Latest Evidence on Post-Prostatectomy Urinary Incontinence
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Epidemiology and Risk Factors
3.2. Pathophysiology
3.2.1. Patients’ Related Factors
3.2.2. Biological Factors
3.2.3. Surgical Factors
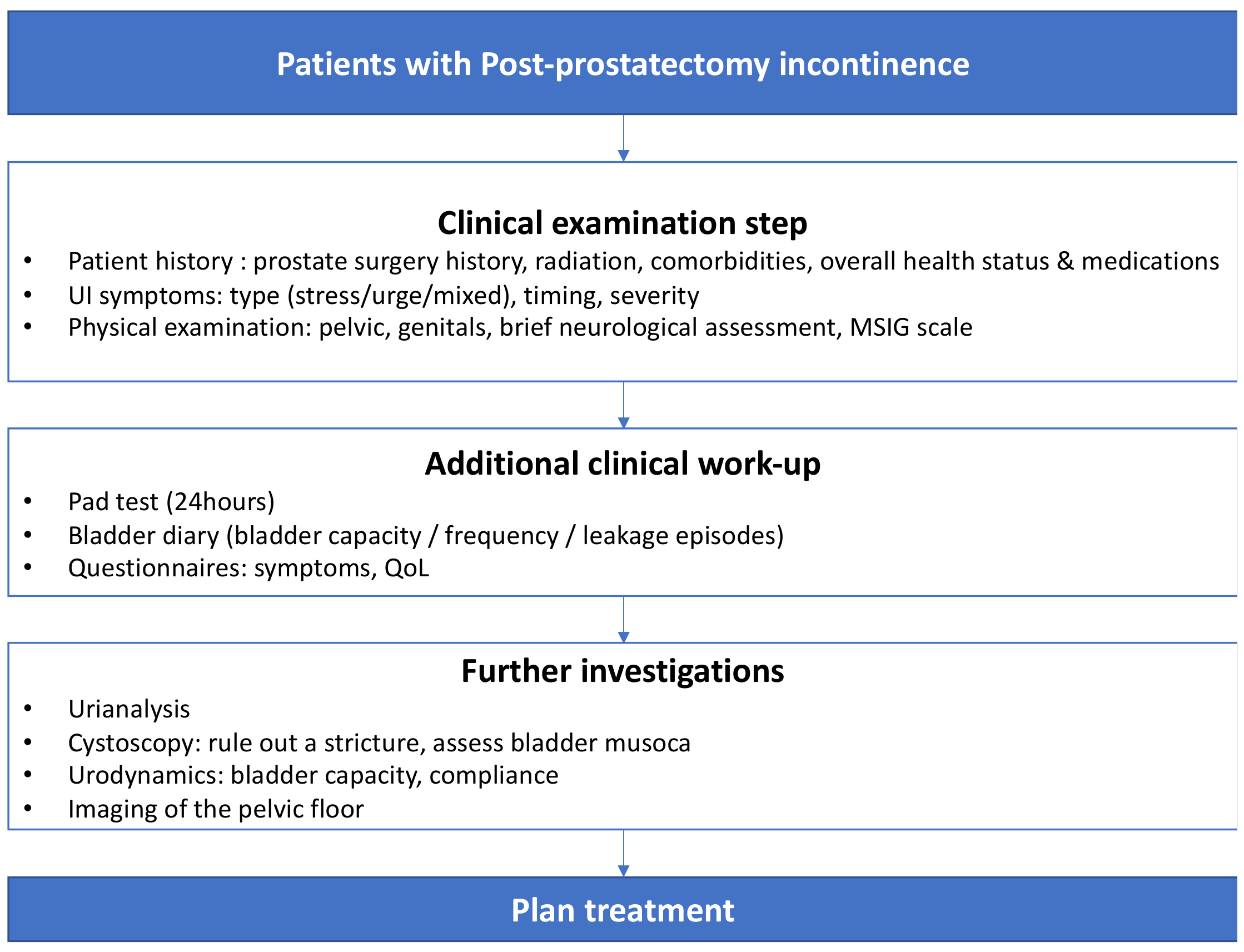
3.3. Diagnostic Work-Up
3.3.1. Medical History and Physical Examination
3.3.2. Questionnaires, Voiding Diaries and Pad Tests
3.3.3. Imaging
3.3.4. Urinalysis
3.3.5. Urethrocystoscopy
3.3.6. Urodynamics
3.4. Conservative Treatment
3.5. Medical Treatment
3.5.1. Duloxetine
3.5.2. Antimuscarinic Drugs
3.5.3. Beta-3 Agonists
| Options | Mechanism of Action | Cure Rate |
|---|---|---|
| Duloxetine | Stimulation of pudendal nerve, leading to increased tension of the urethral sphincter and relaxation of detrusor muscle | Dry rate: 25–89% [116] |
| Antimuscarinic | Antagonizing effect of muscarinic receptor subtypes in the bladder such as bladder contractions | Improvement in urge incontinence: up to 52% [129] |
| Solifenacin | ||
| Tolterodine | Decrease in early urge incontinence after RPRP: up to 53% [128] | |
| Beta-3 Agonists Mirabegron | Stimulation of beta-3 adrenoceptors in smooth muscle cells of detrusor induces detrusor relaxation | Reduced daily micturition: 37% [132] |
3.6. Surgical Treatment
3.6.1. Bulking Agents
3.6.2. Male Slings
3.6.3. Peri-Urethral Balloons
3.6.4. Artificial Urinary Sphincter
| Options | Mechanism of Action | Cure Rate (Dry Rate) |
|---|---|---|
| Peri-Urethral Injectionof Bulking Agents | Increase passive urethral resistance | <30% [135] |
| SLING Non Adjustable AdVance XP™ The Virtue™ IStop TOMS™ Adjustable ATOMS™ Remeex™ Argus™ and ArgusT™ | Relocation and compression of the bulbar urethra with the chance to adjust or not the level of compression on the urethra (adjustable and fixed respectively) | |
| Up to 70% [104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139] | ||
| Up to 50% [143] | ||
| Up to 50% [144] | ||
| Up to 69% [145,146,147,148] Up to 53% [145] Up to 70% [146,147] | ||
| PERI URETHRAL BALLOONS Pro-ACT™ | Adjustable peri-urethral compression | |
| Up to 55% [150] | ||
| ARTIFICICAL URINARY SPHINCTER AMS800™ | Circumferential compression of the urethra by a cuff filled with liquid (placed at the level of the bulbar urethra or with transcorporal approach | |
| Up to 90% [152,153,154] |
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gacci, M.; Baldi, E.; Tamburrino, L.; Detti, B.; Livi, L.; De Nunzio, C.; Tubaro, A.; Gravas, S.; Carini, M.; Serni, S. Quality of Life and Sexual Health in the Aging of PCa Survivors. Int. J. Endocrinol. 2014, 2014, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Litwin, M.; Melmed, G.Y.; Nakazon, T. Life after radical prostatectomy: A longitudinal study. J. Urol. 2001, 166, 587–592. [Google Scholar] [CrossRef]
- Gacci, M.; Simonato, A.; Masieri, L.; Gore, J.L.; Lanciotti, M.; Mantella, A.; Rossetti, M.A.; Serni, S.; Varca, V.; Romagnoli, A.; et al. Urinary and sexual outcomes in long-term (5+ years) prostate cancer disease free survivors after radical prostatectomy. Health Qual. Life Outcomes 2009, 7, 94. [Google Scholar] [CrossRef] [PubMed]
- Gacci, M.; Livi, L.; Paiar, F.; Detti, B.; Litwin, M.; Bartoletti, R.; Giubilei, G.; Cai, T.; Mariani, M.; Carini, M. Quality of life after radical treatment of prostate cancer: Validation of the Italian version of the University of California-Los Angeles Prostate Cancer Index. Urology 2005, 66, 338–343. [Google Scholar] [CrossRef] [PubMed]
- Gacci, M.; Carini, M.; Simonato, A.; Imbimbo, C.; Gontero, P.; Briganti, A.; DE Cobelli, O.; Fulcoli, V.; Martorana, G.; Nicita, G.; et al. Factors predicting continence recovery 1 month after radical prostatectomy: Results of a multicenter survey. Int. J. Urol. 2011, 18, 700–708. [Google Scholar] [CrossRef]
- Gravas, S.; Cornu, J.N.; Gacci, M.; Gratzke, C.; Herrmann, T.R.W.; Mamoulakis, C.; Rieken, M.; Speakman, M.J.; Tikkinen, K.A.O. EAU Guidelines on Management of Non-Neurogenic Male Lower Urinary Tract Symptoms (LUTS), Incl. Benign Prostatic Obstruction (BPO); European Association of Urology: Arnhem, The Netherlands, 2022. [Google Scholar]
- Gacci, M.; Sakalis, V.I.; Karavitakis, M.; Cornu, J.-N.; Gratzke, C.; Herrmann, T.R.; Kyriazis, I.; Malde, S.; Mamoulakis, C.; Rieken, M.; et al. European Association of Urology Guidelines on Male Urinary Incontinence. Eur. Urol. 2022, 82, 387–398. [Google Scholar] [CrossRef]
- Available online:https://uroweb.org/education-events/urowebinar-management-of-post-prostatectomy-incontinence-ppi (accessed on 11 May 2022).
- Kielb, S.; Dunn, R.L.; Rashid, M.G.; Murray, S.; Sanda, M.G.; Montie, J.E.; Wei, J.T. Assessment of early continence recovery after radical prostatectomy: Patient reported symptoms and impairment. J. Urol. 2001, 166, 958–961. [Google Scholar] [CrossRef]
- Jønler, M.; Madsen, F.A.; Rhodes, P.R.; Sall, M.; Messing, E.M.; Bruskewitz, R.C. A prospective study of quantification of urinary incontinence and quality of life in patients undergoing radical retropubic prostatectomy. Urology 1996, 48, 433–440. [Google Scholar] [CrossRef]
- Foote, J.; Yun, S.; Leach, G.E. Postprostatectomy incontinence. Pathophysiology, evaluation, and management. Urol. Clin. North Am. 1991, 18, 229–241. [Google Scholar] [CrossRef]
- Moore, K.N.; Truong, V.; Estey, E.; Voaklander, D.C. Urinary Incontinence after Radical Prostatectomy. J. Wound, Ostomy Cont. Nurs. 2007, 34, 270–279. [Google Scholar] [CrossRef]
- Zorn, K.C.; Gofrit, O.N.; Orvieto, M.A.; Mikhail, A.A.; Zagaja, G.P.; Shalhav, A.L. Robotic-Assisted Laparoscopic Prostatectomy: Functional and Pathologic Outcomes with Interfascial Nerve Preservation. Eur. Urol. 2007, 51, 755–763. [Google Scholar] [CrossRef]
- Patel, V.R.; Thaly, R.; Shah, K. Robotic radical prostatectomy: Outcomes of 500 cases. BJU Int. 2007, 99, 1109–1112. [Google Scholar] [CrossRef]
- Borin, J.F.; Skarecky, D.W.; Narula, N.; Ahlering, T.E. Impact of Urethral Stump Length on Continence and Positive Surgical Margins in Robot-Assisted Laparoscopic Prostatectomy. Urology 2007, 70, 173–177. [Google Scholar] [CrossRef]
- Menon, M.; Shrivastava, A.; Kaul, S.; Badani, K.K.; Fumo, M.; Bhandari, M.; Peabody, J.O. Vattikuti Institute Prostatectomy: Contemporary Technique and Analysis of Results. Eur. Urol. 2007, 51, 648–658. [Google Scholar] [CrossRef]
- Rodriguez, E.; Skarecky, D.W.; Ahlering, T.E. Post–robotic prostatectomy urinary continence: Characterization of perfect continence versus occasional dribbling in pad-free men. Urology 2006, 67, 785–788. [Google Scholar] [CrossRef]
- Sacco, E.; Prayer-Galetti, T.; Pinto, F.; Fracalanza, S.; Betto, G.; Pagano, F.; Artibani, W. Urinary incontinence after radical prostatectomy: Incidence by definition, risk factors and temporal trend in a large series with a long-term follow-up. BJU Int. 2006, 97, 1234–1241. [Google Scholar] [CrossRef]
- Ficazzola, M.A.; Nitti, V.W. The etiology of post-radical prostatectomy incontinence and correlation of symptoms with urodynamic findings. J. Urol. 1998, 160, 1317–1320. [Google Scholar] [CrossRef]
- Chao, R.; Mayo, M.E. Incontinence after Radical Prostatectomy: Detrusor or Sphincter Causes. J. Urol. 1995, 154, 16–18. [Google Scholar] [CrossRef]
- Kleinhans, B.; Gerharz, E.; Melekos, M.; Weingärtner, K.; Kälble, T.; Riedmiller, H. Changes of urodynamic findings after radical retropubic prostatectomy. Eur. Urol. 1999, 35, 217–222. [Google Scholar] [CrossRef]
- Bianco, F.J., Jr.; Scardino, P.T.; Eastham, J.A. Radical prostatectomy: Long-term cancer control and recovery of sexual and urinary function (“trifecta”). Urology 2005, 66, 83–94. [Google Scholar] [CrossRef]
- Winters, J.C.; Appell, R.A.; Rackley, R.R. Urodynamic findings in post prostatectomy incontinence. Neurourol. Urodyn. 1998, 17, 493–498. [Google Scholar] [CrossRef]
- Groutz, A.; Blaivas, J.G.; Chaikin, D.C.; Weiss, J.P.; Verhaaren, M. The pathophysiology of post-radical prostatectomy incontinence: A clinical and video urodynamic study. J. Urol. 2000, 163, 1767–1770. [Google Scholar] [CrossRef] [PubMed]
- Leach, G.E.; Trockman, B.; Wong, A.; Hamilton, J.; Haab, F.; Zimmern, P.E. Post-Prostatectomy Incontinence: Urodynamic Findings and Treatment Outcomes. J. Urol. 1996, 155, 1256–1259. [Google Scholar] [CrossRef] [PubMed]
- Goluboff, E.T.; Chang, D.T.; Olsson, C.A.; Kaplan, S.A. Urological Neurology and Urodynamics: Urodynamics and the Etiology of Post-Prostatectomy Urinary Incontinence: The Initial Columbia Experience. J. Urol. 1995, 153, 1034–1037. [Google Scholar] [CrossRef] [PubMed]
- Desautel, M.G.; Kapoor, R.; Badlani, G.H. Sphincteric incontinence: The primary cause of post-prostatectomy incontinence in patients with prostate cancer. Neurourol. Urodyn. 1997, 16, 153–159. [Google Scholar] [CrossRef]
- Giannantoni, A.; Mearini, E.; Zucchi, A.; Costantini, E.; Mearini, L.; Bini, V.; Porena, M. Bladder and Urethral Sphincter Function after Radical Retropubic Prostatectomy: A Prospective Long-Term Study. Eur. Urol. 2008, 54, 657–664. [Google Scholar] [CrossRef]
- Steiner, M.S. Continence-preserving anatomic radical retropubic prostatectomy. Urology 2000, 55, 427–435. [Google Scholar] [CrossRef]
- Bessede, T.; Sooriakumaran, P.; Takenaka, A.; Tewari, A. Neural supply of the male urethral sphincter: Comprehensive anatomical review and implications for continence recovery after radical prostatectomy. World J. Urol. 2016, 35, 549–565. [Google Scholar] [CrossRef]
- Porena, M.; Mearini, E.; Mearini, L.; Vianello, A.; Giannantoni, A. Voiding Dysfunction after Radical Retropubic Prostatectomy: More than External Urethral Sphincter Deficiency. Eur. Urol. 2007, 52, 38–45. [Google Scholar] [CrossRef]
- Stanford, J.L.; Feng, Z.; Hamilton, A.S.; Gilliland, F.D.; Stephenson, R.A.; Eley, J.W.; Albertsen, P.C.; Harlan, L.C.; Potosky, A.L. Urinary and Sexual Function After Radical Prostatectomy for Clinically Localized Prostate Cancer. JAMA 2000, 283, 354–360. [Google Scholar] [CrossRef]
- Bauer, R.M.; Bastian, P.J.; Gozzi, C.; Stief, C.G. Postprostatectomy Incontinence: All about Diagnosis and Management. Eur. Urol. 2009, 55, 322–333. [Google Scholar] [CrossRef]
- Campodonico, F.; Manuputty, E.E.; Campora, S.; Puntoni, M.; Maffezzini, M. Age Is Predictive of Immediate Postoperative Urinary Continence after Radical Retropubic Prostatectomy. Urol. Int. 2013, 92, 276–281. [Google Scholar] [CrossRef]
- Nilsson, A.E.; Schumacher, M.C.; Johansson, E.; Carlsson, S.; Stranne, J.; Nyberg, T.; Wiklund, N.P.; Steineck, G. Age at surgery, educational level and long-term urinary incontinence after radical prostatectomy. BJU Int. 2011, 108, 1572–1577. [Google Scholar] [CrossRef]
- Barry, M.J.; Gallagher, P.M.; Skinner, J.S.; Fowler, F.J., Jr. Adverse Effects of Robotic-Assisted Laparoscopic Versus Open Retropubic Radical Prostatectomy Among a Nationwide Random Sample of Medicare-Age Men. J. Clin. Oncol. 2012, 30, 513–518. [Google Scholar] [CrossRef]
- Tienza, A.; Akin, Y.; Rassweiler, J.; Gözen, A.S. A match-pair analysis of continence in intermediate and high-risk prostate cancer patients after robot-assisted radical prostatectomy: The role of urine loss ratio and predictive analysis. Prostate Int. 2017, 6, 94–98. [Google Scholar] [CrossRef]
- Shikanov, S.; Desai, V.; Razmaria, A.; Zagaja, G.P.; Shalhav, A.L. Robotic Radical Prostatectomy for Elderly Patients: Probability of Achieving Continence and Potency 1 Year After Surgery. J. Urol. 2010, 183, 1803–1807. [Google Scholar] [CrossRef]
- Cakmak, S.; Canda, A.E.; Ener, K.; Atmaca, A.F.; Altinova, S.; Balbay, M.D. Does Type 2 Diabetes Mellitus Have an Impact on Postoperative Early, Mid-Term and Late-Term Urinary Continence After Robot-Assisted Radical Prostatectomy? J. Endourol. 2019, 33, 201–206. [Google Scholar] [CrossRef]
- Teber, D.; Sofikerim, M.; Ates, M.; Gözen, A.S.; Güven, O.; Sanli, O.; Rassweiler, J. Is Type 2 Diabetes Mellitus a Predictive Factor for Incontinence After Laparoscopic Radical Prostatectomy? A Matched Pair and Multivariate Analysis. J. Urol. 2010, 183, 1087–1091. [Google Scholar] [CrossRef]
- Novara, G.; Ficarra, V.; D’Elia, C.; Secco, S.; Cioffi, A.; Cavalleri, S.; Artibani, W. Evaluating Urinary Continence and Preoperative Predictors of Urinary Continence After Robot Assisted Laparoscopic Radical Prostatectomy. J. Urol. 2010, 184, 1028–1033. [Google Scholar] [CrossRef]
- Ahlering, T.E.; Eichel, L.; Edwards, R.; Skarecky, D.W. Impact of obesity on clinical outcomes in robotic prostatectomy. Urology 2005, 65, 740–744. [Google Scholar] [CrossRef] [Green Version]
- Mikhail, A.A.; Stockton, B.R.; Orvieto, M.A.; Chien, G.W.; Gong, E.M.; Zorn, K.C.; Brendler, C.B.; Zagaja, G.P.; Shalhav, A.L. Robotic-assisted laparoscopic prostatectomy in overweight and obese patients. Urology 2006, 67, 774–779. [Google Scholar] [CrossRef] [PubMed]
- Khaira, H.S.; Bruyère, F.; O’Malley, P.J.; Peters, J.S.; Costello, A.J. Does obesity influence the operative course or complications of robot-assisted laparoscopic prostatectomy. BJU Int. 2006, 98, 1275–1278. [Google Scholar] [CrossRef] [PubMed]
- Castle, E.P.; Atug, F.; Woods, M.; Thomas, R.; Davis, R. Impact of body mass index on outcomes after robot assisted radical prostatectomy. World J. Urol. 2007, 26, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Link, B.A.; Nelson, R.; Josephson, D.Y.; Yoshida, J.S.; Crocitto, L.E.; Kawachi, M.H.; Wilson, T.G. The Impact of Prostate Gland Weight in Robot Assisted Laparoscopic Radical Prostatectomy. J. Urol. 2008, 180, 928–932. [Google Scholar] [CrossRef]
- Skolarus, T.A.; Hedgepeth, R.C.; Zhang, Y.; Weizer, A.Z.; Montgomery, J.S.; Miller, D.C.; Wood, D.P.; Hollenbeck, B.K. Does Robotic Technology Mitigate the Challenges of Large Prostate Size? Urology 2010, 76, 1117–1121. [Google Scholar] [CrossRef]
- Zorn, K.C.; A Wille, M.; Thong, A.; Katz, M.; A Shikanov, S.; Razmaria, A.; Gofrit, O.N.; Zagaja, G.P.; Shalhav, A.L. Continued improvement of perioperative, pathological and continence outcomes during 700 robot-assisted radical prostatectomies. Can. J. Urol. 2009, 16, 4742–4749. [Google Scholar]
- Gacci, M.; Russo, G.I.; De Nunzio, C.; Sebastianelli, A.; Salvi, M.; Vignozzi, L.; Tubaro, A.; Morgia, G.; Serni, S. Meta-analysis of metabolic syndrome and prostate cancer. Prostate Cancer Prostatic Dis. 2017, 20, 146–155. [Google Scholar] [CrossRef]
- Song, C.; Doo, C.K.; Hong, J.-H.; Choo, M.-S.; Kim, C.-S.; Ahn, H. Relationship Between the Integrity of the Pelvic Floor Muscles and Early Recovery of Continence After Radical Prostatectomy. J. Urol. 2007, 178, 208–211. [Google Scholar] [CrossRef]
- Nguyen, L.; Jhaveri, J.; Tewari, A. Surgical Technique to Overcome Anatomical Shortcoming: Balancing Post-Prostatectomy Continence Outcomes of Urethral Sphincter Lengths on Preoperative Magnetic Resonance Imaging. J. Urol. 2008, 179, 1907–1911. [Google Scholar] [CrossRef]
- Hollabaugh, R.S.; Dmochowski, R.R.; Kneib, T.G.; Steiner, M.S. Preservation of Putative Continence Nerves during Radical Retropubic Prostatectomy Leads to More Rapid Return of Urinary Continence. Urology 1998, 51, 960–967. [Google Scholar] [CrossRef]
- Eastham, J.A.; Kattan, M.W.; Rogers, E.; Goad, J.R.; Ohori, M.; Boone, T.B.; Scardino, P.T. Risk factors for urinary incontinence after radical prostatectomy. J. Urol. 1996, 156, 1707–1713. [Google Scholar] [CrossRef]
- Walsh, P. Trends in Treatment of Localized Prostate-Cancer by Radical Prostatectomy—Observations from the Commission-on-Cancer National-Cancer-Database. Urology 1994, 43, 492. [Google Scholar] [CrossRef]
- O’Donnell, P.D.; Finan, B.F. Continence Following Nerve-Sparing Radical Prostatectomy. J. Urol. 1989, 142, 1227–1228. [Google Scholar] [CrossRef]
- Gacci, M.; Ierardi, A.; Rose, A.D.; Tazzioli, S.; Scapaticci, E.; Filippi, S.; Maggi, M.; Nicita, G.; Carini, M.; Montorsi, F. Vardenafil can Improve Continence Recovery after Bilateral Nerve Sparing Prostatectomy: Results of a Randomized, Double Blind, Placebo-Controlled Pilot Study. J. Sex. Med. 2010, 7, 234–243. [Google Scholar] [CrossRef]
- Mandel, P.; Graefen, M.; Michl, U.; Huland, H.; Tilki, D. The effect of age on functional outcomes after radical prostatectomy. Urol. Oncol. Semin. Orig. Investig. 2015, 33, 203.e11–203.e18. [Google Scholar] [CrossRef]
- Wiltz, A.L.; Shikanov, S.; Eggener, S.E.; Katz, M.H.; Thong, A.E.; Steinberg, G.D.; Shalhav, A.L.; Zagaja, G.P.; Zorn, K.C. Robotic Radical Prostatectomy in Overweight and Obese Patients: Oncological and Validated-Functional Outcomes. Urology 2009, 73, 316–322. [Google Scholar] [CrossRef]
- Kadono, Y.; Ueno, S.; Kadomoto, S.; Iwamoto, H.; Takezawa, Y.; Nakashima, K.; Nohara, T.; Izumi, K.; Mizokami, A.; Gabata, T.; et al. Use of preoperative factors including urodynamic evaluations and nerve-sparing status for predicting urinary continence recovery after robot-assisted radical prostatectomy: Nerve-sparing technique contributes to the reduction of postprostatectomy incontin. Neurourol. Urodyn. 2015, 35, 1034–1039. [Google Scholar] [CrossRef]
- Wolin, K.Y.; Luly, J.; Sutcliffe, S.; Andriole, G.L.; Kibel, A.S. Risk of Urinary Incontinence Following Prostatectomy: The Role of Physical Activity and Obesity. J. Urol. 2010, 183, 629–633. [Google Scholar] [CrossRef]
- Corona, G.; Gacci, M.; Baldi, E.; Mancina, R.; Forti, G.; Maggi, M. Androgen Deprivation Therapy in Prostate Cancer: Focusing on Sexual Side Effects. J. Sex. Med. 2012, 9, 887–902. [Google Scholar] [CrossRef]
- Gacci, M.; Corona, G.; Apolone, A.; Lanciotti, M.; Tosi, N.; Giancane, S.; Masieri, L.; Serni, S.; Maggi, M.; Carini, M. Influence of serum testosterone on urinary continence and sexual activity in patients undergoing radical prostatectomy for clinically localized prostate cancer. Prostate Cancer Prostatic Dis. 2010, 13, 168–172. [Google Scholar] [CrossRef]
- Giannantoni, A.; Mearini, E.; Di Stasi, S.M.; Mearini, L.; Bini, V.; Pizzirusso, G.; Porena, M. Assessment of Bladder and Urethral Sphincter Function before and after Radical Retropubic Prostatectomy. J. Urol. 2004, 171, 1563–1566. [Google Scholar] [CrossRef] [PubMed]
- Ficarra, V.; Novara, G.; Rosen, R.C.; Artibani, W.; Carroll, P.R.; Costello, A.; Menon, M.; Montorsi, F.; Patel, V.R.; Stolzenburg, J.-U.; et al. Systematic Review and Meta-analysis of Studies Reporting Urinary Continence Recovery After Robot-assisted Radical Prostatectomy. Eur. Urol. 2012, 62, 405–417. [Google Scholar] [CrossRef] [PubMed]
- Boczko, J.; Erturk, E.; Golijanin, D.; Madeb, R.; Patel, H.; Joseph, J.V. Impact of Prostate Size in Robot-Assisted Radical Prostatectomy. J. Endourol. 2007, 21, 184–188. [Google Scholar] [CrossRef] [PubMed]
- Rudy, D.C.; Woodside, J.R.; Crawford, E.D. Urodynamic Evaluation of incontinence in Patients Undergoing Modified Campbell Radical Retropubic Prostatectomy: A Prospective Study. J. Urol. 1984, 132, 708–712. [Google Scholar] [CrossRef]
- Hammerer, P.; Huland, H. Urodynamic evaluation of changes in urinary control after radical retropubic prostatectomy. J. Urol. 1997, 157, 233–236. [Google Scholar] [CrossRef]
- Iacovelli, V.; Carilli, M.; Sandri, M.; Forte, V.; Cipriani, C.; Bertolo, R.; Vittori, M.; Petta, F.; Maiorino, F.; Signoretti, M.; et al. The role of preoperative prostatic shape in the recovery of urinary continence after robotic radical prostatectomy: A single cohort analysis. Prostate Cancer Prostatic Dis. 2022. [Google Scholar] [CrossRef]
- Matsushita, K.; Kent, M.T.; Vickers, A.J.; von Bodman, C.; Bernstein, M.; Touijer, K.A.; Coleman, J.A.; Laudone, V.T.; Scardino, P.T.; Eastham, J.A.; et al. Preoperative predictive model of recovery of urinary continence after radical prostatectomy. BJU Int. 2015, 116, 577–583. [Google Scholar] [CrossRef]
- Ma, X.; Tang, K.; Yang, C.; Wu, G.; Xu, N.; Wang, M.; Zeng, X.; Hu, Z.; Song, R.; Yuh, B.; et al. Bladder neck preservation improves time to continence after radical prostatectomy: A systematic review and meta-analysis. Oncotarget 2016, 7, 67463–67475. [Google Scholar] [CrossRef]
- Galfano, A.; Ascione, A.; Grimaldi, S.; Petralia, G.; Strada, E.; Bocciardi, A.M. A New Anatomic Approach for Robot-Assisted Laparoscopic Prostatectomy: A Feasibility Study for Completely Intrafascial Surgery. Eur. Urol. 2010, 58, 457–461. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, J.; Yang, Z.; Liu, Q.; Zhang, W.; Qing, Z.; Wang, D. Comparison of Retzius-sparing and conventional robot-assisted laparoscopic radical prostatectomy regarding continence and sexual function: An updated meta-analysis. Prostate Cancer Prostatic Dis. 2021, 25, 47–54. [Google Scholar] [CrossRef]
- Gravas, S.; Cornu, J.N.; Gacci, M.; Gratzke, C.; Herrmann, T.R.W.; Mamoulakis, C.; Rieken, M.; Speakman, M.J.; Tikkinen, K.A.O. EAU Guidelines on Management of Non-Neurogenic Male Lower Urinary Tract Symptoms (LUTS), Incl. Benign prostatic Obstruction (BPO); European Association of Urology: Arnhem, The Netherlands, 2020. [Google Scholar]
- Abrams, P.; Cardozo, L.; Wagg, A.; Wein, A. Incontinence 2017, 6th ed.; The International Consultation on Urological Diseases: Paris, France, 2017. [Google Scholar]
- Martin, J.; Williams, K.; Sutton, A.; Abrams, K.; Assassa, R. Systematic review and meta-analysis of methods of diagnostic assessment for urinary incontinence. Neurourol. Urodyn. 2006, 25, 674–683. [Google Scholar] [CrossRef]
- Morey, A.F.; Singla, N.; Carmel, M.; Klein, A.; Tausch, T.J.; Siegel, J.; Tachibana, I.; Scott, J. Standing cough test for evaluation of post-prostatectomy incontinence: A pilot study. Can. J. Urol. 2017, 24, 8664–8669. [Google Scholar]
- Chan, S.S.C.; Cheung, R.Y.K.; Lai, B.P.Y.; Lee, L.L.; Choy, K.W.; Chung, T.K.H. Responsiveness of the Pelvic Floor Distress Inventory and Pelvic Floor Impact Questionnaire in women undergoing treatment for pelvic floor disorders. Int. Urogynecol. J. 2012, 24, 213–221. [Google Scholar] [CrossRef]
- Kim, J.; Lee, W.; Gioia, K.; Patel, B.; Lucioni, A.; Govier, F.; Kobashi, K. 1576 Is there a relationship between incontinence impact questionnaire 7 score after surgery for stress urinary incontinence and patient-perceived satisfaction and improvement? J. Urol. 2013, 189, e647. [Google Scholar] [CrossRef]
- Tran, M.G.B.; Yip, J.L.Y.; Uveili, K.; Biers, S.M.; Thiruchelvam, N. Patient reported outcome measures in male incontinence surgery. Ind. Mark. Manag. 2014, 96, 521–525. [Google Scholar] [CrossRef]
- Bright, E.; Cotterill, N.; Drake, M.; Abrams, P. Developing and Validating the International Consultation on Incontinence Questionnaire Bladder Diary. Eur. Urol. 2014, 66, 294–300. [Google Scholar] [CrossRef]
- Fayyad, A.M.; Hill, S.R.; Jones, G. Urine production and bladder diary measurements in women with type 2 diabetes mellitus and their relation to lower urinary tract symptoms and voiding dysfunction. Neurourol. Urodyn. 2009, 29, 354–358. [Google Scholar] [CrossRef]
- Homma, Y.; Kakizaki, H.; Yamaguchi, O.; Yamanishi, T.; Nishizawa, O.; Yokoyama, O.; Takeda, M.; Seki, N.; Yoshida, M. Assessment of Overactive Bladder Symptoms: Comparison of 3-Day Bladder Diary and the Overactive Bladder Symptoms Score. Urology 2011, 77, 60–64. [Google Scholar] [CrossRef]
- Stav, K.; Dwyer, P.L.; Rosamilia, A. Women Overestimate Daytime Urinary Frequency: The Importance of the Bladder Diary. J. Urol. 2009, 181, 2176–2180. [Google Scholar] [CrossRef]
- van Brummen, H.; Heintz, A.; van der Vaart, C. The association between overactive bladder symptoms and objective parameters from bladder diary and filling cystometry. Neurourol. Urodyn. 2003, 23, 38–42. [Google Scholar] [CrossRef]
- Burgio, K.L.; Locher, J.L.; Goode, P.S.; Hardin, J.M.; McDowell, B.J.; Dombrowski, M.; Candib, D. Behavioral vs. Drug Treatment for Urge Urinary Incontinence in Older Women. JAMA 1998, 280, 1995–2000. [Google Scholar] [CrossRef]
- O’Sullivan, R.; Karantanis, E.; Stevermuer, T.; Allen, W.; Moore, K. Definition of mild, moderate and severe incontinence on the 24-h pad test. BJOG: Int. J. Obstet. Gynaecol. 2004, 111, 859–862. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Tanda, H.; Nakajima, H.; Nitta, T.; Akagashi, K.; Hanzawa, T.; Tobe, M.; Haga, K.; Uchida, K.; Honma, I. Simple and reliable predictor of urinary continence after radical prostatectomy: Serial measurement of urine loss ratio after catheter removal. Int. J. Urol. 2014, 21, 647–651. [Google Scholar] [CrossRef] [PubMed]
- Shy, M.; Fletcher, S.G. Objective Evaluation of Overactive Bladder: Which Surveys Should I Use? Curr. Bl. Dysfunct. Rep. 2013, 8, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Najjari, L.; Hennemann, J.; Maass, N.; Kirschner-Hermanns, R. Die Perinealsonographie zur Diagnostik der männlichen Belastungsinkontinenz. Der. Urol. 2011, 51, 384–389. [Google Scholar] [CrossRef]
- Goode, P.S.; Locher, J.L.; Bryant, R.L.; Roth, D.L.; Burgio, K.L. Measurement of Postvoid Residual Urine with Portable Transabdominal Bladder Ultrasound Scanner and Urethral Catheterization. Int. Urogynecol. J. 2000, 11, 296–300. [Google Scholar] [CrossRef]
- Griffiths, D.J.; Harrison, G.; Moore, K.; McCracken, P. Variability of post-void residual urine volume in the elderly. Urol. Res. 1996, 24, 23–26. [Google Scholar] [CrossRef]
- Marks, L.S.; Dorey, F.J.; Macairan, M.L.; Park, C.; Dekernion, J.B. Three-dimensional ultrasound device for rapid determination of bladder volume. Urology 1997, 50, 341–348. [Google Scholar] [CrossRef]
- Nygaard, I.E. Postvoid residual volume cannot be accurately estimated by bimanual examination. Int. Urogynecol. J. 1996, 7, 74–76. [Google Scholar] [CrossRef]
- Ouslander, J.G.; Simmons, S.; Tuico, E.; Nigam, J.G.; Fingold, S.; Bates-Jensen, B.; Schnelle, J.F. Use of a Portable Ultrasound Device to Measure Post-Void Residual Volume Among Incontinent Nursing Home Residents. J. Am. Geriatr. Soc. 1994, 42, 1189–1192. [Google Scholar] [CrossRef]
- Stoller, M.L.; Millard, R.J. The Accuracy of a Catheterized Residual Urine. J. Urol. 1989, 141, 15–16. [Google Scholar] [CrossRef]
- Babjuk, M.; Burger, M.; Capoun, O.; Cohen, D.; Compérat, E.M.; Escrig, J.L.D.; Gontero, P.; Liedberg, F.; Masson-Lecomte, A.; Mostafid, A.H.; et al. EAU Guidelines on Non-Muscle invasive Bladder Cancer Ed. In Proceedings of the EAU Annual Congress, Amsterdam, The Netherlands, 1–4 July 2022. [Google Scholar]
- Bonkat, G.; Bartoletti, R.; Bruyère, F.; Cai, T.; Geerlings, S.E.; Köves, B.; Schubert, S.; Pilatz, A.; Veeratterapillay, R.; Wagenlehner, F. EAU Guidelines on Urological Infections Ed. In Proceedings of the EAU Annual Congress, Amsterdam, The Netherlands, 1–4 July 2022. [Google Scholar]
- Palou, J.; Wood, D.; Bochner, B.H.; van der Poel, H.; Al-Ahmadie, H.A.; Yossepowitch, O.; Soloway, M.S.; Jenkins, L.C. ICUD-EAU International Consultation on Bladder Cancer 2012: Urothelial Carcinoma of the Prostate. Eur. Urol. 2013, 63, 81–87. [Google Scholar] [CrossRef]
- Rouprêt, M.; Babjuk, M.; Burger, M.; Capoun, O.; Cohen, D.; Compérat, E.M.; Cowan, N.C.; Dominguez-Escrig, J.L.; Gontero, P.; Mostafid, A.H.; et al. EAU Guidelines on Upper Urinary Tract Urothelial Cell Carcinoma Edn. In Proceedings of the EAU Annual Congress, Amsterdam, The Netherlands, 1–4 July 2022. [Google Scholar]
- Arinzon, Z.; Shabat, S.; Peisakh, A.; Berner, Y. Clinical presentation of urinary tract infection (UTI) differs with aging in women. Arch. Gerontol. Geriatr. 2012, 55, 145–147. [Google Scholar] [CrossRef]
- Moore, E.E.; Jackson, S.L.; Boyko, E.J.; Scholes, D.; Fihn, S.D. Urinary Incontinence and Urinary Tract Infection. Obstet. Gynecol. 2008, 111, 317–323. [Google Scholar] [CrossRef]
- Ouslander, J.G.; Schapira, M.; Schnelle, J.F.; Uman, G.; Fingold, S.; Tuico, E.; Nigam, J.G. Does Eradicating Bacteriuria Affect the Severity of Chronic Urinary Incontinence in Nursing Home Residents? Ann. Intern. Med. 1995, 122, 749. [Google Scholar] [CrossRef]
- Gravas, S.; Cornu, J.N.; Gacci, M.; Gratzke, C.; Herrmann, T.R.W.; Mamoulakis, C.; Rieken, M.; Speakman, M.J.; Tikkinen, K.A.O. EAU Guidelines on Management of Non-Neurogenic Male Lower Urinary Tract Symptoms (LUTS), Incl. Benign Prostatic Obstruction (BPO); European Association of Urology: Arnhem, The Netherlands, 2021. [Google Scholar]
- Chung, A.S.J.; Suarez, O.A.; McCammon, K.A. AdVance male sling. Transl. Androl. Urol. 2017, 6, 674–681. [Google Scholar] [CrossRef]
- Bauer, R.M.; Gozzi, C.; Roosen, A.; Khoder, W.; Trottmann, M.; Waidelich, R.; Stief, C.G.; Soljanik, I. Impact of the ‘Repositioning Test’ on Postoperative Outcome of Retroluminar Transobturator Male Sling Implantation. Urol. Int. 2013, 90, 334–338. [Google Scholar] [CrossRef]
- Hennessey, D.B.; Hoag, N.; Gani, J. Impact of bladder dysfunction in the management of post radical prostatectomy stress urinary incontinence—A review. Transl. Androl. Urol. 2017, 6, S103–S111. [Google Scholar] [CrossRef]
- Imamura, M.; Williams, K.; Wells, M.; McGrother, C. Lifestyle interventions for the treatment of urinary incontinence in adults. Cochrane Database Syst. Rev. 2015, 2015, CD003505. [Google Scholar] [CrossRef]
- Macaulay, M.; Broadbridge, J.; Gage, H.; Williams, P.; Birch, B.; Moore, K.N.; Cottenden, A.; Fader, M.J. A trial of devices for urinary incontinence after treatment for prostate cancer. BJU Int. 2015, 116, 432–442. [Google Scholar] [CrossRef]
- A Anderson, C.; Omar, M.I.; E Campbell, S.; Hunter, K.F.; Cody, J.D.; Glazener, C.M. Conservative management for postprostatectomy urinary incontinence. Cochrane Database Syst. Rev. 2015, 1, CD001843. [Google Scholar] [CrossRef] [PubMed]
- Kannan, P.; Winser, S.J.; Fung, B.; Cheing, G. Effectiveness of Pelvic Floor Muscle Training Alone and in Combination with Biofeedback, Electrical Stimulation, or Both Compared to Control for Urinary Incontinence in Men Following Prostatectomy: Systematic Review and Meta-Analysis. Phys. Ther. 2018, 98, 932–945. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sciarra, A.; Viscuso, P.; Arditi, A.; Mariotti, G.; De Berardinis, E.; Di Pierro, G.B.; Canale, V.; Gentilucci, A.; Busetto, G.M.; Maggi, M.; et al. A biofeedback-guided programme or pelvic floor muscle electric stimulation can improve early recovery of urinary continence after radical prostatectomy: A meta-analysis and systematic review. Int. J. Clin. Pr. 2021, 75, e14208. [Google Scholar] [CrossRef] [PubMed]
- Goonewardene, S.S.; Gillatt, D.; Persad, R. A systematic review of PFE pre-prostatectomy. J. Robot. Surg. 2018, 12, 397–400. [Google Scholar] [CrossRef] [PubMed]
- Marchioni, M.; Primiceri, G.; Castellan, P.; Schips, L.; Mantica, G.; Chapple, C.; Papalia, R.; Porpiglia, F.; Scarpa, R.M.; Esperto, F. Conservative management of urinary incontinence following robot-assisted radical prostatectomy. Minerva Urol. Nephrol. 2020, 72, 555–562. [Google Scholar] [CrossRef]
- Dubbelman, Y.; Groen, J.; Wildhagen, M.; Rikken, B.; Bosch, R. The recovery of urinary continence after radical retropubic prostatectomy: A randomized trial comparing the effect of physiotherapist-guided pelvic floor muscle exercises with guidance by an instruction folder only. BJU Int. 2010, 106, 515–522. [Google Scholar] [CrossRef]
- Løvvik, A.; Müller, S.; Patel, H.R.H. Pharmacological Treatment of Post-Prostatectomy Incontinence: What is the Evidence? Drugs Aging 2016, 33, 535–544. [Google Scholar] [CrossRef]
- Kotecha, P.; Sahai, A.; Malde, S. Use of Duloxetine for Postprostatectomy Stress Urinary Incontinence: A Systematic Review. Eur. Urol. Focus 2020, 7, 618–628. [Google Scholar] [CrossRef]
- Alan, C.; Eren, A.E.; Ersay, A.R.; Kocoglu, H.; Basturk, G.; Demirci, E. Efficacy of Duloxetine in the Early Management of Urinary Continence after Radical Prostatectomy. Curr. Urol. 2015, 8, 43–48. [Google Scholar] [CrossRef]
- Filocamo, M.T.; Marzi, V.L.; Del Popolo, G.; Cecconi, F.; Villari, D.; Marzocco, M.; Nicita, G. Pharmacologic Treatment in Postprostatectomy Stress Urinary Incontinence. Eur. Urol. 2007, 51, 1559–1564. [Google Scholar] [CrossRef]
- Cornu, J.-N.; Merlet, B.; Ciofu, C.; Mouly, S.; Peyrat, L.; Sèbe, P.; Yiou, R.; Vallancien, G.; Debrix, I.; Laribi, K.; et al. Duloxetine for Mild to Moderate Postprostatectomy Incontinence: Preliminary Results of a Randomised, Placebo-Controlled Trial. Eur. Urol. 2011, 59, 148–154. [Google Scholar] [CrossRef]
- Serra, A.C.; Rubio-Briones, J.; Payás, M.P.; Juan, I.I.; Ramón-Borja, J.C.; Narbón, E.S. Postprostatectomy Established Stress Urinary Incontinence Treated with Duloxetine. Urology 2011, 78, 261–266. [Google Scholar] [CrossRef]
- Fink, K.G.; Huber, J.; Würnschimmel, E.; Schmeller, N.T. The use of Duloxetine in the treatment of male stress urinary incontinence. Wien. Med. Wochenschr. 2008, 158, 116–118. [Google Scholar] [CrossRef]
- Schlenker, B.; Gratzke, C.; Reich, O.; Schorsch, I.; Seitz, M.; Stief, C.G. Preliminary Results on the Off-Label Use of Duloxetine for the Treatment of Stress Incontinence after Radical Prostatectomy or Cystectomy. Eur. Urol. 2006, 49, 1075–1078. [Google Scholar] [CrossRef]
- Zahariou, A.; Papaioannou, P.; Kalogirou, G. Is HCl Duloxetine Effective in the Management of Urinary Stress Incontinence after Radical Prostatectomy? Urol. Int. 2006, 77, 9–12. [Google Scholar] [CrossRef]
- Neff, D.; Guise, A.; Guralnick, M.L.; Langenstroer, P.; See, W.A.; Jacobsohn, K.M.; O’Connor, R.C. Duloxetine for the treatment of post-prostatectomy stress urinary incontinence. Can. Urol. Assoc. J. 2013, 7, 260–262. [Google Scholar] [CrossRef]
- Peyronnet, B.; Brucker, B.M. Management of Overactive Bladder Symptoms after Radical Prostatectomy. Curr. Urol. Rep. 2018, 19, 95. [Google Scholar] [CrossRef]
- Liss, M.A.; Morales, B.; Skarecky, D.; Ahlering, T.E. Phase 1 Clinical Trial of Vesicare™ (Solifenacin) in the Treatment of Urinary Incontinence after Radical Prostatectomy. J. Endourol. 2014, 28, 1241–1245. [Google Scholar] [CrossRef]
- Yang, S.W.; Na, Y.G.; Song, K.H.; Shin, J.H.; Chang, Y.S.; Park, J.M.; Lee, C.L.; Lim, J.S. Lower Urinary Tract Symptoms and Efficacy of Anticholinergic Drugs in Patients Remaining Disease-Free After Radical Retropubic Prostatectomy. Urol. J. 2016, 28, 2684–2689. [Google Scholar]
- Mitropoulos, D.; Papadoukakis, S.; Zervas, A.; Alamanis, C.; Giannopoulos, A. Efficacy of tolterodine in preventing urge incontinence immediately after prostatectomy. Int. Urol. Nephrol. 2006, 38, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Shim, M.; Kim, J.; Park, S.; Choi, S.-K.; Lee, S.M.; Huh, K.O.; Song, C.; Choo, M.-S.; Ahn, H. The Therapeutic Effect of Solifenacin Succinate on the Recovery from Voiding Dysfunction After Radical Prostatectomy in Men with Clinically Localized Prostate Cancer: A Prospective, Randomized, Controlled Study. Urology 2015, 85, 1123–1129. [Google Scholar] [CrossRef] [PubMed]
- Bianco, F.J.; Albala, D.M.; Belkoff, L.H.; Miles, B.J.; Peabody, J.O.; He, W.; Bradt, J.S.; Haas, G.P.; Ahlering, T.E. A Randomized, Double-Blind, Solifenacin Succinate versus Placebo Control, Phase 4, Multicenter Study Evaluating Urinary Continence after Robotic Assisted Radical Prostatectomy. J. Urol. 2015, 193, 1305–1310. [Google Scholar] [CrossRef] [PubMed]
- De Nunzio, C.; Brucker, B.; Bschleipfer, T.; Cornu, J.-N.; Drake, M.J.; Fusco, F.; Gravas, S.; Oelke, M.; Peyronnet, B.; Tutolo, M.; et al. Beyond Antimuscarinics: A Review of Pharmacological and Interventional Options for Overactive Bladder Management in Men. Eur. Urol. 2021, 79, 492–504. [Google Scholar] [CrossRef]
- Sebastianelli, A.; I Russo, G.; A Kaplan, S.; McVary, K.T.; Moncada, I.; Gravas, S.; Chapple, C.; Morgia, G.; Serni, S.; Gacci, M. Systematic review and meta-analysis on the efficacy and tolerability of mirabegron for the treatment of storage lower urinary tract symptoms/overactive bladder: Comparison with placebo and tolterodine. Int. J. Urol. 2017, 25, 196–205. [Google Scholar] [CrossRef]
- Tubaro, A.; Batista, J.E.; Nitti, V.W.; Herschorn, S.; Chapple, C.R.; Blauwet, M.B.; Siddiqui, E.; Huang, M.; Oelke, M. Efficacy and safety of daily mirabegron 50 mg in male patients with overactive bladder: A critical analysis of five phase III studies. Ther. Adv. Urol. 2017, 9, 137–154. [Google Scholar] [CrossRef]
- Sandhu, J.S.; Breyer, B.; Comiter, C.; Eastham, J.A.; Gomez, C.; Kirages, D.J.; Kittle, C.; Lucioni, A.; Nitti, V.; Stoffel, J.T.; et al. Incontinence after Prostate Treatment: AUA/SUFU Guideline. J. Urol. 2019, 202, 369–378. [Google Scholar] [CrossRef]
- Nguyen, L.; Leung, L.Y.; Walker, R.; Nitkunan, T.; Sharma, D.; Seth, J. The use of urethral bulking injections in post-prostatectomy stress urinary incontinence: A narrative review of the literature. Neurourol. Urodyn. 2019, 38, 2060–2069. [Google Scholar] [CrossRef]
- Choinière, R.; Violette, P.D.; Morin, M.; Tu, L.M.; Guyatt, G.H.; Reed, C.; Philie, C.-A.; Legault, B.; Beaudry, M.-M.; Ahmed, M.M.; et al. Evaluation of Benefits and Harms of Surgical Treatments for Post–radical Prostatectomy Urinary Incontinence: A Systematic Review and Meta-analysis. Eur. Urol. Focus 2021, 8, 1042–1052. [Google Scholar] [CrossRef]
- Meisterhofer, K.; Herzog, S.A.; Strini, K.A.; Sebastianelli, L.; Bauer, R.; Dalpiaz, O. Male Slings for Postprostatectomy Incontinence: A Systematic Review and Meta-analysis. Eur. Urol. Focus 2019, 6, 575–592. [Google Scholar] [CrossRef]
- Del Giudice, F.; Huang, J.; Li, S.; Sorensen, S.; Enemchukwu, E.; Maggi, M.; Salciccia, S.; Ferro, M.; Crocetto, F.; Pandolfo, S.D.; et al. Contemporary trends in the surgical management of urinary incontinence after radical prostatectomy in the United States. Prostate Cancer Prostatic Dis. 2022, 1–7. [Google Scholar] [CrossRef]
- Del Favero, L.; Tasso, G.; Deruyver, Y.; Tutolo, M.; Beels, E.; Schillebeeckx, C.; De Ridder, D.; Van der Aa, F. Long-term Functional Outcomes and Patient Satisfaction After AdVance and AdVanceXP Male Sling Surgery. Eur. Urol. Focus 2022, 8, 1408–1414. [Google Scholar] [CrossRef] [PubMed]
- Abrams, P.; Constable, L.D.; Cooper, D.; MacLennan, G.; Drake, M.J.; Harding, C.; Mundy, A.; McCormack, K.; McDonald, A.; Norrie, J.; et al. Outcomes of a Noninferiority Randomised Controlled Trial of Surgery for Men with Urodynamic Stress Incontinence After Prostate Surgery (MASTER). Eur. Urol. 2021, 79, 812–823. [Google Scholar] [CrossRef] [PubMed]
- Sacco, E.; Gandi, C.; Marino, F.; Totaro, A.; Di Gianfrancesco, L.; Palermo, G.; Pierconti, F.; Racioppi, M.; Bassi, P. Artificial urinary sphincter significantly better than fixed sling for moderate post-prostatectomy stress urinary incontinence: A propensity score-matched study. BJU Int. 2020, 127, 229–237. [Google Scholar] [CrossRef]
- Khouri, R.K., Jr.; Ortiz, N.M.; Baumgarten, A.S.; Ward, E.E.; VanDyke, M.E.; Hudak, S.J.; Morey, A.F. Artificial Urinary Sphincter Outperforms Sling for Moderate Male Stress Urinary Incontinence. Urology 2020, 141, 168–172. [Google Scholar] [CrossRef]
- McCall, A.N.; Rivera, M.E.; Elliott, D.S. Long-term Follow-up of the Virtue Quadratic Male Sling. Urology 2016, 93, 213–216. [Google Scholar] [CrossRef]
- Malval, B.; Rebibo, J.-D.; Baron, M.; Nouhaud, F.-X.; Pfister, C.; Cornu, J.-N.; Grise, P. Long-term outcomes of I-Stop TOMS™ male sling implantation for post-prostatectomy incontinence management. Prog. En Urol. 2017, 27, 1084–1090. [Google Scholar] [CrossRef]
- Angulo, J.C.; Ruiz, S.; Lozano, M.; Arance, I.; Virseda, M.; Lora, D. Systematic review and meta-analysis comparing Adjustable Transobturator Male System (ATOMS) and male Readjustment Mechanical External (REMEEX) system for post-prostatectomy incontinence. World J. Urol. 2020, 39, 1083–1092. [Google Scholar] [CrossRef]
- Constable, L.; Abrams, P.; Cooper, D.; Kilonzo, M.; Cotterill, N.; Harding, C.; Drake, M.J.; Pardoe, M.N.; McDonald, A.; Smith, R.; et al. Synthetic sling or artificial urinary sphincter for men with urodynamic stress incontinence after prostate surgery: The MASTER non-inferiority RCT. Health Technol. Assess. 2022, 26, 1–152. [Google Scholar] [CrossRef]
- Siracusano, S.; Visalli, F.; Favro, M.; Tallarigo, C.; Saccomanni, M.; Kugler, A.; Diminutto, A.; Talamini, R.; Artibani, W. Argus-T Sling in 182 Male Patients: Short-term Results of a Multicenter Study. Urology 2017, 110, 177–183. [Google Scholar] [CrossRef]
- Mühlstädt, S.; Angulo, J.C.; Mohammed, N.; Schumann, A.; Fornara, P. Complications of the urinary incontinence system ATOMS: Description of risk factors and how to prevent these pitfalls. World J. Urol. 2019, 38, 1795–1803. [Google Scholar] [CrossRef]
- Cornu, J.-N.; Sebe, P.; Ciofu, C.; Peyrat, L.; Cussenot, O.; Haab, F. Mid-term evaluation of the transobturator male sling for post-prostatectomy incontinence: Focus on prognostic factors. BJU Int. 2010, 108, 236–240. [Google Scholar] [CrossRef] [PubMed]
- Munier, P.; Nicolas, M.; Tricard, T.; Droupy, S.; Costa, P.; Saussine, C. What if artificial urinary sphincter is not possible? Feasibility and effectiveness of ProACT for patients with persistent stress urinary incontinence after radical prostatectomy treated by sling. Neurourol. Urodyn. 2020, 39, 1417–1422. [Google Scholar] [CrossRef]
- Van der Aa, F.; Drake, M.J.; Kasyan, G.R.; Petrolekas, A.; Cornu, J.-N. The Artificial Urinary Sphincter after a Quarter of a Century: A Critical Systematic Review of Its Use in Male Non-neurogenic Incontinence. Eur. Urol. 2013, 63, 681–689. [Google Scholar] [CrossRef]
- Plata, M.; Zuluaga, L.; Santander, J.; Salazar, M.; Castaño, J.C.; Benavides-Martínez, J.A.; Garzón, D.L.; Schlesinger, R.; Serrano, B.; Echeverry, M.; et al. Performance of the artificial urinary sphincter implantation in men with urinary incontinence: Results from a contemporary long-term real-world nationwide analysis. Neurourol. Urodyn. 2022, 41, 1573–1581. [Google Scholar] [CrossRef]
- Deruyver, Y.; Schillebeeckx, C.; Beels, E.; De Ridder, D.; Van der Aa, F. Long-term outcomes and patient satisfaction after artificial urinary sphincter implantation. World J. Urol. 2021, 40, 497–503. [Google Scholar] [CrossRef]
- Tutolo, M.; Cornu, J.; Bauer, R.M.; Ahyai, S.; Bozzini, G.; Heesakkers, J.; Drake, M.J.; Tikkinen, K.; Launonen, E.; Larré, S.; et al. Efficacy and safety of artificial urinary sphincter (AUS): Results of a large multi-institutional cohort of patients with mid-term follow-up. Neurourol. Urodyn. 2018, 38, 710–718. [Google Scholar] [CrossRef]
- Mock, S.; Dmochowski, R.R.; Brown, E.T.; Reynolds, W.; Kaufman, M.R.; Milam, D.F. The Impact of Urethral Risk Factors on Transcorporeal Artificial Urinary Sphincter Erosion Rates and Device Survival. J. Urol. 2015, 194, 1692–1696. [Google Scholar] [CrossRef]
- Beaugerie, A.; Poinard, F.; Denormandie, A.; Cotte, J.; Reus, C.; Mozer, P.; Chartier-Kastler, E. Comparative Urodynamic Study in Cadaver of Urethral Pressure Profilometry between the UroMems Artificial Urinary Sphincter and the AMS800™; Elsevier: Amsterdam, The Netherlands, 2022; EAU 2022, Abstract A0586. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gacci, M.; De Nunzio, C.; Sakalis, V.; Rieken, M.; Cornu, J.-N.; Gravas, S. Latest Evidence on Post-Prostatectomy Urinary Incontinence. J. Clin. Med. 2023, 12, 1190. https://doi.org/10.3390/jcm12031190
Gacci M, De Nunzio C, Sakalis V, Rieken M, Cornu J-N, Gravas S. Latest Evidence on Post-Prostatectomy Urinary Incontinence. Journal of Clinical Medicine. 2023; 12(3):1190. https://doi.org/10.3390/jcm12031190
Chicago/Turabian StyleGacci, Mauro, Cosimo De Nunzio, Vasileios Sakalis, Malte Rieken, Jean-Nicolas Cornu, and Stavros Gravas. 2023. "Latest Evidence on Post-Prostatectomy Urinary Incontinence" Journal of Clinical Medicine 12, no. 3: 1190. https://doi.org/10.3390/jcm12031190









