Towards Optimum Mandibular Reconstruction for Dental Occlusal Rehabilitation: From Preoperative Virtual Surgery to Autogenous Particulate Cancellous Bone and Marrow Graft with Custom-Made Titanium Mesh—A Retrospective Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Inclusion Criteria and Exclusion Criteria
2.3. Mandibular Defect Classification
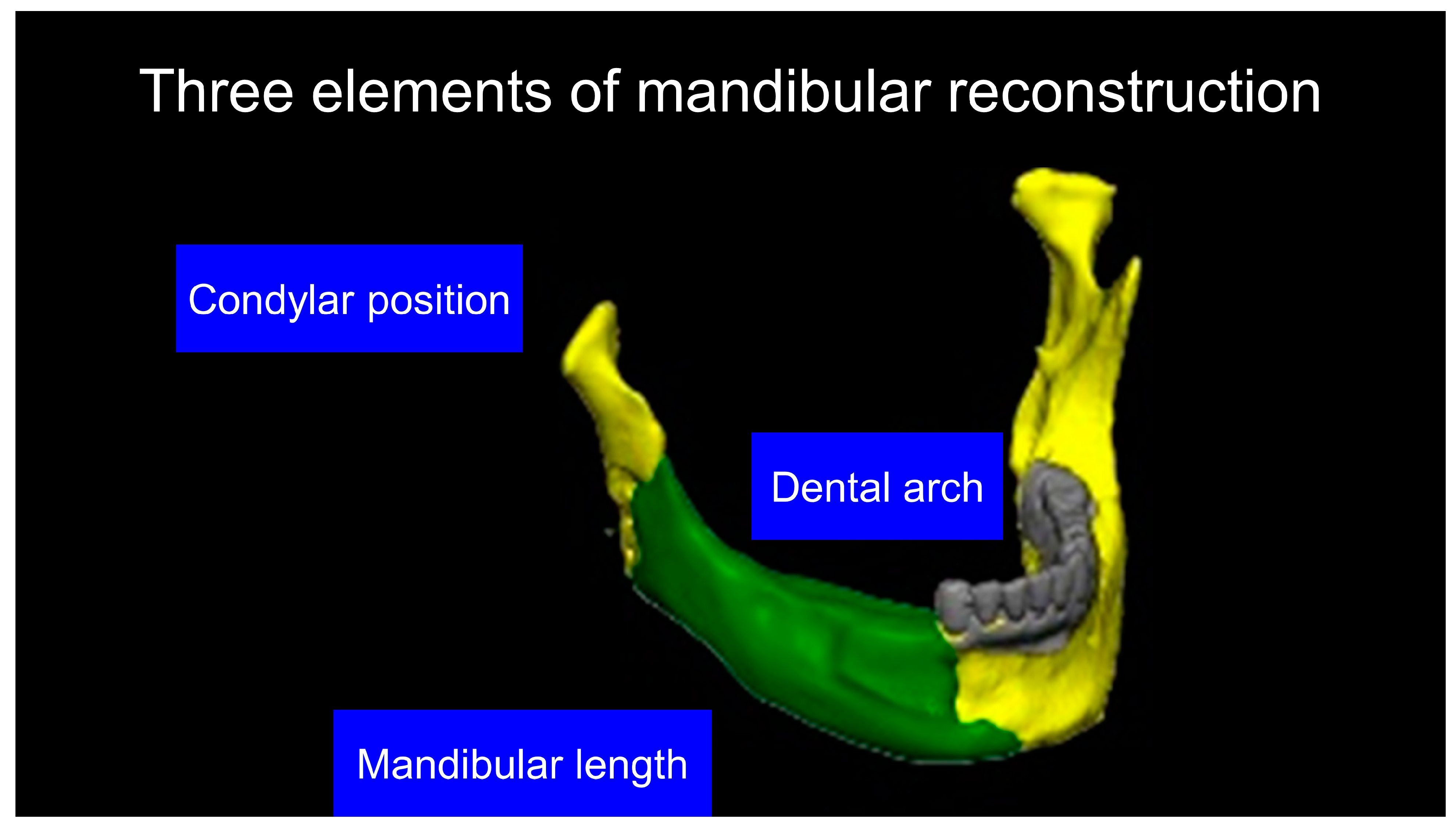
2.4. Mandibular Data Acquisition and the Making of a Virtual Neo-Mandible for Optimum Dental Occlusion
2.5. 3D Printing Model Fabrication and Titanium Mesh Pre-Bending
2.6. Positioning Gauge and Cutting Guide
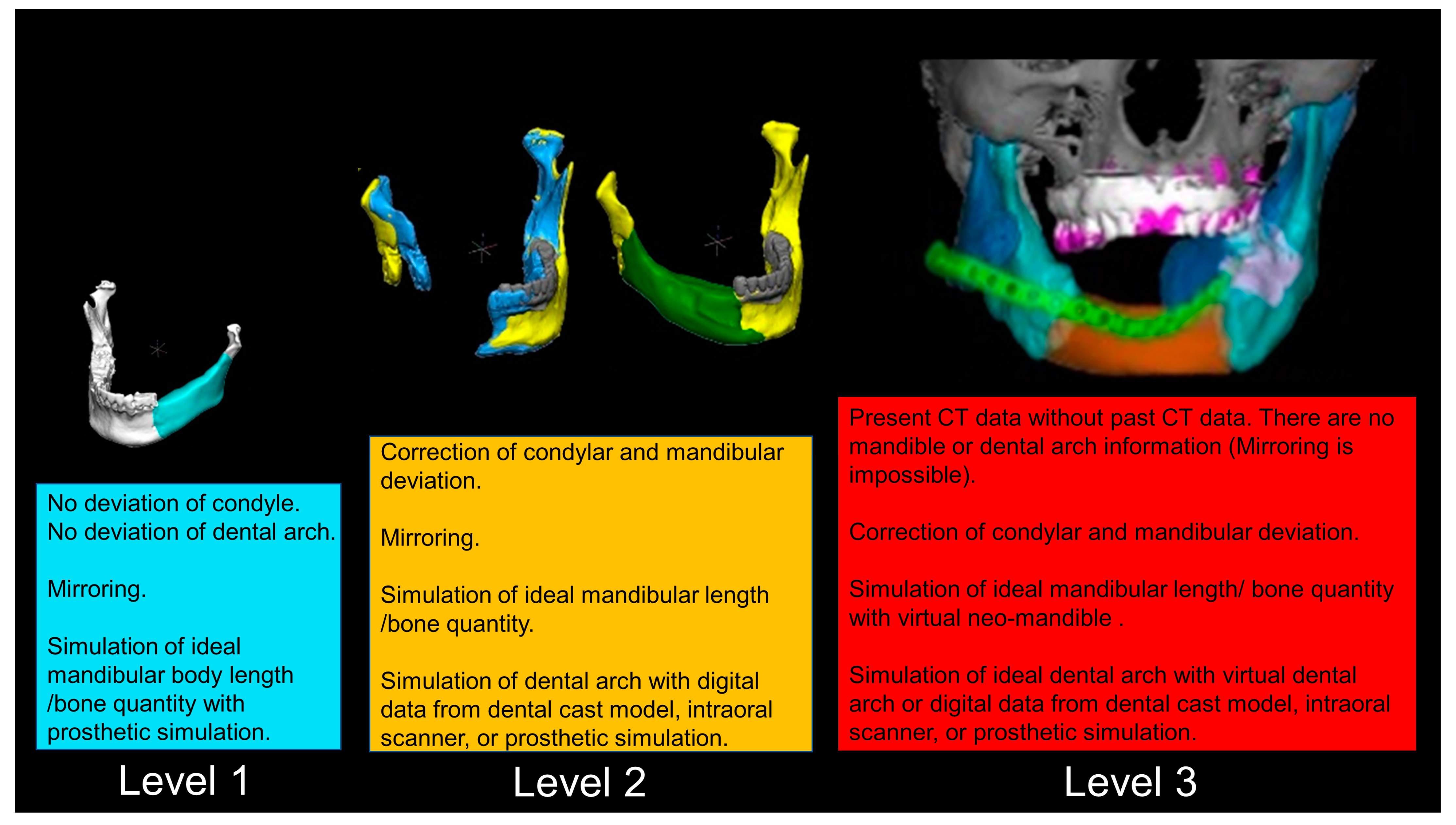
2.7. The Detailed Virtual Reconstruction Technique
2.7.1. Level 1
2.7.2. Level 2
2.7.3. Level 3
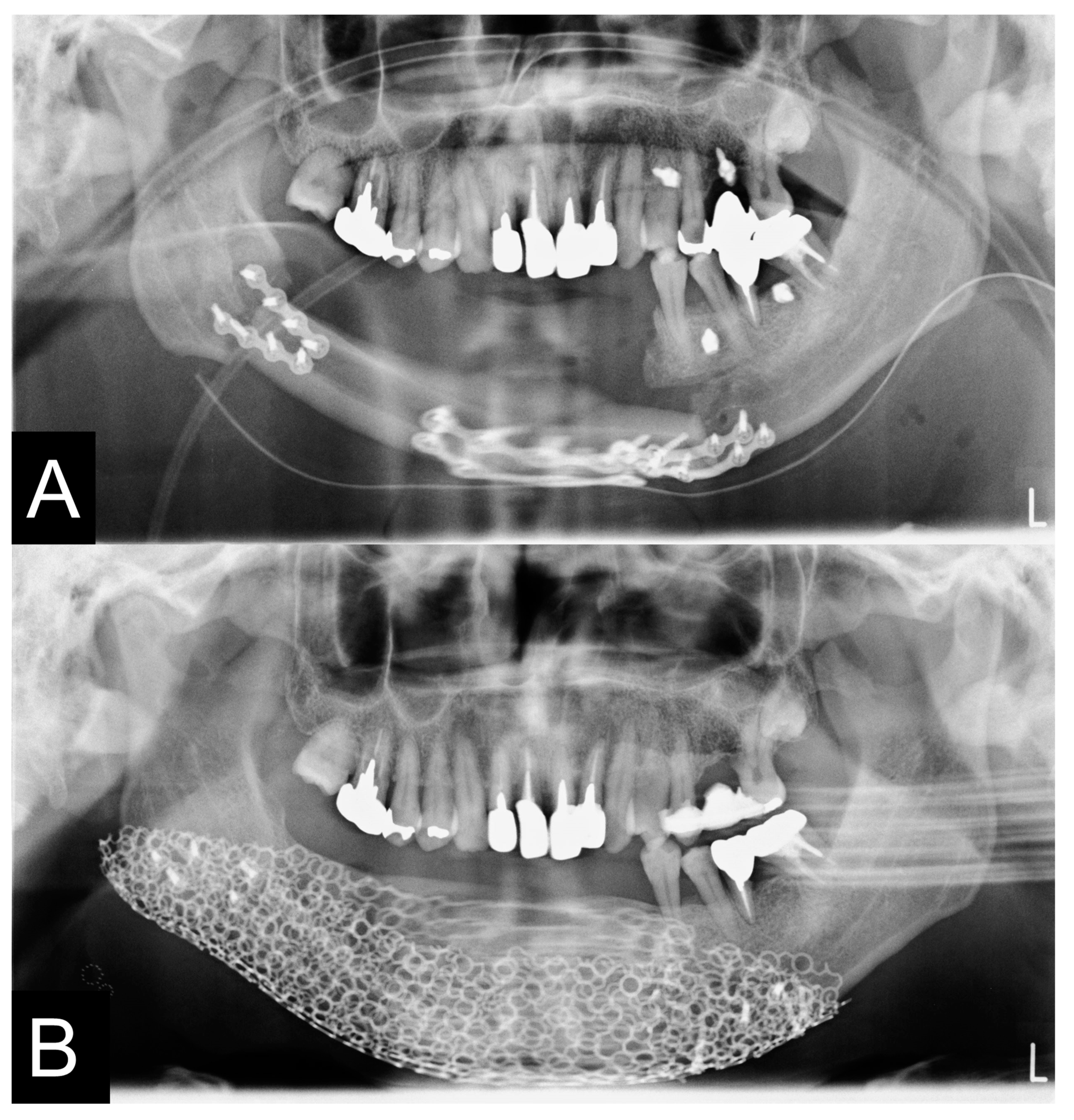
2.8. Clinical Procedures
2.9. Evaluation of Mandible
2.10. Statistical Analysis
3. Results
3.1. Classification of Simulation
3.2. Operation
3.3. Evaluation of Reconstructed Mandible
3.4. Postoperative Complication
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shah, J.P.; Gil, Z. Current concepts in management of oral cancer--surgery. Oral Oncol. 2009, 45, 394–401. [Google Scholar] [CrossRef]
- Pogrel, M.A.; Podlesh, S.; Anthony, J.P.; Alexander, J. A comparison of vascularized and nonvascularized bone grafts for reconstruction of mandibular continuity defects. J. Oral Maxillofac. Surg. 1997, 55, 1200–1206. [Google Scholar] [CrossRef] [PubMed]
- Vu, D.D.; Schmidt, B.L. Quality of life evaluation for patients receiving vascularized versus nonvascularized bone graft reconstruction of segmental mandibular defects. J. Oral Maxillofac. Surg. 2008, 66, 1856–1863. [Google Scholar] [CrossRef] [PubMed]
- Goh, B.T.; Lee, S.; Tideman, H.; Stoelinga, P.J. Mandibular reconstruction in adults: A review. Int. J. Oral Maxillofac. Surg. 2008, 37, 597–605. [Google Scholar] [CrossRef] [PubMed]
- Kumar, B.P.; Venkatesh, V.; Kumar, K.A.J.; Yadav, B.Y.; Mohan, S.R. Mandibular reconstruction: Overview. J. Maxillofac. Oral Surg. 2016, 15, 425–441. [Google Scholar] [CrossRef]
- Chiapasco, M.; Biglioli, F.; Autelitano, L.; Romeo, E.; Brusati, R. Clinical outcome of dental implants placed in fibula-free flaps used for the reconstruction of maxillo-mandibular defects following ablation for tumors or osteoradionecrosis. Clin. Oral Implant. Res. 2006, 17, 220–228. [Google Scholar] [CrossRef]
- Rasmusson, L.; Thor, A.; Sennerby, L. Stability evaluation of implants integrated in grafted and nongrafted maxillary bone: A clinical study from implant placement to abutment connection. Clin. Implant Dent. Relat. Res. 2012, 14, 61–66. [Google Scholar] [CrossRef]
- Wetzels, J.G.H.; Meijer, G.J.; Koole, R.; Adang, E.M.; Merkx, M.A.W.; Speksnijder, C.M. Costs and clinical outcomes of implant placement during ablative surgery and postponed implant placement in curative oral oncology: A five-year retrospective cohort study. Clin. Oral Implant. Res. 2017, 28, 1433–1442. [Google Scholar] [CrossRef]
- Brown, J.S.; Lowe, D.; Kanatas, A.; Schache, A. Mandibular reconstruction with vascularised bone flaps: A systematic review over 25 years. Br. J. Oral Maxillofac. Surg. 2017, 55, 113–126. [Google Scholar] [CrossRef]
- Kakarala, K.; Shnayder, Y.; Tsue, T.T.; Girod, D.A. Mandibular reconstruction. Oral Oncol. 2018, 77, 111–117. [Google Scholar] [CrossRef]
- Petrovic, I.; Panchal, H.; De Souza Franca, P.D.; Hernandez, M.; McCarthy, C.C.; Shah, J.P. A systematic review of validated tools assessing functional and aesthetic outcomes following fibula free flap reconstruction of the mandible. Head Neck. 2019, 41, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Smolka, K.; Kraehenbuehl, M.; Eggensperger, N.; Hallermann, W.; Thoren, H.; Iizuka, T.; Smolka, W. Fibula free flap reconstruction of the mandible in cancer patients: Evaluation of a combined surgical and prosthodontic treatment concept. Oral Oncol. 2008, 44, 571–581. [Google Scholar] [CrossRef]
- Alberga, J.M.; Vosselman, N.; Korfage, A.; Delli, K.; Witjes, M.J.H.; Raghoebar, G.M.; Vissink, A. What is the optimal timing for implant placement in oral cancer patients? A scoping literature review. Oral Dis. 2021, 27, 94–110. [Google Scholar] [CrossRef]
- Bauer, E.; Mazul, A.; Zenga, J.; Graboyes, E.M.; Jackson, R.; Puram, S.V.; Doering, M.; Pipkorn, P. Complications after soft Tissue With Plate vs Bony mandibular reconstruction: A systematic review and meta-analysis. Otolaryngol Head Neck Surg. 2021, 164, 501–511. [Google Scholar] [CrossRef] [PubMed]
- Petrovic, I.; Rosen, E.B.; Matros, E.; Huryn, J.M.; Shah, J.P. Oral rehabilitation of the cancer patient: A formidable challenge. J. Surg. Oncol. 2018, 117, 1729–1735. [Google Scholar] [CrossRef]
- Hundepool, A.C.; Dumans, A.G.; Hofer, S.O.; Fokkens, N.J.; Rayat, S.S.; van der Meij, E.H.; Schepman, K.P. Rehabilitation after mandibular reconstruction with fibula free-flap: Clinical outcome and quality of life assessment. Int. J. Oral Maxillofac. Surg. 2008, 37, 1009–1013. [Google Scholar] [CrossRef]
- Garrett, N.; Roumanas, E.D.; Blackwell, K.E.; Freymiller, E.; Abemayor, E.; Wong, W.K.; Gerratt, B.; Berke, G.; Beumer, J.; Kapur, K.K. Efficacy of conventional and implant-supported mandibular resection prostheses: Study overview and treatment outcomes. J. Prosthet Dent. 2006, 96, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Roumanas, E.D.; Garrett, N.; Blackwell, K.E.; Freymiller, E.; Abemayor, E.; Wong, W.K.; Beumer, J.; Fueki, K.; Fueki, W.; Kapur, K.K. Masticatory and swallowing threshold performances with conventional and implant-supported prostheses after mandibular fibula free-flap reconstruction. J. Prosthet Dent. 2006, 96, 289–297. [Google Scholar] [CrossRef]
- Boyne, P.J. Restoration of osseous defects in maxillofacial casualities. J. Am. Dent. Assoc. 1969, 78, 767–776. [Google Scholar] [CrossRef]
- Carlson, E.R.; Marx, R.E. Part II. Mandibular reconstruction using cancellous cellular bone grafts. J. Oral Maxillofac. Surg. 1996, 54, 889–897. [Google Scholar] [CrossRef]
- Dumbach, J.; Rodemer, H.; Spitzer, W.J.; Steinhäuser, E.W. Mandibular reconstruction with cancellous bone, hydroxylapatite and titanium mesh. J. Craniomaxillofac. Surg. 1994, 22, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Iino, M.; Fukuda, M.; Nagai, H.; Hamada, Y.; Yamada, H.; Nakaoka, K.; Mori, Y.; Chikazu, D.; Saijo, H.; Seto, I.; et al. Evaluation of 15 mandibular reconstructions with Dumbach Titan Mesh-System and particulate cancellous bone and marrow harvested from bilateral posterior ilia. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2009, 107, e1–e8. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, I.; Yamashita, Y.; Yamamoto, N.; Nogami, S.; Yamauchi, K.; Yoshiga, D.; Kaneuji, T.; Takahashi, T. Evaluation of mandibular reconstruction with particulate cancellous bone marrow and titanium mesh after mandibular resection due to tumor surgery. Implant Dent. 2014, 23, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Yamada, H.; Nakaoka, K.; Horiuchi, T.; Kumagai, K.; Ikawa, T.; Shigeta, Y.; Imamura, E.; Iino, M.; Ogawa, T.; Hamada, Y. Mandibular reconstruction using custom-made titanium mesh tray and particulate cancellous bone and marrow harvested from bilateral posterior ilia. J. Plast Surg. Hand Surg. 2014, 48, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Dawood, A.; Marti Marti, B.; Sauret-Jackson, V.; Darwood, A. 3D printing in dentistry. Br. Dent J. 2015, 219, 521–529. [Google Scholar] [CrossRef]
- Zhou, L.B.; Shang, H.T.; He, L.S.; Bo, B.; Liu, G.C.; Liu, Y.P.; Zhao, J.L. Accurate reconstruction of discontinuous mandible using a reverse engineering/computer-aided design/rapid prototyping technique: A preliminary clinical study. J. Oral Maxillofac. Surg. 2010, 68, 2115–2121. [Google Scholar] [CrossRef]
- Yamada, H.; Nakaoka, K.; Sonoyama, T.; Kumagai, K.; Ikawa, T.; Shigeta, Y.; Harada, N.; Kawamura, N.; Ogawa, T.; Hamada, Y. Clinical Usefulness of Mandibular Reconstruction Using Custom-Made titanium Mesh Tray and Autogenous Particulate cancellous Bone and Marrow Harvested from Tibia and/or Ilia. J. Craniofac. Surg. 2016, 27, 586–592. [Google Scholar] [CrossRef]
- Khalifa, G.A.; Abd El Moniem, N.A.; Elsayed, S.A.; Qadry, Y. Segmental mirroring: Does it eliminate the need for intraoperative readjustment of the virtually pre-bent reconstruction plates and is it economically valuable? J. Oral Maxillofac. Surg. 2016, 74, 621–630. [Google Scholar] [CrossRef]
- Shen, Y.; Sun, J.; Li, J.; Ji, T.; Li, M.M.; Huang, W.; Hu, M. Using computer simulation and stereomodel for accurate mandibular reconstruction with vascularized iliac crest flap. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2012, 114, 175–182. [Google Scholar] [CrossRef]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P.; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. PLOS Med. 2007, 4, e296. [Google Scholar] [CrossRef]
- Brown, J.S.; Barry, C.; Ho, M.; Shaw, R. A new classification for mandibular defects after oncological resection. Lancet Oncol. 2016, 17, e23–e30. [Google Scholar] [CrossRef]
- Doi, A.; Takahashi, H.; Syuto, B.; Katayama, M.; Nagashima, H.; Okumura, M. Tailor-made plate design and manufacturing system for treating bone fractures in small animals. J. Adv. Comput. Intell. Intell. Inform. 2013, 17, 588–597. [Google Scholar] [CrossRef]
- Kamio, T.; Hayashi, K.; Onda, T.; Takaki, T.; Shibahara, T.; Yakushiji, T.; Shibui, T.; Kato, H. Utilizing a low-cost desktop 3D printer to develop a “one-stop 3D printing lab” for oral and maxillofacial surgery and dentistry fields. 3D Print Med. 2018, 4, 6. [Google Scholar] [CrossRef]
- Iino, M.; Ishii, H.; Sato, J.; Seto, K. Histological evaluation of autogenous iliac particulate cancellous bone and marrow grafted to alveolar clefts--a preliminary report of five young adult cases. Cleft Palate Craniofac. J. 2000, 37, 55–60. [Google Scholar] [CrossRef]
- Kristensen, K.D.; Schmidt, B.; Stoustrup, P.; Pedersen, T.K. Idiopathic condylar resorptions: 3-dimensional condylar bony deformation, signs and symptoms. Am. J. Orthod Dentofac. Orthop. 2017, 152, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Kanda, Y. Investigation of the freely available easy-to-use software “EZR” for medical statistics. Bone Marrow Transplant. 2013, 48, 452–458. [Google Scholar] [CrossRef]
- Wu, T.F.; Liu, J.Y.; Li, Y.S.; Liu, B. Matching locating holes in multiple plates to record bone position for accurate reconstruction after segmental mandibulectomy. Int. J. Oral Maxillofac. Surg. 2019, 48, 1516–1519. [Google Scholar] [CrossRef]
- Etemad-Shahidi, Y.; Qallandar, O.B.; Evenden, J.; Alifui-Segbaya, F.; Ahmed, K.E. Accuracy of 3-dimensionally printed full-arch dental models: A systematic review. J. Clin. Med. 2020, 9, 3357. [Google Scholar] [CrossRef]
- Davies, J.C.; Chan, H.H.L.; Jozaghi, Y.; Goldstein, D.P.; Irish, J.C. Analysis of simulated mandibular reconstruction using a segmental mirroring technique. J. Craniomaxillofac. Surg. 2019, 47, 468–472. [Google Scholar] [CrossRef]
- Schrag, C.; Chang, Y.M.; Tsai, C.Y.; Wei, F.C. Complete rehabilitation of the mandible following segmental resection. J. Surg. Oncol. 2006, 94, 538–545. [Google Scholar] [CrossRef] [PubMed]
- Hjelm, N.; Ortlip, T.E.; Topf, M.; Luginbuhl, A.; Curry, J.; Krein, H.; Heffelfinger, R. Functional outcomes of temporomandibular joint reconstruction with vascularized tissue. Am. J. Otolaryngol. 2019, 40, 691–695. [Google Scholar] [CrossRef] [PubMed]
- Chao, J.W.; Rohde, C.H.; Chang, M.M.; Kutler, D.I.; Friedman, J.; Spector, J.A. Oral rehabilitation outcomes after free fibula reconstruction of the mandible without condylar restoration. J. Craniofac. Surg. 2014, 25, 415–417. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, I.; Funaki, K.; Yamauchi, K.; Kodama, T.; Takahashi, T. Alveolar ridge reconstruction with titanium mesh and autogenous particulate bone graft: Computed tomography-based evaluations of augmented bone quality and quantity. Clin Implant. Dent. Relat. Res. 2012, 14, 304–311. [Google Scholar] [CrossRef]
- Hakim, S.G.; Kimmerle, H.; Trenkle, T.; Sieg, P.; Jacobsen, H.C. Masticatory rehabilitation following upper and lower jaw reconstruction using vascularised free fibula flap and enossal implants-19 years of experience with a comprehensive concept. Clin Oral. Investig. 2015, 19, 525–534. [Google Scholar] [CrossRef] [PubMed]
- MacIntosh, R.B.; Obwegeser, H.L. Preprosthetic surgery: A scheme for its effective employment. J. Oral Surg. 1967, 25, 397–413. [Google Scholar]
- Iizuka, T.; Häfliger, J.; Seto, I.; Rahal, A.; Mericske-Stern, R.; Smolka, K. Oral rehabilitation after mandibular reconstruction using an osteocutaneous fibula free flap with endosseous implants. Factors affecting the functional outcome in patients with oral cancer. Clin Oral Implant. Res. 2005, 16, 69–79. [Google Scholar] [CrossRef]
- Hoshi, I.; Kawai, T.; Kurosu, S.; Minamino, T.; Onodera, K.; Miyamoto, I.; Yamada, H. Custom-made titanium mesh tray for mandibular reconstruction using an electron beam melting system. Materials 2021, 14, 6556. [Google Scholar] [CrossRef]














| Case | Gender | Age | Diagnosis of Primary Disease | Primary Reconstruction | Bone Defect | Classification Of Js Brown | Schematic of Mandibular Defect |
|---|---|---|---|---|---|---|---|
| 1 | M | 23 | Ameloblastic carcinoma | None | Marginal bone defects | Marginal bone defect |  |
| 2 | M | 73 | SCC Gingiva | Surgical plate | Segmental bone defects | Body-chin Class II |  |
| 3 | F | 78 | SCC Floor of mouth | Rectus abdominis musculocutaneous flap and surgical plate | Segmental bone defects | Body-chin Class III |  |
| 4 | M | 70 | SCC Floor of mouth | Anterior cervical flap | Marginal bone defects | Marginal bone defect |  |
| 5 | F | 69 | SCC Gingiva | Surgical plate | Segmental bone defects | Ramus-body Class I |  |
| 6 | M | 69 | Osteoblastoma of the mandible | None | Marginal bone defects | Marginal bone defect |  |
| 7 | M | 73 | SCC Gingiva | None | Marginal bone defects | Marginal bone defect |  |
| 8 | M | 69 | Ameloblastoma | Simultaneous surgery | Segmental bone defects | Ramus-body Class I |  |
| 9 | M | 58 | Mucoepidermoid carcinoma Floor of mouth | Vascularized fibular bone graft | Segmental bone defects | Ramus-body-chin Class III |  |
| 10 | M | 60 | Ameloblastoma | Fractured surgical plate | Segmental bone defects | Ramus-body Class I |  |
| 11 | F | 62 | SCC Gingiva | Fractured surgical plate | Segmental bone defects | Ramus-body Class II |  |
| 12 | M | 81 | SCC Gingiva | None | Marginal bone defects | Marginal bone defect |  |
| 13 | F | 50 | Odontogenic myxoma | Surgical plate | Segmental bone defects | Body-chin Class II |  |
| 14 | M | 74 | SCC Gingiva | Surgical plate | Segmental bone defects | Body-chin Class III |  |
| 15 | M | 62 | SCC Gingiva | Vascularized scapular bone graft (non-union) | Segmental bone defects | Body-chin Class I |  |
| 16 | F | 45 | Ameloblastoma | Surgical plate | Segmental bone defects | Ramus-body Class I |  |
| 17 | M | 17 | Ossifying fibroma | None | Mandibular partial resection | Marginal bone defect |  |
| 18 | M | 63 | Ameloblastoma | Fractured surgical plate | Segmental bone defects | Ramus-body-chin Class III |  |
| Case | Virtual Reconstruction Technique | Preoperative Simulation of PCBM Amount | The Reality of PCBM Quantity | Preoperative Length (mm) | Postoperative Length (mm) | A Preoperative Sum of the Condylar Axial Angles (Degree) | A Postoperative Sum of the Condylar Axial Angles (Degree) |
|---|---|---|---|---|---|---|---|
| 1 | Level 1 | 2.2 cc | 6.4 cc | 26.7 mm | 25.8 mm | 126° | 127° |
| 2 | Level 2 | 11.3 cc | 20 cc | 45.4 mm | 50.4 mm | 141° | 151° |
| 3 | Level 2 | 11.9 cc | 23 cc | 36.0 mm | 36.0 mm | 150° | 151° |
| 4 | Level 1 | 6.51 cc | 10 cc | 37.7 mm | 37.7 mm | 148° | 147° |
| 5 | Level 2 | 11.3 cc | 17 cc | 46.0 mm | 53.5 mm | 153° | 142° |
| 6 | Level 1 | 14.2 cc | 30 cc | 63.3 mm | 63.3 mm | 143° | 143° |
| 7 | Level 1 | 8.2 cc | 10 cc | 54.6 mm | 54.6 mm | 131° | 131° |
| 8 | Level 1 | 16.8 cc | 17 cc | 69.0 mm | 69.0 mm | 129° | 128° |
| 9 | Level 2 | 20 cc | 40 cc | 63.4 mm | 65.7 mm | 106° | 132° |
| 10 | Level 2 | 19.3 cc | 40 cc | 70.7 mm | 77.7 mm | 129° | 136° |
| 11 | Level 2 | 10 cc | 15 cc | 56.3 mm | 53.1 mm | 136° | 140° |
| 12 | Level 1 | 8 cc | 14 cc | 40.6 mm | 40.6 mm | 149° | 149° |
| 13 | Level 2 | 10 cc | 19 cc | 35.3 mm | 42.4 mm | 150° | 145° |
| 14 | Level 3 | 10.4 cc | 20 cc | 56.3 mm | 59.6 mm | 172° | 161° |
| 15 | Level 3 | 10.6 cc | 24 cc | 54.5 mm | 61.0 mm | 197° | 197° |
| 16 | Level 2 | 9.6 cc | 21 cc | 30.8 mm | 36.0 mm | 164° | 160° |
| 17 | Level 1 | 5.1 cc | 10 cc | 44.5 mm | 44.5 mm | 122° | 122° |
| 18 | Level 2 | 17.7 cc | 32 cc | 54.5 mm | 72.5 mm | 137° | 140° |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Onodera, K.; Miyamoto, I.; Hoshi, I.; Kawamata, S.; Takahashi, N.; Shimazaki, N.; Kondo, H.; Yamada, H. Towards Optimum Mandibular Reconstruction for Dental Occlusal Rehabilitation: From Preoperative Virtual Surgery to Autogenous Particulate Cancellous Bone and Marrow Graft with Custom-Made Titanium Mesh—A Retrospective Study. J. Clin. Med. 2023, 12, 1122. https://doi.org/10.3390/jcm12031122
Onodera K, Miyamoto I, Hoshi I, Kawamata S, Takahashi N, Shimazaki N, Kondo H, Yamada H. Towards Optimum Mandibular Reconstruction for Dental Occlusal Rehabilitation: From Preoperative Virtual Surgery to Autogenous Particulate Cancellous Bone and Marrow Graft with Custom-Made Titanium Mesh—A Retrospective Study. Journal of Clinical Medicine. 2023; 12(3):1122. https://doi.org/10.3390/jcm12031122
Chicago/Turabian StyleOnodera, Kei, Ikuya Miyamoto, Isao Hoshi, Shinsuke Kawamata, Noriaki Takahashi, Nobuko Shimazaki, Hisatomo Kondo, and Hiroyuki Yamada. 2023. "Towards Optimum Mandibular Reconstruction for Dental Occlusal Rehabilitation: From Preoperative Virtual Surgery to Autogenous Particulate Cancellous Bone and Marrow Graft with Custom-Made Titanium Mesh—A Retrospective Study" Journal of Clinical Medicine 12, no. 3: 1122. https://doi.org/10.3390/jcm12031122
APA StyleOnodera, K., Miyamoto, I., Hoshi, I., Kawamata, S., Takahashi, N., Shimazaki, N., Kondo, H., & Yamada, H. (2023). Towards Optimum Mandibular Reconstruction for Dental Occlusal Rehabilitation: From Preoperative Virtual Surgery to Autogenous Particulate Cancellous Bone and Marrow Graft with Custom-Made Titanium Mesh—A Retrospective Study. Journal of Clinical Medicine, 12(3), 1122. https://doi.org/10.3390/jcm12031122






