noble-Haeusslein, L.J.; John, C.M. Characterization and Functionality of Proliferative Human Sertoli Cells. Cell Transplant. 2011, 20, 619–635. [Google Scholar] [CrossRef] [Green Version]
- Portela, J.M.D.; De Winter-Korver, C.M.; Van Daalen, S.K.M.; Meißner, A.; De Melker, A.A.; Repping, S.; Van Pelt, A.M.M. Assessment of fresh and cryopreserved testicular tissues from (pre)pubertal boys during organ culture as a strategy for in vitro spermatogenesis. Hum. Reprod. 2019, 34, 2443–2455. [Google Scholar] [CrossRef]
- Castrillon, D.H.; Quade, B.J.; Wang, T.Y.; Quigley, C.; Crum, C.P. The human VASA gene is specifically expressed in the germ cell lineage. Proc. Natl. Acad. Sci. USA 2000, 97, 9585–9590. [Google Scholar] [CrossRef] [Green Version]
- Kashiwabara, S.-I.; Arai, Y.; Kodaira, K.; Baba, T. Acrosin biosynthesis in meiotic and postmelotic spermatogenic cells. Biochem. Biophys. Res. Commun. 1990, 173, 240–245. [Google Scholar] [CrossRef]
- Zuo, X.; Rong, B.; Li, L.; Lv, R.; Lan, F.; Tong, M.-H. The histone methyltransferase SETD2 is required for expression of acrosin-binding protein 1 and protamines and essential for spermiogenesis in mice. J. Biol. Chem. 2018, 293, 9188–9197. [Google Scholar] [CrossRef] [Green Version]
- Ventelä, S.; Mulari, M.; Okabe, M.; Tanaka, H.; Nishimune, Y.; Toppari, J.; Parvinen, M. Regulation of acrosome formation inmice expressing green fluorescent protein as a marker. Tissue Cell 2000, 32, 501–507. [Google Scholar] [CrossRef]
- Schill, W.B.; Engel, W.; Flörke-Gerloff, S.; Töpfer-Petersen, E.; Müller-Esterl, W. Acrosin and the acrosome in human spermatogenesis. Hum. Genet. 1983, 65, 61–67. [Google Scholar] [CrossRef]
- Kallajoki, M.; Parvinen, M.; Suominen, J. Expression of Acrosin during Mouse Spermatogenesis: A Biochemical and Immunocytochemical Analysis by a Monoctonal Antibody C 11 H1. Biol. Reprod. 1986, 35, 157–165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sassone-Corsi, P. Coupling gene expression to cAMP signalling: Role of CREB and CREM. Int. J. Biochem. Cell Biol. 1998, 30, 27–38. [Google Scholar] [CrossRef]
- Behr, R.; Weinbauer, G.F. Germ Cell-Specific Cyclic Adenosine 3′,5′-Monophosphate Response Element Modulator Expression in Rodent and Primate Testis Is Maintained Despite Gonadotropin Deficiency*. Endocrinology 1999, 140, 2746–2754. [Google Scholar] [CrossRef] [PubMed]
- Don, J.; Stelzer, G. The expanding family of CREB/CREM transcription factors that are involved with spermatogenesis. Mol. Cell. Endocrinol. 2002, 187, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Blendy, J.A.; Kaestner, K.H.; Weinbauer, G.F.; Nieschlag, E.; Schütz, G. Severe impairment of permatogenesis in mice lacking the CREM gene. Nature 1996, 380, 162–165. [Google Scholar] [CrossRef]
- Weinbauer, G.F.; Behr, R.; Bergmann, M.; Nieschlag, E. Testicular cAMP responsive element modulator (CREM) protein is expressed in round spermatids but is absent or reduced in men with round spermatid maturation arrest. Mol. Hum. Reprod. 1998, 4, 9–15. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Zhou, S.; Liao, L.; Chen, X.; Meistrich, M.; Xu, J. Jmjd1a Demethylase-regulated Histone Modification Is Essential for cAMP-response Element Modulator-regulated Gene Expression and Spermatogenesis. J. Biol. Chem. 2010, 285, 2758–2770. [Google Scholar] [CrossRef] [Green Version]
- Jazireian, P.; Favaedi, R.; Sadighi Gilani, M.A.; Shahhoseini, M. Dynamic Expression and Chromatin Incorporation of ACT and CREM Transcription Factors in Testis Tissues of Infertile Men. Cell J. 2021, 23, 736–741. [Google Scholar] [CrossRef]
- Allen, C.M.; Lopes, F.; Mitchell, R.T.; Spears, N. How does chemotherapy treatment damage the prepubertal testis? Reproduction 2018, 156, R209–R233. [Google Scholar] [CrossRef]
- Delkhosh, A.; Delashoub, M.; Tehrani, A.A.; Bahrami, A.M.; Niazi, V.; Shoorei, H.; Banimohammad, M.; Kalarestaghi, H.; Shokoohi, M.; Agabalazadeh, A.; et al. Upregulation of FSHR and PCNA by administration of coenzyme Q10 on cyclophosphamide-induced premature ovarian failure in a mouse model. J. Biochem. Mol. Toxicol. 2019, 33, e22398. [Google Scholar] [CrossRef] [PubMed]
- Oduwole, O.O.; Huhtaniemi, I.T.; Misrahi, M. The Roles of Luteinizing Hormone, Follicle-Stimulating Hormone and Testosterone in Spermatogenesis and Folliculogenesis Revisited. Int. J. Mol. Sci. 2021, 22, 12735. [Google Scholar] [CrossRef] [PubMed]
- Dimitriadis, F.; Tsiampali, C.; Chaliasos, N.; Tsounapi, P.; Takenaka, A.; Sofikitis, N. The Sertoli cell as the orchestra conductor of spermatogenesis: Spermatogenic cells dance to the tune of testosterone. Hormones 2015, 14, 479–503. [Google Scholar] [CrossRef]
- Pieri, N.C.G.; Mançanares, A.C.F.; de Souza, A.F.; Fernandes, H.; Diaza, A.M.G.; Bressan, F.F.; Roballo, K.C.S.; Casals, J.B.; Binelli, M.; Ambrósio, C.E.; et al. Xenotransplantation of canine spermatogonial stem cells (cSSCs) regulated by FSH promotes spermatogenesis in infertile mice. Stem Cell Res. Ther. 2019, 10, 135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanjo, H.; Komeya, M.; Sato, T.; Abe, T.; Katagiri, K.; Yamanaka, H.; Ino, Y.; Arakawa, N.; Hirano, H.; Yao, T.; et al. In vitro mouse spermatogenesis with an organ culture method in chemically defined medium. PLoS ONE 2018, 13, e0192884. [Google Scholar] [CrossRef] [Green Version]
- Hasegawa, H.; Terada, Y.; Ugajin, T.; Yaegashi, N.; Sato, K. A novel culture system for mouse spermatid maturation which produces elongating spermatids capable of inducing calcium oscillation during fertilization and embryonic development. J. Assist. Reprod. Genet. 2010, 27, 565–570. [Google Scholar] [CrossRef] [Green Version]
- Komeya, M.; Kimura, H.; Nakamura, H.; Yokonishi, T.; Sato, T.; Kojima, K.; Hayashi, K.; Katagiri, K.; Yamanaka, H.; Sanjo, H.; et al. Long-term ex vivo maintenance of testis tissues producing fertile sperm in a microfluidic device. Sci. Rep. 2016, 6, 21472. [Google Scholar] [CrossRef] [Green Version]
- Sato, T.; Katagiri, K.; Kojima, K.; Komeya, M.; Yao, M.; Ogawa, T. In Vitro Spermatogenesis in Explanted Adult Mouse Testis Tissues. PLoS ONE 2015, 10, e0130171. [Google Scholar] [CrossRef]
- Yokonishi, T.; McKey, J.; Ide, S.; Capel, B. Sertoli cell ablation and replacement of the spermatogonial niche in mouse. Nat. Commun. 2020, 11, 40. [Google Scholar] [CrossRef] [Green Version]
- Dubois, W.; Callard, G.V. Culture of intact Sertoli/germ cell units and isolated sertoli cells fromSqualus testis. II. Stimulatory effects of insulin and IGF-I on DNA synthesis in premeiotic stages. J. Exp. Zool. 1993, 267, 233–244. [Google Scholar] [CrossRef]
- Parks, J.; Lee, D.; Huang, S.; Kaproth, M. Prospects for spermatogenesis in vitro. Theriogenology 2003, 59, 73–86. [Google Scholar] [CrossRef] [PubMed]
- Reda, A.; Hou, M.; Winton, T.; Chapin, R.; Söder, O.; Stukenborg, J.-B. In vitro differentiation of rat spermatogonia into round spermatids in tissue culture. Mol. Hum. Reprod. 2016, 22, 601–612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hue, D.; Staub, C.; Perrard-Sapori, M.-H.; Weiss, M.; Nicolle, J.-C.; Vigier, M.; Durand, P. Meiotic Differentiation of Germinal Cells in Three-Week Cultures of Whole Cell Population from Rat Seminiferous Tubules1. Biol. Reprod. 1998, 59, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Merhi, R.A.; Guillaud, L.; Delouis, C.; Cotinot, C. Establishment and Characterization of Immortalized Ovine Sertoli Cell Lines. Vitr. Cell. Dev. Biol. Anim. 2001, 37, 581. [Google Scholar] [CrossRef]
- Sato, Y.; Yoshida, K.; Nozawa, S.; Yoshiike, M.; Arai, M.; Otoi, T.; Iwamoto, T. Establishment of adult mouse Sertoli cell lines by using the starvation method. Reproduction 2013, 145, 505–516. [Google Scholar] [CrossRef] [Green Version]
- Xie, X.; Franěk, R.; Pšenička, M.; Chen, F.; Kašpar, V. Optimization of in vitro culture conditions of common carp germ cells for purpose of surrogate production. Front. Vet. Sci. 2022, 9, 1036495. [Google Scholar] [CrossRef]
- Nasiri, Z.; Hosseini, S.; Hajian, M.; Abedi, P.; Bahadorani, M.; Baharvand, H.; Nasr-Esfahani, M. Effects of different feeder layers on short-term culture of prepubertal bovine testicular germ cells In-vitro. Theriogenology 2012, 77, 1519–1528. [Google Scholar] [CrossRef]
- Nasimi, F.S.; Zahri, S.; Ahmadian, S.; Bagherzadeh, A.; Yamchi, N.N.; Haghighi, L.; Bedate, A.M.; Khalilzadeh, B.; Rahbarghazi, R.; Mahdipour, M. Estradiol modulated differentiation and dynamic growth of CD90+ spermatogonial stem cells toward Sertoli-like cells. Life Sci. 2021, 286, 120041. [Google Scholar] [CrossRef]
- La Vignera, S.; Condorelli, R.A.; Cimino, L.; Cannarella, R.; Giacone, F.; Calogero, A.E. Early Identification of Isolated Sertoli Cell Dysfunction in Prepubertal and Transition Age: Is It Time? J. Clin. Med. 2019, 8, 636. [Google Scholar] [CrossRef] [Green Version]
- Young, J.; Chanson, P.; Salenave, S.; Noël, M.; Brailly, S.; O’Flaherty, M.; Schaison, G.; Rey, R.A. Testicular Anti-Müllerian Hormone Secretion Is Stimulated by Recombinant Human FSH in Patients with Congenital Hypogonadotropic Hypogonadism. J. Clin. Endocrinol. Metab. 2005, 90, 724–728. [Google Scholar] [CrossRef]
| OA (n = 12) | NOA (n = 34) | p | |
|---|---|---|---|
| Age (years) | 31 ± 4.1 | 33 ± 2.6 | n.s. * |
| Total testis volume (cc) | 36 ± 6 | 18 ± 14 | <0.05 |
| FSH (IU/L) | 3.6 ± 1.2 | 18.2 ± 9.3 | <0.05 |
| Total testosterone (ng/mL) | 4.2 ± 1.2 | 2.6 ± 1.6 | n.s. * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aydos, O.S.; Yukselten, Y.; Ozkan, T.; Ozkavukcu, S.; Tuten Erdogan, M.; Sunguroglu, A.; Aydos, K. Co-Culture of Cryopreserved Healthy Sertoli Cells with Testicular Tissue of Non-Obstructive Azoospermia (NOA) Patients in Culture Media Containing Follicle-Stimulating Hormone (FSH)/Testosterone Has No Advantage in Germ Cell Maturation. J. Clin. Med. 2023, 12, 1073. https://doi.org/10.3390/jcm12031073
Aydos OS, Yukselten Y, Ozkan T, Ozkavukcu S, Tuten Erdogan M, Sunguroglu A, Aydos K. Co-Culture of Cryopreserved Healthy Sertoli Cells with Testicular Tissue of Non-Obstructive Azoospermia (NOA) Patients in Culture Media Containing Follicle-Stimulating Hormone (FSH)/Testosterone Has No Advantage in Germ Cell Maturation. Journal of Clinical Medicine. 2023; 12(3):1073. https://doi.org/10.3390/jcm12031073
Chicago/Turabian StyleAydos, O. Sena, Yunus Yukselten, Tulin Ozkan, Sinan Ozkavukcu, Meltem Tuten Erdogan, Asuman Sunguroglu, and Kaan Aydos. 2023. "Co-Culture of Cryopreserved Healthy Sertoli Cells with Testicular Tissue of Non-Obstructive Azoospermia (NOA) Patients in Culture Media Containing Follicle-Stimulating Hormone (FSH)/Testosterone Has No Advantage in Germ Cell Maturation" Journal of Clinical Medicine 12, no. 3: 1073. https://doi.org/10.3390/jcm12031073




